Influence of Anodizing Parameters on Surface Morphology and Surface-Free Energy of Al2O3 Layers Produced on EN AW-5251 Alloy
Abstract
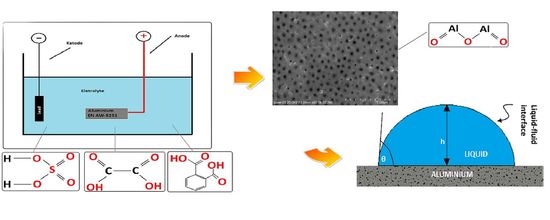
:1. Introduction
2. Materials and Methods
2.1. Research Material
2.2. Research Methodology
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Davis, J.R. Aluminum and Aluminum Alloys; ASM International: Metals Park, OH, USA, 1993. [Google Scholar]
- Davis, J.R. Corrosion of Aluminum and Aluminum Alloys; ASM International: Metals Park, OH, USA, 1999. [Google Scholar]
- Diggle, J.W.; Downie, T.C.; Goulding, C.W. Anodic oxide films on aluminum. Chem. Rev. 1969, 69, 365–405. [Google Scholar] [CrossRef]
- Thompson, G.E. Porous anodic alumina: Fabrication, characterization and applications. Thin Solid Films 1997, 297, 192–201. [Google Scholar] [CrossRef]
- Sulka, G.D. Highly ordered anodic porous alumina formation by self-organized anodizing. In Nanostructured Materials in Electrochemistry; Eftekhari, A., Ed.; Wiley-VCH Verlag GmbH & Co., KGaA: Weinheim, Germany, 2008; pp. 8–20. [Google Scholar]
- Lee, W.; Ji, R.; Gosele, U.; Nielsch, K. Fast fabrication of long-range ordered porous alumina membranes by hard anodization. Nat. Mater. 2006, 5, 741–747. [Google Scholar] [CrossRef] [PubMed]
- Stepniowski, W.J.; Moneta, M.; Karczewski, K.; Michalska-Domanska, M.; Czujko, T.; Mol, J.M.; Buijnsters, J.G. Fabrication of copper nanowires via electrodeposition in anodic aluminum oxide templates formed by combined hard anodizing and electrochemical barrier layer thinning. J. Electroanal. Chem. 2018, 809, 59–66. [Google Scholar] [CrossRef]
- Sattler, K. Handbook of Nanophysics/Functional Nanomaterials; CRC Press: Boca Raton, FL, USA, 2011; p. 787. [Google Scholar]
- Liu, P.; Singh, V.P.; Rajaputra, S. Barrier layer nonuniformity effects in anodized aluminum oxide nanopores on ITO substrates. Nanotechnology 2010, 21, 115303. [Google Scholar] [CrossRef] [PubMed]
- Fratila-Apachitei, L.E.; Tichelaar, F.D.; Thompson, G.E.; Terryn, H.; Skeldon, P.; Duszczyk, J.; Katgerman, L. A transmission electron microscopy study of hard anodic oxide layers on AlSi(Cu) alloys. Electrochim. Acta 2004, 49, 3169–3177. [Google Scholar] [CrossRef]
- Runge, J.M. The Metallurgy of Anodizing Aluminum—Connecting Science to Practice, 1st ed.; Springer International Publishing: Cham, Switzerland, 2018. [Google Scholar]
- Zhang, L.; Cho, H.S.; Li, F.; Metzger, R.M.; Doyle, W.D. Cellular growth of highly ordered porous anodic films on aluminium. J. Mater. Sci. Lett. 1998, 17, 291–294. [Google Scholar] [CrossRef]
- Jia, Y.; Zhou, H.; Luo, P.; Luo, S.; Chen, J.; Kuang, Y. Preparation and characteristics of well-aligned macroporous films on aluminum by high voltage anodization in mixed acid. Surf. Coat. Technol. 2006, 201, 513–518. [Google Scholar] [CrossRef]
