Removal of Hazardous Oxyanions from the Environment Using Metal-Oxide-Based Materials
Abstract
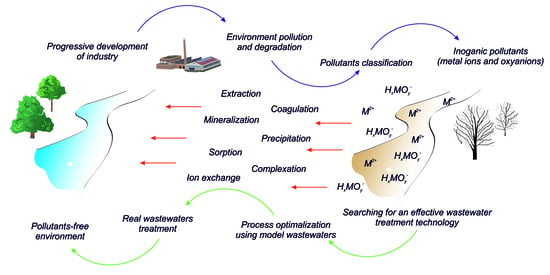
:1. Introduction
2. Arsenic Oxyanions
2.1. Arsenic Pollution
2.2. Characteristic of Arsenic Oxyanions in Aquatic Environment
2.3. Adsorbents for Arsenic Removal from Water Sources
3. Vanadium Oxyanions
3.1. Vanadium Pollution
3.2. Characteristic of Vanadium Oxyanions in Aquatic Environment
3.3. Oxide-Based Material for Vanadium Oxyanions Adsorption
4. Boron Oxyanions
4.1. Boron Pollution and Its Behavior in Aquatic Media
4.2. Materials for Effective Adsorption of Boron Oxyanions
5. Tungsten Oxyanions
5.1. Tungsten as an Environmental Threat and Its Performance in Water
5.2. Adsorption of Tungsten Species
6. Molybdenum Oxyanions
6.1. Molybdenum Pollution and Behavior of Molybdenum Species in Water
6.2. Adsorbents for Molybdenum Removal from Water Environment
7. Conclusions
- The role of sorption material, and in fact its physicochemical parameters designed at the synthesis stage—the presence of functional groups exhibiting a positive charge, facilitating the binding of negatively charged oxyanions;
- The influence of the pH of the adsorption environment on the nature of functional groups of the sorption material and the form of oxyanions in aqueous solutions, so important analyzing their potential interactions;
- Selectivity tests of sorption materials towards various metal oxyanions, which in the scientific papers published so far are effectively omitted, inversely as in case of sorption of metal cations,
- The effect of the presence of other components of wastewater on the sorption efficiency of a particular oxyanion group.
Funding
Conflicts of Interest
References
- Adegoke, H.I.; Adekola, F.A.; Fatoki, O.S.; Ximba, B.J. Sorptive interaction of oxyanions with iron oxides: A review. Pol. J. Environ. Stud. 2013, 22, 7–24. [Google Scholar]
- Cornelis, G.; Johnson, C.A.; Van Gerven, T.; Vandecasteele, C. Leaching mechanisms of oxyanionic metalloid and metal species in alkaline solid wastes: A review. Appl. Geochem. 2008, 23, 955–976. [Google Scholar] [CrossRef]
- Kailasam, V.; Rosenberg, E. Oxyanion removal and recovery using silica polyamine composites. Hydrometallurgy 2012, 129–130, 97–104. [Google Scholar] [CrossRef]
- Verbinnen, B.; Block, C.; Van Caneghem, J.; Vandecasteele, C. Recycling of spent adsorbents for oxyanions and heavy metal ions in the production of ceramics. Waste Manag. 2015, 45, 407–411. [Google Scholar] [CrossRef]
- Hajji, S.; Montes-Hernandez, G.; Sarret, G.; Tordo, A.; Morin, G.; Ona-nguema, G.; Bureau, S.; Turkia, T.; Mzoughi, N. Arsenite and chromate sequestration onto ferrihydrite, siderite and goethite nanostructured minerals: Isotherms from flow-through reactor experiments and XAS measurements. J. Hazard. Mater. 2019, 362, 358–367. [Google Scholar] [CrossRef]
- Wang, Y.; Ding, S.; Shi, L.; Gong, M.; Xu, S.; Zhang, C. Simultaneous measurements of cations and anions using diffusive gradients in thin films with a ZrO-Chelex mixed binding layer. Anal. Chim. Acta 2017, 972, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Mon, M.; Bruno, R.; Ferrando-Soria, J.; Armentano, D.; Pardo, E. Metal-organic framework technologies for water remediation: Towards a sustainable ecosystem. J. Mater. Chem. A 2018, 6, 4912–4947. [Google Scholar] [CrossRef]
