Enhanced Electrochemical Performances of Cobalt-Doped Li2MoO3 Cathode Materials
Abstract
:1. Introduction
2. Materials and Methods
2.1. Preparation of the Li2Mo1−xCoxO3 Powder
2.2. Physical Characterization
2.3. Electrochemical Tests
3. Results and Discussion
3.1. Characteristics of the as-Prepared Li2Mo1−xCoxO3
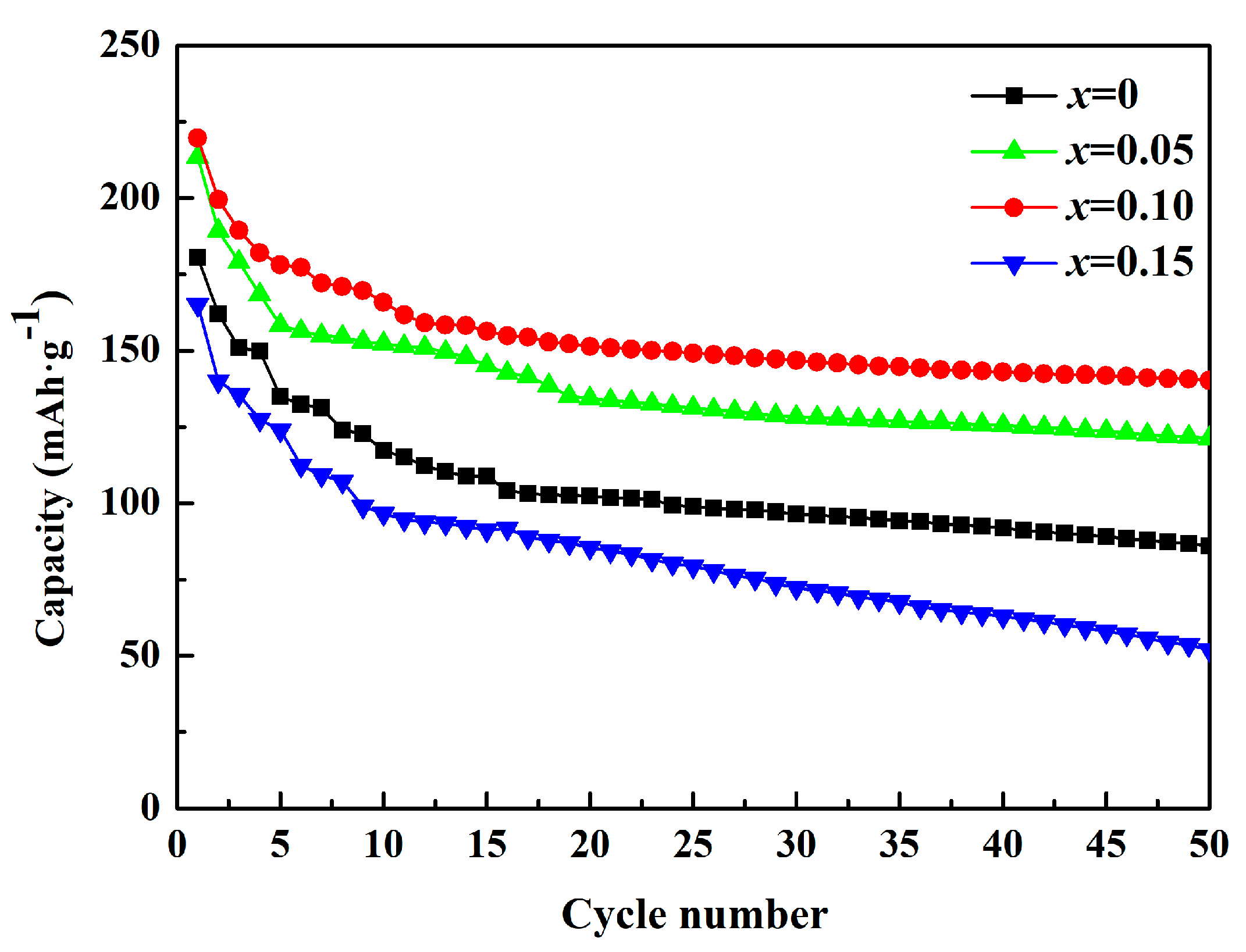
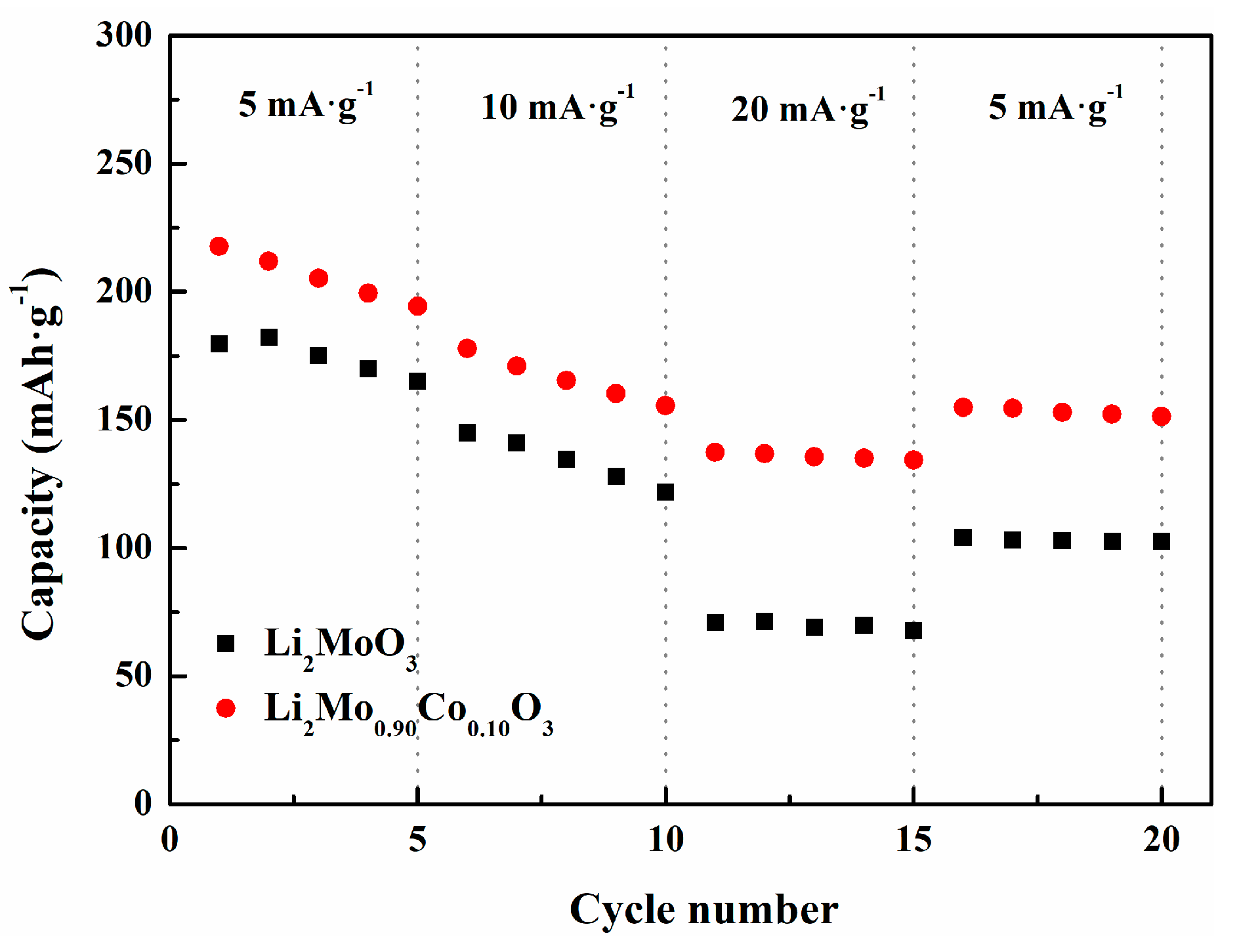
3.2. Electrochemical Performances
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Wang, Y.; Gu, H.T.; Song, J.H.; Feng, Z.; Zhou, X.; Zhou, Y.N.; Wang, K.; Xie, J. Suppressing Mn reduction of Li-rich Mn-based cathodes by F-doping for advanced lithium-ion batteries. J. Phys. Chem. C 2018, 122, 27836–27842. [Google Scholar] [CrossRef]
- Ma, J.; Zhou, Y.N.; Gao, Y.; Kong, Q.; Wang, Z.; Yang, X.Q.; Chen, L. Molybdenum substitution for improving the charge compensation and activity of Li2MnO3. Chem. Eur. J. 2014, 20, 8723–8730. [Google Scholar] [CrossRef] [PubMed]
- Zhao, W.; Xiong, L.; Xu, Y.; Xiao, X.; Wang, J.; Ren, Z. Magnesium substitution to improve the electrochemical performance of layered Li2MnO3 positive-electrode material. J. Power Sources 2016, 330, 37–44. [Google Scholar] [CrossRef]
- Bareño, J.; Balasubramanian, M.; Kang, S.H.; Wen, J.G.; Lei, C.H.; Pol, S.V.; Petrov, I.; Abraham, D.P. Long-range and local structure in the layered oxide Li1.2Co0.4Mn0.4O2. Chem. Mater. 2011, 23, 2039–2050. [Google Scholar] [CrossRef]
- Zhang, Q.; Peng, T.; Zhan, D.; Hu, X. Synthesis and electrochemical property of xLi2MnO3·(1 − x)LiMnO2 composite cathode materials derived from partially reduced Li2MnO3. J. Power Sources 2014, 250, 40–49. [Google Scholar] [CrossRef]
