Thermoelectric Properties of Scandium Sesquitelluride
Abstract
:1. Introduction
2. Materials and Methods
2.1. Synthesis
2.2. Characterization
3. Results and Discussion
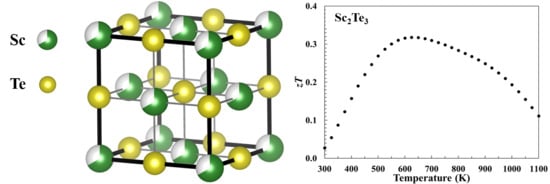
3.1. Phase Analysis
3.2. Electronic Transport Properties
3.3. Thermal Transport Properties
3.4. Dimensionless Thermoelectric Figure of Merit
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Fleurial, J.-P. Thermoelectric power generation materials: Technology and application opportunities. JOM 2009, 61, 74–85. [Google Scholar] [CrossRef]
- Wood, C. Materials for thermoelectric energy conversion. Rep. Prog. Phys. 1988, 51, 459–539. [Google Scholar] [CrossRef]
- Yang, J.; Caillat, T. Thermoelectric materials for space and automotive power generation. MRS Bull. 2006, 31, 224–229. [Google Scholar] [CrossRef]
- Vining, C.B.; Fleurial, J.-P. Silicon-Germanium: An Overview of Recent Development; AIP Press: Albuquerque, NM, USA, 1993; pp. 87–120. [Google Scholar]
- Snyder, G.J.; Toberer, E.S. Complex Thermoelectric Materials. Nat. Mater. 2008, 7, 105–114. [Google Scholar] [CrossRef] [PubMed]
- May, A.F.; Fleurial, J.-P.; Snyder, G.J. Thermoelectric performance of lanthanum telluride produced via mechanical alloying. Phys. Rev. B 2008, 78, 125205. [Google Scholar] [CrossRef]
- Cheikh, D.; Hogan, B.E.; Vo, T.; von Allmen, P.; Lee, K.; Smiadak, D.M.; Zevalkink, A.; Dunn, B.S.; Fleurial, J.-P.; Bux, S.K. Praseodymium Telluride: A High Temperature, High ZT Thermoelectric Material. Joule 2018, 2, 698–709. [Google Scholar] [CrossRef]
- Vickerv, R.C.; Muir, H.M. Thermoelectric properties of rare earth chalcogenides. Adv. Energy Convers. 1961, 1, 179–186. [Google Scholar] [CrossRef]
- Ma, J.M.; Clarke, S.M.; Zeier, W.G.; Vo, T.; von Allmen, P.; Snyder, G.J.; Kaner, R.B.; Fleurial, J.-P.; Bux, S.K. Mechanochemical synthesis and high temperature thermoelectric properties of calcium-doped lanthanum telluride La3−xCaxTe4. J. Mater. Chem. C 2015, 3, 10459–10466. [Google Scholar] [CrossRef]
- Taher, S.M.; Gruber, J.B. Thermoelectric efficiency of rare earth sesquisulfides. Mat. Res. Bull. 2001, 16, 1407–1412. [Google Scholar] [CrossRef]
- Cutler, M.; Leavy, J.F.; Fitzpatrick, R.L. Electronic Transport in Semimetallic Cerium Sulfide. Phys. Rev 1964, 133, A1143. [Google Scholar] [CrossRef]
- May, A.F.; Singh, D.J.; Snyder, G.J. Influence of band structure on the large thermoelectric performance of lanthanum telluride. Phys. Rev. B. 2009, 9, 153101. [Google Scholar] [CrossRef]
- Vo, T.; von Allmen, P.; Huang, C.-H.; Ma, J.M.; Bux, S.K.; Fleurial, J.-P. Electronic and thermoelectric properties of Ce3Te4 and La3Te4 computed with density functional theory with on-site Coulomb interaction correction. Appl. Phys. 2014, 116, 133701. [Google Scholar] [CrossRef]
- Connelly, N.G.; Damhus, T.; Hartshorn, R.M.; Hutton, A.T. Elements in the Periodic Table. In Nomenclature of Inorganic Chemistry, 1st ed.; The Royal Society of Chemistry: Cambridge, UK, 2005; ISBN 0-85404-438-8. [Google Scholar]