- Baouab, M.H.V.; Gauthier, R.; Bernard, C. Sorption of chromium oxy-anions onto cationized lignocellulosic material. J. Appl. Polym. Sci. 2003, 87, 1660–1665. [Google Scholar]
- Hristovski, K.D.; Markovski, J. Science of the total environment engineering metal (hydr)oxide sorbents for removal of arsenate and similar weak-acid oxyanion contaminants: A critical review with emphasis on factors governing sorption processes. Sci. Total Environ. 2017, 598, 258–271. [Google Scholar] [CrossRef]
- Ungureanu, G.; Filote, C.; Santos, S.C.R.; Boaventura, R.A.R.; Volf, I.; Botelho, C.M.S. Antimony oxyanions uptake by green marine macroalgae. J. Environ. Chem. Eng. 2016, 4, 3441–3450. [Google Scholar] [CrossRef]
- Chung, J.; Ahn, C.H.; Chen, Z.; Rittmann, B.E. Bio-reduction of arsenate using a hydrogen-based membrane biofilm reactor. Chemosphere 2006, 65, 24–34. [Google Scholar] [CrossRef]
- Gupta, V.K.; Fakhri, A.; Kumar, A.; Agarwal, S.; Naji, M. Optimization by response surface methodology for vanadium(V) removal from aqueous solutions using PdO-MWCNTs nanocomposites. J. Mol. Liq. 2017, 234, 117–123. [Google Scholar] [CrossRef]
- Kołodyńska, D.; Budnyak, T.M.; Hubicki, Z.; Tertykh, V.A. Sol–gel derived organic—Inorganic hybrid ceramic materials for heavy metal removal. In Sol-Gel Based Nanoceramic Materials: Preparation, Properties and Applications; Mishra, A.K., Ed.; Springer International Publishing: Berlin, Germany, 2017; pp. 253–274. [Google Scholar]
- Atia, A.A. Adsorption of chromate and molybdate by cetylpyridinium bentonite. Appl. Clay Sci. 2008, 41, 73–84. [Google Scholar] [CrossRef]
- Deng, B.; Caviness, M.; Gu, Z. Arsenic removal by activated carbon-based materials. In Advances in Arsenic Research; O’Day, P.A., Vlassopoulos, D., Meng, X., Benning, L.G., Eds.; American Chemical Society: Washington, DC, USA, 2005; pp. 284–293. [Google Scholar]
- Santonastaso, G.F.; Erto, A.; Bortone, I.; Chianese, S.; Di Nardo, A.; Musmarra, D. Experimental and simulation study of the restoration of a thallium (I)-contaminated aquifer by Permeable Adsorptive Barriers (PABs). Sci. Total Environ. 2018, 630, 62–71. [Google Scholar] [CrossRef]
- Melendres, C.A.; Hahn, F.; Bowmaker, G.A. Oxyanion adsorption and competition at a gold electrode. Electrochim. Acta 2000, 46, 9–13. [Google Scholar] [CrossRef]
- Cumberland, S.L.; Strouse, G.F. Analysis of the nature of oxyanion adsorption on gold nanomaterial surfaces. Langmuir 2002, 18, 269–276. [Google Scholar] [CrossRef]
- Stevenson, K.J.; Gao, X.; Hatchett, D.W.; White, H.S. Voltammetric measurement of anion adsorption on Ag(111). J. Electroanal. Chem. 1998, 447, 43–51. [Google Scholar] [CrossRef]
- Zhu, H.; Jia, Y.; Wu, X.; Wang, H. Removal of arsenic from water by supported nano zero-valent iron on activated carbon. J. Hazard. Mater. 2009, 172, 1591–1596. [Google Scholar] [CrossRef]
- Figueiredo, H.; Quintelas, C. Tailored zeolites for the removal of metal oxyanions: Overcoming intrinsic limitations of zeolites. J. Hazard. Mater. 2014, 274, 287–299. [Google Scholar] [CrossRef]
- Bissen, M.; Frimmel, F.H. Arsenic—A review. Part II: Oxidation of arsenic and its removal in water treatment. Acta Hydrochim. Hydrobiol. 2003, 31, 97–107. [Google Scholar] [CrossRef]
- Baccile, N.; Falco, C.; Titirici, M.-M. Characterization of biomass and its derived char using 13 C-solid state nuclear magnetic resonance. Green Chem. 2014, 16, 4839–4869. [Google Scholar] [CrossRef]