- Lanz, P.; Sommer, H.; Schulz, D.M.; Novák, P. Oxygen Release from high-energy xLi2MnO3·(1 − x)LiMO2 (M = Mn, Ni, Co): Electrochemical, differential electrochemical mass spectrometric, in situ pressure, and in situ temperature characterization. Electrochim. Acta 2013, 93, 114–119. [Google Scholar] [CrossRef]
- Kim, S.J.; Kim, M.C.; Kwak, D.H.; Kim, D.M.; Lee, G.H.; Choe, H.S.; Park, K.W. Highly stable TiO2 coated Li2MnO3 cathode materials for lithium-ion batteries. J. Power Sources 2016, 304, 119–127. [Google Scholar] [CrossRef]
- Wang, F.; Xiao, S.; Li, M.; Wang, X.; Zhu, Y.; Wu, Y.; Shirakawa, A.; Pe, J. A nanocomposite of Li2MnO3 coated by FePO4 as cathode material for lithium ion batteries. J. Power Sources 2015, 287, 416–421. [Google Scholar] [CrossRef]
- Xiao, R.; Li, H.; Chen, L. Density functional investigation on Li2MnO3. Chem. Mater. 2012, 24, 4242–4251. [Google Scholar] [CrossRef]
- Zheng, J.; Gu, M.; Xiao, J.; Zuo, P.; Wang, C.; Zhang, J.G. Corrosion/fragmentation of layered composite cathode and related capacity/voltage fading during cycling process. Nano Lett. 2013, 13, 3824–3830. [Google Scholar] [CrossRef] [PubMed]
- Bettge, M.; Li, Y.; Gallagher, K.; Zhu, Y.; Wu, Q.; Lu, W.; Bloom, I.; Abraham, D.P. Voltage fade of layered oxides: Its measurement and impact on energy density. J. Electrochem. Soc. 2013, 160, A2046–A2055. [Google Scholar] [CrossRef]
- Pearce, P.E.; Perez, A.J.; Rousse, G.; Saubanère, M.; Batuk, D.; Foix, D.; McCalla, E.; Abakumov, A.M.; Tendeloo, G.V.; Doublet, M.L.; et al. Evidence for anionic redox activity in a tridimensional-ordered Li-rich positive electrode β-Li2IrO3. Nat. Mater. 2017, 16, 580–586. [Google Scholar] [CrossRef]
- Miura, Y.; Yasui, Y.; Sato, M.; Igawa, N.; Kakurai, K. New-Ttype phase transition of Li2RuO3 with honeycomb structure. J. Phys. Soc. Jpn. 2007, 76, 033705. [Google Scholar] [CrossRef]