- Ahmad, Z. The Properties and Application of Scandium-Reinforced Aluminum. JOM 2003, 55, 35–39. [Google Scholar] [CrossRef]
- Parker, B.A.; Zhou, Z.F.; Nolle, S.P. The Effect of Small Addition of Scandium on the Properties of the Aluminum Alloys. J. Mater. Sci. 1995, 30, 452–458. [Google Scholar] [CrossRef]
- Sawtell, R.R.; Jensen, C.L. Mechanical Properties and Microstructure of Al-Mg-Sc Alloys. Metal. Trans. A 1990, 21, 421–430. [Google Scholar] [CrossRef]
- Jindal, V.; De, P.K.; Venkateswarlu, K. Effect of Al3Sc precipitates on the work hardening behavior of aluminum–scandium alloys. Mater. Lett. 2006, 60, 3373–3375. [Google Scholar] [CrossRef]
- Wygant, B.R.; Jarvis, K.A.; Chemelewski, W.D.; Mabayoje, O.; Celio, H.; Mullins, C.B. Structural and Catalytic Effects of Iron- and Scandium-Doping on a Strontium Cobalt Oxide Electrocatalyst for Water Oxidation. ACS Catal. 2016, 6, 1122–1133. [Google Scholar] [CrossRef]
- Yin, Y.M.; Xiong, M.W.; Yang, N.T.; Tong, Z.; Guo, Y.Q.; Ma, Z.F.; Sun, E.; Yamanis, J.; Jing, B.Y. Investigation on thermal, electrical, and electrochemical properties of scandium-doped Pr0.6Sr0.4(Co0.2Fe0.8)(1-x)ScxO3-d as cathode for IT-SOFC. Int. J. Hydrogen Energy 2011, 36, 3989–3996. [Google Scholar] [CrossRef]
- Zeng, P.; Ran, R.; Shao, Z.; Yu, H.; Liu, S. Effects of Scandium Doping Concentration on The Properties of Strontium Cobalt Oxide Membranes. Braz. J. Chem. Eng. 2009, 26, 563–574. [Google Scholar] [CrossRef]
- Han, L.; Christensen, D.V.; Bhowmik, B.; Simonsen, S.B.; Hung, L.T.; Abdellahi, E.; Chen, Y.Z.; Nong, N.V.; Linderoth, S.; Pryds, N. Scandium-doped zinc cadmium oxide as a new stable n-type oxide thermoelectric material. J. Mater. Chem. A 2016, 4, 12221–12231. [Google Scholar] [CrossRef] [Green Version]
- Menga, Q.S.; Fana, W.H.; Chena, R.X.; Munirb, Z.A. Thermoelectric properties of Sc- and Y-doped Mg2Si prepared by field-activated and pressure-assisted reactive sintering. J. Alloys Compd. 2011, 509, 7922–7926. [Google Scholar] [CrossRef]
- Saha, B.; Garbrecht, M.; Perez-Taborda, J.A.; Fawey, M.A.; Koh, Y.R.; Shakouri, A.; Martin-Gonzalez, M.; Hultman, L.; Sands, T.D. Compensation of native donor doping in ScN: Carrier concentration control and p-type ScN. Appl. Phys. Lett. 2017, 110, 252104. [Google Scholar] [CrossRef] [Green Version]
- Kerdsongpanya, S.; Van Nong, N.; Pryds, N.; Žukauskaitė, A.; Jensen, J.; Birch, J.; Lu, J.; Hultman, L.; Wingqvist, G.; Eklund, P. Anomalously high thermoelectric power factor in epitaxial ScN thin films. Appl. Phys. Lett. 2011, 99, 232113. [Google Scholar] [CrossRef] [Green Version]
- Men’kov, A.A.; Komissarova, L.N.; Simanov, Y.P.; Spitsyn, V.I. On Scandium Chalkogenides. Dolk. Akad. Nauk SSSR 1961, 141, 364–367. [Google Scholar]
