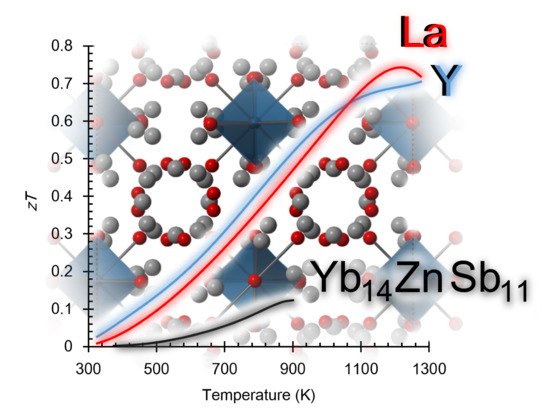
Seebeck and Figure of Merit Enhancement by Rare Earth Doping in Yb14-xRExZnSb11 (x = 0.5)
Abstract
:1. Introduction
2. Materials and Methods
2.1. Synthesis
2.2. Consolidation of Powder
2.3. Electron Microprobe Analysis and Wavelength Dispersive Spectroscopy
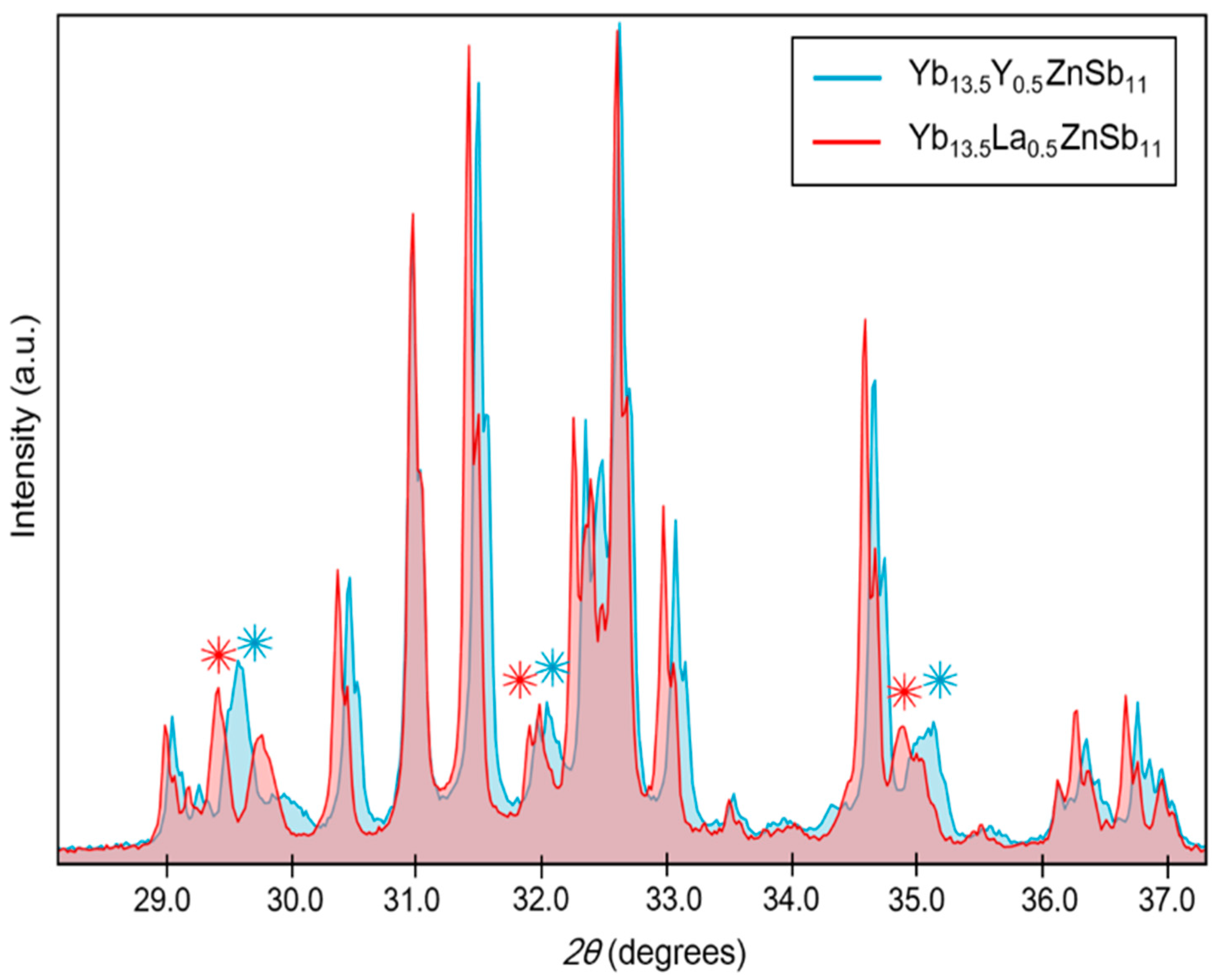
2.4. Powder X-Ray Diffraction
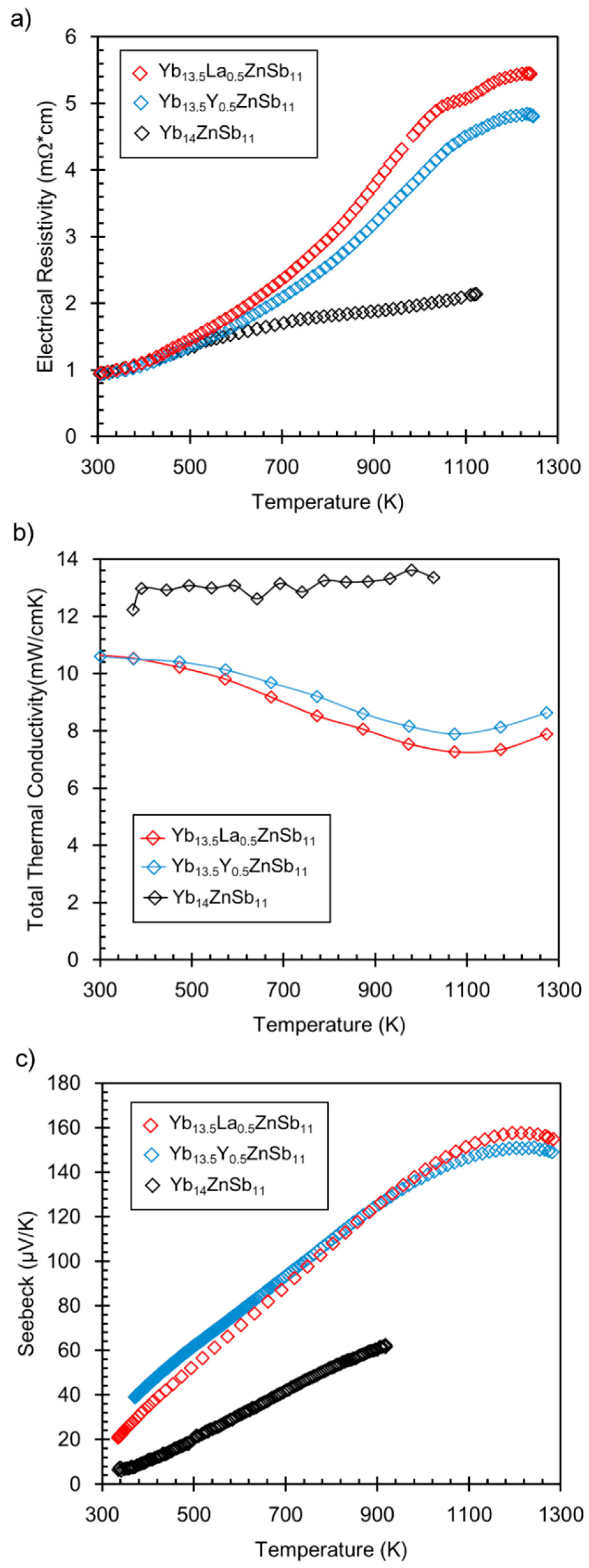
2.5. Electrical Resistivity, Hall Effect, and Seebeck Coefficient
2.6. Thermal Conductivity
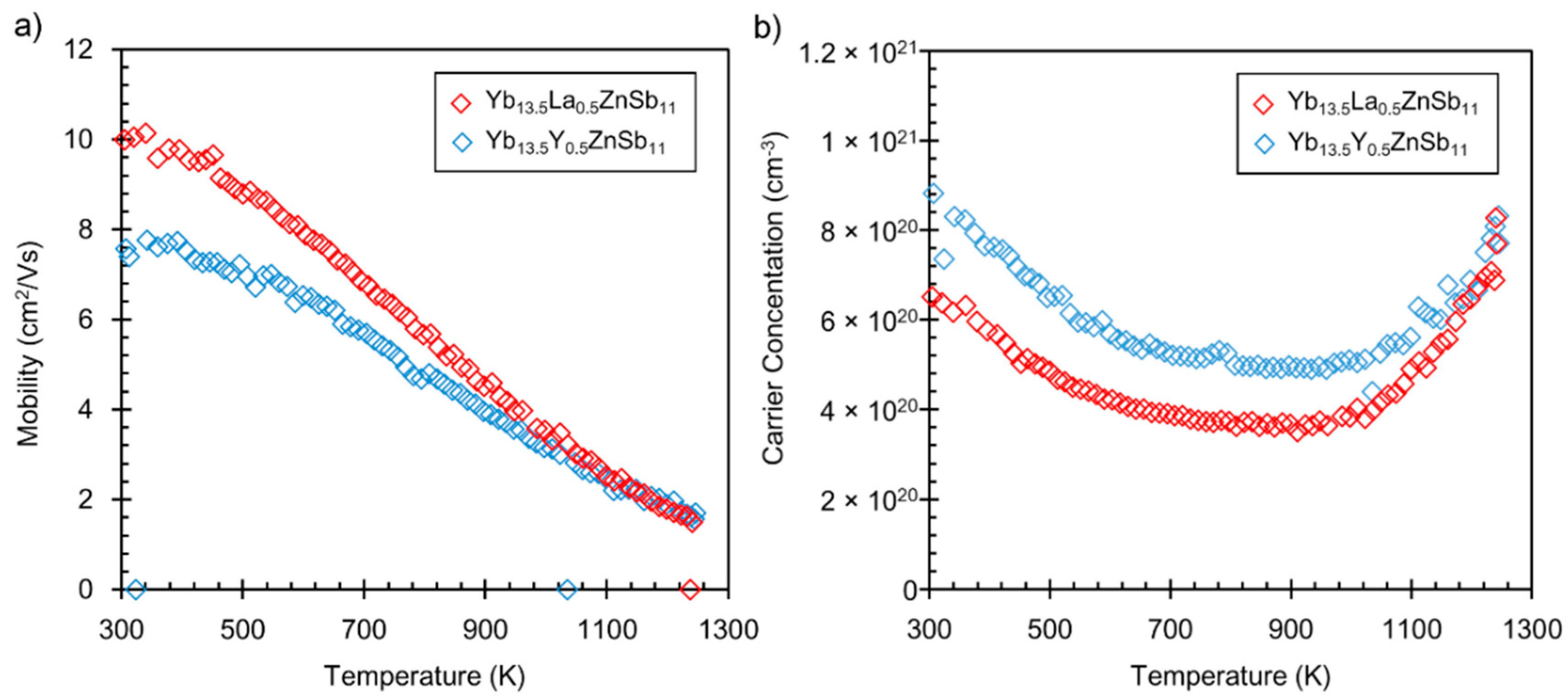
3. Results
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Kauzlarich, S.M.; Brown, S.R.; Jeffrey Snyder, G. Zintl phases for thermoelectric devices. Dalt. Trans. 2007, 2099. [Google Scholar] [CrossRef] [PubMed]
- Hu, Y.; Cerretti, G.; Kunz Wille, E.L.; Bux, S.K.; Kauzlarich, S.M. The remarkable crystal chemistry of the Ca14AlSb11 structure type, magnetic and thermoelectric properties. J. Solid State Chem. 2019, 271, 88–102. [Google Scholar] [CrossRef]
- Kauzlarich, S.M.; Zevalkink, A.; Toberer, E.; Snyder, G.J. Zintl phases: Recent developments in thermoelectrics and future outlook. RSC Energy Environ. Ser. 2017, 2017, 1–26. [Google Scholar]
- Toberer, E.S.; May, A.F.; Snyder, G.J. Zintl Chemistry for Designing High Efficiency Thermoelectric Materials. Chem. Mater. 2010, 22, 624–634. [Google Scholar] [CrossRef]
- Brown, S.R.; Kauzlarich, S.M.; Gascoin, F.; Jeffrey Snyder, G. Yb14MnSb11: New high efficiency thermoelectric material for power generation. Chem. Mater. 2006, 18, 1873–1877. [Google Scholar] [CrossRef]