- Arunkumar, P.; Jeong, W.J.; Won, S.; Im, W.B. Improved electrochemical reversibility of over-lithiated layered Li2RuO3 cathodes: Understanding aliovalent Co3+ substitution with excess lithium. J. Power Sources 2016, 324, 428–438. [Google Scholar] [CrossRef]
- Takahashi, Y.; Kijima, N.; Hayakawa, H.; Awaka, J.; Akimoto, J. Single-crystal synthesis and structure refinement of Li2MoO3. J. Phys. Chem. Solids 2008, 69, 1518–1520. [Google Scholar] [CrossRef]
- Ma, J.; Gao, Y.R.; Wang, Z.X.; Chen, L. Structural and electrochemical stability of Li-rich layer structured Li2MoO3 in air. J. Power Sources 2014, 258, 314–320. [Google Scholar] [CrossRef]
- Ma, J.; Zhou, Y.N.; Gao, Y.R.; Yu, X.; Kong, Q.; Gu, L.; Wang, Z.; Yang, X.; Chen, L. Feasibility of using Li2MoO3 in constructing Li-rich high energy density cathode materials. Chem. Mater. 2014, 26, 3256–3262. [Google Scholar] [CrossRef]
- Self, E.C.; Zou, L.; Zhang, M.J.; Opfer, R.; Ruther, R.E.; Veith, G.M.; Song, B.; Wang, C.; Wang, F.; Huq, A.; et al. Synthesis and electrochemical and structural investigations of oxidatively stable Li2MoO3 and xLi2MoO3·(1 − x)LiMO2 composite cathodes. Chem. Mater. 2018, 30, 5061–5068. [Google Scholar] [CrossRef]
- Lee, J.; Urban, A.; Li, X.; Su, D.; Hautier, G.; Ceder, G. Unlocking the potential of cation-disordered oxides for rechargeable lithium batteries. Science 2014, 343, 519–522. [Google Scholar] [CrossRef]
- Kumakura, S.; Shirao, Y.; Kubota, K.; Komaba, S. Preparation and electrochemical properties of Li2MoO3/C composites for rechargeable Li-ion batteries. Phys. Chem. Chem. Phys. 2016, 18, 28556–28563. [Google Scholar] [CrossRef] [PubMed]