- Chao, H.P.; Lee, C.K.; Juang, L.C.; Han, Y.L. Sorption of organic compounds, oxyanions, and heavy metal ions on surfactant modified titanate nanotubes. Ind. Eng. Chem. Res. 2013, 52, 9843–9850. [Google Scholar] [CrossRef]
- Hemming, G.; Reeder, R.J.; Hart, S.R. Growth-step-selective incorporation of boron on the calcite surface. Geochim. Cosmochim. Acta 1998, 62, 2915–2922. [Google Scholar] [CrossRef]
- Hiemstra, T.; Van Riemsdijk, W.H. Fluoride adsorption on goethite in relation to different types of surface sites. J. Colloid Interface Sci. 2000, 225, 94–104. [Google Scholar] [CrossRef]
- Iwai, T.; Hashimoto, Y. Adsorption of tungstate (WO4) on birnessite, ferrihydrite, gibbsite, goethite and montmorillonite as affected by pH and competitive phosphate (PO4) and molybdate (MoO4) oxyanions. Appl. Clay Sci. 2017, 143, 372–377. [Google Scholar] [CrossRef]
- Manning, B.A.; Fendorf, S.E.; Goldberg, S. Surface structures and stability of arsenic(III) on goethite: Spectroscopic evidence for inner-sphere complexes. Environ. Sci. Technol. 1998, 32, 2383–2388. [Google Scholar] [CrossRef]
- Hiemstra, T.; Van Riemsdijk, W.H. Surface structural adsorption modeling of competitive binding of oxanions by metal (hydr)oxides. J. Colloid Interface Sci. 1999, 210, 182–193. [Google Scholar] [CrossRef]
- Matis, K.A.; Zouboulis, A.I.; Zamboulis, D.; Valtadoru, A.V. Sorption of As(V) by goethite particles and study of their flocculation. Water Air Soil Pollut. 1999, 111, 297–316. [Google Scholar] [CrossRef]
- Li, Z.; Bowman, R.S. Retention of inorganic oxyanions by organo-kaolinite. Water Res. 2001, 35, 3771–3776. [Google Scholar] [CrossRef]
- Sherlala, A.I.A.; Raman, A.A.A.; Bello, M.M. Synthesis and characterization of magnetic graphene oxide for arsenic removal from aqueous solution. Environ. Technol. 2018, 1–9. [Google Scholar] [CrossRef]
- Xu, N.; Christodoulatos, C.; Braida, W. Modeling the competitive effect of phosphate, sulfate, silicate, and tungstate anions on the adsorption of molybdate onto goethite. Chemosphere 2006, 64, 1325–1333. [Google Scholar] [CrossRef]
- Laudadio, E.D.; Bennett, J.W.; Green, C.M.; Mason, S.E.; Hamers, R.J. Impact of phosphate adsorption on complex cobalt oxide nanoparticle dispersibility in aqueous media. Environ. Sci. Technol. 2018, 52, 10186–10195. [Google Scholar] [CrossRef]
- Davis, S.A.; Misra, M. Transport model for the adsorption of oxyanions of selenium(IV) and arsenic(V) from water onto lanthanum-and aluminum-based oxides. J. Colloid Interface Sci. 1997, 188, 340–350. [Google Scholar] [CrossRef]
- Ravenscroft, P.; Brammer, H.; Richards, K. Arsenic Pollution; Environmental Chemistry: West Sussex, UK, 2009. [Google Scholar]
- Smedley, P.L.; Kinniburgh, D.G. A review of the source, behaviour and distribution of arsenic in natural waters. Appl. Geochem. 2002, 17, 517–568. [Google Scholar] [CrossRef]
- Peng, F.F.; Di, P. Removal of arsenic from aqueous solution by adsorbing colloid flotation. Ind. Eng. Chem. Res. 1994, 33, 922–928. [Google Scholar] [CrossRef]
- Van Halem, D.; Bakker, S.A.; Amy, G.L.; Van Dijk, J.C. Arsenic in drinking water: A worldwide water quality concern for water supply companies. Drink. Water Eng. Sci. 2009, 2, 29–34. [Google Scholar] [CrossRef]
- World Health Organization. Arsenic. 2019. Available online: https://www.who.int/news-room/fact-sheets/detail/arsenic (accessed on 27 January 2018).