- Hu, Y.; Wang, J.; Kawamura, A.; Kovnir, K.; Kauzlarich, S.M. Yb14MgSb11 and Ca14MgSb11—New Mg-Containing Zintl Compounds and Their Structures, Bonding, and Thermoelectric Properties. Chem. Mater. 2015, 27, 343–351. [Google Scholar] [CrossRef]
- Fisher, I.R.; Bud’ko, S.L.; Song, C.; Canfield, P.C.; Ozawa, T.C.; Kauzlarich, S.M. Yb14ZnSb11: Charge balance in Zintl compounds as a route to intermediate Yb valence. Phys. Rev. Lett. 2000, 85, 1120–1123. [Google Scholar] [CrossRef] [PubMed]
- Holm, A.P.; Ozawa, T.C.; Kauzlarich, S.M.; Morton, S.A.; Dan Waddill, G.; Tobin, J.G. X-ray photoelectron spectroscopy studies of Yb14MnSb11 and Yb14ZnSb11. J. Solid State Chem. 2005, 178, 262–269. [Google Scholar] [CrossRef]
- Brown, S.R.; Toberer, E.S.; Ikeda, T.; Cox, C.A.; Gascoin, F.; Kauzlarich, S.M.; Snyder, G.J. Improved Thermoelectric Performance in Yb14Mn1−xZnxSb11 by the Reduction of Spin-Disorder Scattering. Chem. Mater. 2008, 20, 3412–3419. [Google Scholar] [CrossRef]
- Sampathkumaran, E. V Intermediate valence in rare earth systems. Hyperfine Interact. 1986, 27, 183–192. [Google Scholar] [CrossRef]
- Kunz Wille, E.L.; Jo, N.H.; Fettinger, J.C.; Canfield, P.C.; Kauzlarich, S.M. Single crystal growth and magnetic properties of the mixed valent Yb containing Zintl phase, Yb14MgSb11. Chem. Commun. 2018, 54, 12946–12949. [Google Scholar] [CrossRef] [PubMed]