- Yu, Z.; Yu, T.; Li, W.; Hao, J.; Liu, H.; Sun, N.; Lu, M.; Ma, J. Improved electrochemical performances of carbon-coated Li2MoO3 cathode materials for Li-ion batteries. Int. J. Electrochem. Sci. 2018, 13, 4504–4511. [Google Scholar] [CrossRef]
- Yuan, B.; Liao, S.X.; Xin, Y.; Zhong, Y.; Shi, X.; Li, L.; Guo, X. Cobalt-doped lithium-rich cathode with superior electrochemical performance for lithium-ion batteries. RSC Adv. 2015, 5, 2947–2951. [Google Scholar] [CrossRef]
- Oz, E.; Demirel, S.; Altin, S. Fabrication and electrochemical properties of LiCo1−xRuxO2 cathode materials for Li-ion battery. J. Alloys Compd. 2016, 671, 24–33. [Google Scholar] [CrossRef]
- Tang, Z.; Wang, Z.; Li, X.; Peng, W. Preparation and electrochemical properties of Co-doped and none-doped Li[LixMn0.65(1−x)Ni0.35(1−x)]O2 cathode materials for lithium battery batteries. J. Power Sources 2012, 204, 187–192. [Google Scholar] [CrossRef]
- Song, J.; Shao, G.; Shi, M.; Ma, Z.; Song, W.; Wang, C.; Liu, S. The effect of doping Co on the electrochemical properties of LiFePO4/C nanoplates synthesized by solvothermal route. Solid State Ion. 2013, 253, 39–46. [Google Scholar] [CrossRef]
- Kim, Y. First principles investigation of the structure and stability of LiNiO2 doped with Co and Mn. J. Mater. Sci. 2012, 47, 7558–7563. [Google Scholar] [CrossRef]
- Becker, D.; Cherkashinin, G.; Hausbrand, R.; Jaegermann, W. Adsorption of diethyl carbonate on LiCoO2 thin films: Formation of the electrochemical interface. J. Phys. Chem. C 2014, 118, 962–967. [Google Scholar] [CrossRef]
- Guan, J.; Li, Y.; Guo, Y.; Su, R.; Gao, G.; Song, H.; Yuan, H.; Liang, B.; Guo, Z. Mechanochemical process enhanced cobalt and lithium recycling from wasted lithium-ion batteries. ACS Sustain. Chem. Eng. 2017, 5, 1026–1032. [Google Scholar] [CrossRef]