- White, J.G.; Dismukes, J.P. The Crystal Structure of Scandium Sesquitelluride. Inorg. Chem. 1965, 4, 1760–1763. [Google Scholar] [CrossRef]
- Borup, K.A.; Toberer, E.S.; Zoltan, L.D.; Nakatsukasa, G.; Errico, M.; Fleurial, J.-P.; Iversen, B.B.; Snyder, G.J. Measurement of the electrical resistivity and Hall coefficient at high temperatures. Rev. Sci. Instrum. 2012, 83, 123902. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wood, C.; Zoltan, D.; Stapfer, G. Measurement of Seebeck coefficient using a light pulse. Rev. Sci. Instrum. 1985, 56, 719–722. [Google Scholar] [CrossRef]
- Li, G.; Gladden, J.R. High temperature resonant ultrasound spectroscopy: A review. Int. J. Spectrosc. 2010, 2010. [Google Scholar] [CrossRef]
- ASTM C1548-02. Standard Test Method for Dynamic Young’s Modulus, Shear Modulus, and Poisson’s Ratio by Impulse Excitation of Vibration; ASTM International: West Conshohocken, PA, USA, 2002. [Google Scholar]
- Goldsmid, H.J.; Sharp, J.W. Estimation of the thermal band gap of a semiconductor from seebeck measurements. J. Electron. Mater. 1999, 28, 869–872. [Google Scholar] [CrossRef]
- Kim, H.S.; Gibbs, Z.M.; Tang, Y.; Wang, H.; Snyder, G.J. Characterization of Lorenz number with Seebeck coefficient measurement. APL Mater. 2015, 3, 041506. [Google Scholar] [CrossRef]
- Zeier, W.; Zevalkink, A.; Gibbs, Z.M.; Hautier, G.; Kanatzidis, M.G.; Snyder, G.J. Thinking like a chemist: Intuition in thermoelectric materials. Angew. Chem. Int. Ed. 2016, 55, 6826–6841. [Google Scholar] [CrossRef] [PubMed]
- Miller, S.A.; Gorai, P.; Ortiz, B.R.; Goyal, A.; Gao, D.; Barnett, S.A.; Mason, T.O.; Snyder, G.J.; Lv, Q.; Stevanović, V.; et al. Capturing Anharmonicity in a Lattice Thermal Conductivity Model for High-Throughput Predictions. Chem. Mater. 2017, 29, 2494–2501. [Google Scholar] [CrossRef]




| Parameters | PrTe1.46 | LaTe1.46 | Sc2Te3 |
|---|---|---|---|
| Young’s modulus, Y (GPa) | 68.6 | 70.1 | 65.4 |
| Shear modulus, G (GPa) | 27.1 | 27.6 | 26.7 |
| Bulk modulus, B (GPa) | 49.3 | 50.9 | 39.6 |
| Long melocity, vL (m/s) | 3543 | 3642 | 3773 |
| Transverse velocity, vT (m/s) | 1994 | 2042 | 2247 |
| Density, ρ (g/cm3) | 6.804 | 6.607 | 5.285 |
| Volume CTE (10−5/K) | 5.06 | 4.62 | 3.60 |
| Grüneisen parameter, γ | 1.96 | 1.89 | 1.03 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cheikh, D.; Lee, K.; Peng, W.; Zevalkink, A.; Fleurial, J.-P.; Bux, S.K. Thermoelectric Properties of Scandium Sesquitelluride. Materials 2019, 12, 734. https://doi.org/10.3390/ma12050734
Cheikh D, Lee K, Peng W, Zevalkink A, Fleurial J-P, Bux SK. Thermoelectric Properties of Scandium Sesquitelluride. Materials. 2019; 12(5):734. https://doi.org/10.3390/ma12050734
Chicago/Turabian StyleCheikh, Dean, Kathleen Lee, Wanyue Peng, Alexandra Zevalkink, Jean-Pierre Fleurial, and Sabah K. Bux. 2019. "Thermoelectric Properties of Scandium Sesquitelluride" Materials 12, no. 5: 734. https://doi.org/10.3390/ma12050734