- Stone, M.B.; Garlea, V.O.; Gillon, B.; Cousson, A.; Christianson, A.D.; Lumsden, M.D.; Nagler, S.E.; Mandrus, D.; Sales, B.C. Excitations and magnetization density distribution in the dilute ferromagnetic semiconductor Yb14 MnSb11. Phys. Rev. B 2017, 95, 020412. [Google Scholar] [CrossRef]
- Young, D.M.; Torardi, C.C.; Olmstead, M.M.; Kauzlarich, S.M. Exploring the Limits of the Zintl Concept for the A14MPn11 Structure Type with M = Zn, Cd. Chem. Mater. 1995, 7, 93–101. [Google Scholar] [CrossRef]
- Toberer, E.S.; Brown, S.R.; Ikeda, T.; Kauzlarich, S.M.; Jeffrey Snyder, G. High thermoelectric efficiency in lanthanum doped Yb14MnSb11. Appl. Phys. Lett. 2008, 93, 062110. [Google Scholar] [CrossRef]
- Hu, Y.; Chen, C.-W.; Cao, H.; Makhmudov, F.; Grebenkemper, J.H.; Abdusalyamova, M.N.; Morosan, E.; Kauzlarich, S.M. Tuning Magnetism of [MnSb4]9− Cluster in Yb14MnSb11 through Chemical Substitutions on Yb Sites: Appearance and Disappearance of Spin Reorientation. J. Am. Chem. Soc. 2016, 138, 12422–12431. [Google Scholar] [CrossRef] [PubMed]
- Grebenkemper, J.H.; Kauzlarich, S.M. Magnetic and structural effects of partial Ce substitution in Yb14 MnSb11. APL Mater. 2015, 3, 041503. [Google Scholar] [CrossRef]
- Sales, B.C.; Khalifah, P.; Enck, T.P.; Nagler, E.J.; Sykora, R.E.; Jin, R.; Mandrus, D. Kondo Lattice Behavior in the Ordered Dilute Magnetic Semiconductor Yb14-xLaxMnSb11. Phys. Rev. B 2005, 72. [Google Scholar] [CrossRef]
- Prakash, J.; Stoyko, S.; Voss, L.; Bobev, S. On the Extended Series of Quaternary Zintl Phases Ca13REMnSb11 (RE = La–Nd, Sm, Gd–Dy). Eur. J. Inorg. Chem. 2016, 2016, 2912–2922. [Google Scholar] [CrossRef]