- Li, X.; Qu, M.; Yu, Z. Structural and electrochemical performances of Li4Ti5−xZrxO12 as anode material for lithium-ion batteries. J. Alloys Compd. 2009, 487, L12–L17. [Google Scholar] [CrossRef]
- Zhao, R.; Hung, I.M.; Li, Y.T.; Chen, H.; Lin, C.P. Synthesis and properties of Co-doped LiFePO4 as cathode material via a hydrothermal route for lithium-ion batteries. J. Alloys Compd. 2012, 513, 282–288. [Google Scholar] [CrossRef]
- Yabuuchi, N.; Tahara, Y.; Komaba, S.; Kitada, S.; Kajiya, Y. Synthesis and electrochemical properties of Li4MoO5-NiO binary system as positive electrode materials for rechargeable lithium batteries. Chem. Mater. 2016, 28, 416–419. [Google Scholar] [CrossRef]
- Kim, D.Y.; Ahn, H.J.; Kim, J.S.; Kim, I.P.; Kweon, J.H.; Nam, T.H.; Kim, K.W.; Ahn, J.H.; Hong, S.H. The Electrochemical properties of nano-sized cobalt powder as an anode material for lithium batteries. Electron. Mater. Lett. 2009, 5, 183–186. [Google Scholar] [CrossRef]









| Samples | Rs/Ω | Rf/Ω | Rct/Ω | DLi+ (cm2·s−1) |
|---|---|---|---|---|
| Li2MoO3 | 6.21 | 32.73 | 478.75 | 3.89 × 10−17 |
| Li2Mo0.90Co0.10O3 | 5.89 | 25.79 | 105.70 | 1.94 × 10−16 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yu, Z.; Hao, J.; Li, W.; Liu, H. Enhanced Electrochemical Performances of Cobalt-Doped Li2MoO3 Cathode Materials. Materials 2019, 12, 843. https://doi.org/10.3390/ma12060843
Yu Z, Hao J, Li W, Liu H. Enhanced Electrochemical Performances of Cobalt-Doped Li2MoO3 Cathode Materials. Materials. 2019; 12(6):843. https://doi.org/10.3390/ma12060843
Chicago/Turabian StyleYu, Zhiyong, Jishen Hao, Wenji Li, and Hanxing Liu. 2019. "Enhanced Electrochemical Performances of Cobalt-Doped Li2MoO3 Cathode Materials" Materials 12, no. 6: 843. https://doi.org/10.3390/ma12060843