- Kubica, M.; Skoneczny, W.; Bara, M. Analysis of Al2O3 Nanostructure Using Scanning Microscopy. Scanning 2018, 2018, 8459768. [Google Scholar] [CrossRef] [PubMed]
- Bara, M.; Skoneczny, W.; Hajduga, M. Ceramic-graphite surface layers obtained by the duplex method on an aluminium alloy substrate. Chem. Process. Eng. 2009, 30, 431–442. [Google Scholar]
- Wang, Q.; Zhang, B.W.; Qu, M.N.; Zhang, J.Y.; He, D.Y. Fabrication of superhydrophobic surfaces on engineering material surfaces with stearic acid. Appl. Surf. Sci. 2008, 254, 2009–2012. [Google Scholar] [CrossRef]
- Zhang, X.; Shi, F.; Niu, J.; Jiang, Y.G.; Wang, Z.Q. Superhydrophobic surfaces: From structural control to functional application. J. Mater. Chem. 2008, 18, 621–633. [Google Scholar] [CrossRef]
- Sooksaena, P.; Chulasinonta, O.; Janmata, P.; Thovasakula, W. Chemical treatment on aluminum alloy for hydrophobic surfaces. Mater. Today-Proc. 2017, 4, 6528–6533. [Google Scholar] [CrossRef]
- Skoneczny, W.; Niedźwiedź, M.; Bara, M. The Effect of Production Parameters of Oxide Layers on Their Nanostructure, Nanomorphology, and Surface Free Energy. Appl. Sci. 2018, 8, 2251. [Google Scholar] [CrossRef]
- Korzekwa, J.; Tenne, R.; Skoneczny, W.; Dercz, G. Two-step method for preparation of Al2O3 /IF-WS2 nanoparticles composite coating. Phys. Status Solidi (A) Appl. Mater. Sci. 2013, 210, 2292–2297. [Google Scholar] [CrossRef]
- Whyman, G.; Bormashenko, E.; Stein, T. The rigorous derivation of Young, Cassie–Baxter and Wenzel equations and the analysis of the contact angle hysteresis phenomenon. Chem. Phys. Lett. 2008, 450, 355–359. [Google Scholar] [CrossRef]






| Sample | Current Density j (A/dm2) | Process Time t (min) | Electrolyte Temperature T (K) |
|---|---|---|---|
| A | 1 | 20 | 283 |
| B | 1 | 20 | 293 |
| C | 1 | 20 | 303 |
| D | 1 | 20 | 313 |
| E | 2 | 60 | 293 |
| F | 3 | 60 | 293 |
| G | 3 | 60 | 298 |
| H | 3 | 60 | 303 |
| I | 4 | 45 | 303 |
| J | 4 | 45 | 298 |
| K | 4 | 20 | 283 |
| L | 4 | 20 | 313 |
| Sample | Thickness (µm) | Deviation (µm) |
|---|---|---|
| A | 6.5 | 0.6 |
| B | 5.6 | 0.4 |
| C | 5.3 | 0.7 |
| D | 3.3 | 0.6 |
| E | 36.6 | 1.1 |
| F | 52.9 | 1.7 |
| G | 51.4 | 1 |
| H | 50.2 | 1 |
| I | 53.5 | 1.5 |
| J | 53.5 | 2.5 |
| K | 26.3 | 1.5 |
| L | 23.6 | 0.6 |
| Sample | Porosity (%) | Deviation (%) | Pore Density (Number of Pores/µm2) | Deviation (Number of Pores/µm2) | Average Area of Nanopores (nm2) | Deviation (nm2) |
|---|---|---|---|---|---|---|
| A | 4.2 | 0.16 | 59.2 | 2.26 | 706.9 | 26.93 |
| B | 4.4 | 0.19 | 34.5 | 1.49 | 1275.2 | 55.07 |
| C | 4.8 | 0.20 | 83.9 | 3.50 | 568.6 | 23.69 |
| D | 3.3 | 0.11 | 182.8 | 6.11 | 183 | 6.10 |