- Toby, B.H.; Von Dreele, R.B. GSAS-II: The genesis of a modern open-source all purpose crystallography software package. J. Appl. Crystallogr. 2013, 46, 544–549. [Google Scholar] [CrossRef]
- Kourkoumelis, N. Recent developments. ICDD Annu. Spring Meet. Powder Diffr. 2013, 28, 137–148. [Google Scholar]
- Borup, K.A.; De Boor, J.; Wang, H.; Drymiotis, F.; Gascoin, F.; Shi, X.; Chen, L.; Fedorov, M.I.; Müller, E.; Iversen, B.B.; et al. Measuring thermoelectric transport properties of materials. Energy Environ. Sci. 2015, 8, 423–435. [Google Scholar] [CrossRef] [Green Version]
- Ravi, V.; Firdosy, S.; Caillat, T.; Brandon, E.; Van Der Walde, K.; Maricic, L.; Sayir, A. Thermal expansion studies of selected high-temperature thermoelectric materials. J. Electron. Mater. 2009, 38, 1433–1442. [Google Scholar] [CrossRef]
- Cox, C.A.; Toberer, E.S.; Levchenko, A.A.; Brown, S.R.; Snyder, G.J.; Navrotsky, A.; Kauzlarich, S.M. Structure, Heat Capacity, and High-Temperature Thermal Properties of Yb14Mn1−xAlxSb11. Chem. Mater. 2009, 21, 1354–1360. [Google Scholar] [CrossRef]
- Grebenkemper, J.H.; Hu, Y.; Barrett, D.; Gogna, P.; Huang, C.-K.; Bux, S.K.; Kauzlarich, S.M. High Temperature Thermoelectric Properties of Yb14MnSb11 Prepared from Reaction of MnSb with the Elements. Chem. Mater. 2015, 27, 5791–5798. [Google Scholar] [CrossRef]
- Honig, R.E. Vapor Pressure Data for the More Common Elements. RCA Rev. 1957, 18, 195–204. [Google Scholar]