- Su, C.; Puls, R.W. Arsenate and arsenite removal by zerovalent iron: Effects of phosphate, silicate, carbonate, borate, sulfate, chromate, molybdate, and nitrate, relative to chloride. Environ. Sci. Technol. 2001, 35, 4562–4568. [Google Scholar] [CrossRef]
- Pena, M.E.; Korfiatis, G.P.; Patel, M.; Lippincott, L.; Meng, X. Adsorption of As(V) and As(III) by nanocrystalline titanium dioxide. Water Res. 2005, 39, 2327–2337. [Google Scholar] [CrossRef]
- Meng, X.; Bang, S.; Korfiatis, G.P. Effects of silicate, sulfate, and carbonate on arsenic removal by ferric chloride. Water Res. 2000, 34, 1255–1261. [Google Scholar] [CrossRef]
- Dambies, L. Existing and prospective sorption technologies for the removal of arsenic in water. Sep. Sci. Technol. 2005, 39, 603–627. [Google Scholar] [CrossRef]
- Jeong, Y.; Fan, M.; Singh, S.; Chuang, C.L.; Saha, B.; van Leeuwen, J. Evaluation of iron oxide and aluminum oxide as potential arsenic(V) adsorbents. Chem. Eng. Process. Process Intensif. 2007, 46, 1030–1039. [Google Scholar] [CrossRef]
- Lin, T.F.; Wu, J.K. Adsorption of arsenite and arsenate within activated alumina grains: Equilibrium and kinetics. Water Res. 2001, 35, 2049–2057. [Google Scholar] [CrossRef]
- Manna, B.R.; Dey, S.; Debnath, S.; Ghosh, U.C. Removal of arsenic from groundwater using crystalline hydrous ferric oxide (CHFO). Water Qual. Res. J. Can. 2003, 38, 193–210. [Google Scholar] [CrossRef]
- Mohan, D.; Pittman, C.U. Arsenic removal from water/wastewater using adsorbents—A critical review. J. Hazard. Mater. 2007, 142, 1–53. [Google Scholar] [CrossRef]
- Ren, Z.; Zhang, G.; Paul Chen, J. Adsorptive removal of arsenic from water by an iron-zirconium binary oxide adsorbent. J. Colloid Interface Sci. 2011, 358, 230–237. [Google Scholar] [CrossRef]
- Oscarson, D.W.; Huang, P.M.; Defosse, C.; Herbillon, A. Oxidative power of Mn(IV) and Fe(III) oxides with respect to As(III) in terrestrial and aquatic environments. Nature 1981, 291, 50–51. [Google Scholar] [CrossRef]
- Su, C.; Puls, R.W. Arsenate and arsenite sorption on magnetite: Relations to groundwater arsenic treatment using zerovalent iron and natural attenuation. Water Air Soil Pollut. 2008, 193, 65–78. [Google Scholar] [CrossRef]
- Bissen, M.; Vieillard-Baron, M.M.; Schindelin, A.J.; Frimmel, F.H. TiO2-catalyzed photooxidation of arsenite to arsenate in aqueous samples. Chemosphere 2001, 44, 751–757. [Google Scholar] [CrossRef]
- Zhang, F.S.; Itoh, H. Photocatalytic oxidation and removal of arsenite from water using slag-iron oxide-TiO2 adsorbent. Chemosphere 2006, 65, 125–131. [Google Scholar] [CrossRef]
- Gupta, K.; Ghosh, U.C. Arsenic removal using hydrous nanostructure iron(III)-titanium(IV) binary mixed oxide from aqueous solution. J. Hazard. Mater. 2009, 161, 884–892. [Google Scholar] [CrossRef]
- Gładysz-Płaska, A.; Skwarek, E.; Budnyak, T.M.; Kołodyńska, D. Metal ions removal using nano oxide PyroloxTM material. Nanoscale Res. Lett. 2017, 12, 1–9. [Google Scholar] [CrossRef]