| E | 5.7 | 0.21 | 66.7 | 2.46 | 858.1 | 31.61 |
| F | 8.2 | 0.38 | 59 | 2.73 | 1378.9 | 63.90 |
| G | 7.5 | 0.33 | 40.2 | 1.77 | 1849.1 | 81.36 |
| H | 14.6 | 0.61 | 72.9 | 3.01 | 2004.7 | 83.76 |
| I | 12.3 | 0.46 | 52.2 | 1.95 | 2358.7 | 88.22 |
| J | 11.5 | 0.46 | 55.2 | 2.21 | 2086.6 | 83.46 |
| K | 3.6 | 0.21 | 36.3 | 2.12 | 997.1 | 58.16 |
| L | 10.3 | 0,41 | 59.27 | 2.36 | 1744 | 69.42 |
| Sample | Contact Angle (Distilled Water) (°) | Deviation (°) | Contact Angle (Diiodomethane) (°) | Deviation (°) |
|---|---|---|---|---|
| A | 90.8 | 2.70 | 59.05 | 2.26 |
| B | 65.49 | 5.98 | 48.4 | 4.75 |
| C | 82.25 | 5.14 | 47.74 | 4.38 |
| D | 83.18 | 4.85 | 44.74 | 4.25 |
| E | 71.8 | 3.25 | 47.15 | 5.56 |
| F | 61.66 | 5.52 | 48.18 | 2.69 |
| G | 73.86 | 2.83 | 46.84 | 3.23 |
| H | 69.55 | 6.50 | 46.25 | 3.17 |
| I | 79.82 | 3.08 | 51.35 | 4.02 |
| J | 75.13 | 2.51 | 59.05 | 3.76 |
| K | 83.4 | 2.29 | 48.4 | 2.29 |
| L | 84.3 | 3.42 | 47.74 | 3.74 |
| Sample | Contact Angle (α-Bromonaphthalene) (°) | Deviation (°) | Contact Angle (Glycerin) (°) | Deviation (°) |
|---|---|---|---|---|
| A | 39.88 | 4.44 | 84.25 | 7.69 |
| B | 33.05 | 2.80 | 73.80 | 2.55 |
| C | 37.68 | 3.23 | 80.22 | 5.24 |
| D | 34.36 | 4.37 | 83.62 | 4.41 |
| E | 29.71 | 4.48 | 69.59 | 4.51 |
| F | 30.09 | 2.70 | 72.40 | 3.29 |
| G | 24.97 | 4.12 | 71.95 | 4.70 |
| H | 33.63 | 1.78 | 72.01 | 2.44 |
| I | 29.54 | 1.57 | 74.46 | 3.58 |
| J | 29.91 | 2.36 | 73.40 | 3.71 |
| K | 32.40 | 3.79 | 75.75 | 3.33 |
| L | 31.07 | 2.37 | 74.25 | 3.37 |
| Sample | SFE Owens-Wendt (mJ/m2) |
|---|---|
| A | 31.36 |
| B | 46.51 |
| C | 40.39 |
| D | 39.04 |
| E | 43.66 |
| F | 49.12 |
| G | 40.84 |
| H | 43.08 |
| I | 39.08 |
| J | 41.32 |
| K | 38.42 |
| L | 38.24 |
| Sample | Atomic Aluminum Content (%) | Error of Aluminum Content (%) | Atomic Oxygen Content (%) | Error of Oxygen Content (%) |
|---|---|---|---|---|
| A | 72.66 | ±0.39 | 26.88 | ±1.15 |
| K | 59.94 | ±0.31 | 39.94 | ±0.93 |
| H | 57.13 | ±0.29 | 42.36 | ±0.86 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bara, M.; Niedźwiedź, M.; Skoneczny, W. Influence of Anodizing Parameters on Surface Morphology and Surface-Free Energy of Al2O3 Layers Produced on EN AW-5251 Alloy. Materials 2019, 12, 695. https://doi.org/10.3390/ma12050695
Bara M, Niedźwiedź M, Skoneczny W. Influence of Anodizing Parameters on Surface Morphology and Surface-Free Energy of Al2O3 Layers Produced on EN AW-5251 Alloy. Materials. 2019; 12(5):695. https://doi.org/10.3390/ma12050695
Chicago/Turabian StyleBara, Marek, Mateusz Niedźwiedź, and Władysław Skoneczny. 2019. "Influence of Anodizing Parameters on Surface Morphology and Surface-Free Energy of Al2O3 Layers Produced on EN AW-5251 Alloy" Materials 12, no. 5: 695. https://doi.org/10.3390/ma12050695