- Shannon, R.D. IUCr Revised effective ionic radii and systematic studies of interatomic distances in halides and chalcogenides. Acta Crystallogr. Sect. A 1976, 32, 751–767. [Google Scholar] [CrossRef]
- Hu, Y.; Bux, S.K.; Grebenkemper, J.H.; Kauzlarich, S.M. The effect of light rare earth element substitution in Yb14MnSb11 on thermoelectric properties. J. Mater. Chem. C 2015, 3, 10566–10573. [Google Scholar] [CrossRef]
- Wilson, D.K.; Saparov, B.; Bobev, S. Synthesis, Crystal Structures and Properties of the Zintl Phases Sr2ZnP2, Sr2ZnAs2, A2ZnSb2 and A2ZnBi2 (A = Sr and Eu). Z. Anorg. Allg. Chem. 2011, 637, 2018–2025. [Google Scholar] [CrossRef]
- Varma, C.M. Mixed-valence compounds. Rev. Mod. Phys. 1976, 48, 219–238. [Google Scholar] [CrossRef]
- Stewart, G.R. Heavy-fermion systems. Rev. Mod. Phys. 1984, 56, 755–787. [Google Scholar] [CrossRef]
- Vasilyeva, I.G.; Nikolaev, R.E.; Abdusaljamova, M.N.; Kauzlarich, S.M. Thermochemistry study and improved thermal stability of Yb14MnSb11 alloyed by Ln3+ (La–Lu). J. Mater. Chem. C 2016, 4, 3342–3348. [Google Scholar] [CrossRef]
- Zevalkink, A.; Smiadak, D.M.; Blackburn, J.L.; Ferguson, A.J.; Chabinyc, M.L.; Delaire, O.; Wang, J.; Kovnir, K.; Martin, J.; Schelhas, L.T.; et al. A practical field guide to thermoelectrics: Fundamentals, synthesis, and characterization. Appl. Phys. Rev. 2018, 5, 21303. [Google Scholar] [CrossRef]



| As Loaded | Yb | RE | Zn | Sb | |
|---|---|---|---|---|---|
| Main Phase | Yb13.5Y0.5ZnSb11 | 13.7(2) | 0.35(1) | 0.85(5) | 11.0(1) |
| Yb13.5La0.5ZnSb11 | 13.7(2) | 0.48(5) | 0.91(5) | 11.0(1) | |
| Secondary Phase | Yb13.5Y0.5ZnSb11 | 1.96(2) | 0.04(1) | 0.78(2) | 2.00(2) |
| Yb13.5La0.5ZnSb11 | 1.95(2) | 0.08(1) | 0.79(2) | 2.00(2) |
| As Loaded | a (Å) | c (Å) | V (Å3) | wR (Overall) | RF2/RF (14-1-11 Phase) |
|---|---|---|---|---|---|
| Yb13.5Y0.5ZnSb11 | 16.5939(4) | 21.9309(7) | 6038.9(3) | 20.812% | 14.122%/9.616% |
| Yb13.5La0.5ZnSb11 | 16.6412(4) | 21.9188(6) | 6070.0(3) | 19.914% | 12.328%/8.316% |
| Sample | T (K) | m* (m0) | µ0 (cm2/V∙s) | κL (mW/cm∙K) |
|---|---|---|---|---|
| Yb13.5Y0.5ZnSb11 | 400 | 1.40 | 18.95 | 2.4 |
| 800 | 1.47 | 7.61 | 3.2 | |
| 1200 | 2.07 | 2.11 | 3.8 | |
| Yb13.5La0.5ZnSb11 | 400 | 1.18 | 24.44 | 3.4 |
| 800 | 1.15 | 9.3 | 3.4 | |
| 1200 | 2.07 | 2.08 | 3.5 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kunz Wille, E.L.; Grewal, N.S.; Bux, S.K.; Kauzlarich, S.M. Seebeck and Figure of Merit Enhancement by Rare Earth Doping in Yb14-xRExZnSb11 (x = 0.5). Materials 2019, 12, 731. https://doi.org/10.3390/ma12050731
Kunz Wille EL, Grewal NS, Bux SK, Kauzlarich SM. Seebeck and Figure of Merit Enhancement by Rare Earth Doping in Yb14-xRExZnSb11 (x = 0.5). Materials. 2019; 12(5):731. https://doi.org/10.3390/ma12050731
Chicago/Turabian StyleKunz Wille, Elizabeth L., Navtej S. Grewal, Sabah K. Bux, and Susan M. Kauzlarich. 2019. "Seebeck and Figure of Merit Enhancement by Rare Earth Doping in Yb14-xRExZnSb11 (x = 0.5)" Materials 12, no. 5: 731. https://doi.org/10.3390/ma12050731