- Oscarson, D.W.; Huang, P.M.; Hammer, U.T.; Liaw, W.K. Oxidation and sorption of arsenite by manganese dioxide as influenced by surface coatings of iron and aluminum oxides and calcium carbonate. Water Air Soil Pollut. 1982, 20, 233–244. [Google Scholar] [CrossRef]
- Lei, M.; Qin, P.; Peng, L.; Ren, Y.; Sato, T.; Zeng, Q.; Yang, Z.; Chai, L. Using Fe-Mn binary oxide three-dimensional nanostructure to remove arsenic from aqueous systems. Water Sci. Technol. Water Supply 2016, 16, 516–524. [Google Scholar] [CrossRef]
- Gupta, K.; Biswas, K.; Ghosh, U.C. Nanostructure iron(III)-zirconium(IV) binary mixed oxide: Synthesis, characterization, and physicochemical aspects of arsenic(III) sorption from the aqueous solution. Ind. Eng. Chem. Res. 2008, 47, 9903–9912. [Google Scholar] [CrossRef]
- Gupta, K.; Basu, T.; Ghosh, U.C. Sorption characteristics of arsenic(V) for removal from water using agglomerated nanostructure iron(III)-zirconium(IV) bimetal mixed oxide. J. Chem. Eng. Data 2009, 54, 2222–2228. [Google Scholar] [CrossRef]
- Erdoğan, H.; Yalçinkaya, Ö.; Türker, A.R. Determination of inorganic arsenic species by hydride generation atomic absorption spectrometry in water samples after preconcentration/separation on nano ZrO2/B2O3 by solid phase extraction. Desalination 2011, 280, 391–396. [Google Scholar] [CrossRef]
- Oram, B. The pH of Water. Water Research Center Website. 2019. Available online: https://www.water-research.net/index.php/ph (accessed on 27 January 2019).
- Kwon, O.H.; Kim, J.O.; Cho, D.W.; Kumar, R.; Baek, S.H.; Kurade, M.B.; Jeon, B.H. Adsorption of As(III), As(V) and Cu(II) on zirconium oxide immobilized alginate beads in aqueous phase. Chemosphere 2016, 160, 126–133. [Google Scholar] [CrossRef]
- Youngran, J.; Maohong, F.; Van Leeuwen, J.; Belczyk, J.F. Effect of competing solutes on arsenic(V) adsorption using iron and aluminum oxides. J. Environ. Sci. 2007, 19, 910–919. [Google Scholar] [CrossRef]
- Mojiri, A.; Hui, W.; Arshad, A.K.; Ruslan, A.; Ridzuan, M.; Hamid, N.H.A.; Farraji, H.; Gholami, A.; Vakili, A.H. Vanadium(V) removal from aqueous solutions using a new composite adsorbent (BAZLSC): Optimization by response surface methodology. Adv. Environ. Res. 2017, 6, 173–187. [Google Scholar]
- Wright, M.T.; Stollenwerk, K.G.; Belitz, K. Assessing the solubility controls on vanadium in groundwater, northeastern San Joaquin Valley, CA. Appl. Geochem. 2014, 48, 41–52. [Google Scholar] [CrossRef]
- Sharififard, H.; Soleimani, M.; Zokaee Ashtiani, F. Application of nanoscale iron oxide-hydroxide-impregnated activated carbon (Fe-AC) as an adsorbent for vanadium recovery from aqueous solutions. Desalin. Water Treat. 2016, 57, 15714–15723. [Google Scholar] [CrossRef]
- Omidinasab, M.; Rahbar, N.; Ahmadi, M.; Kakavandi, B.; Ghanbari, F.; Kyzas, G.Z.; Martinez, S.S.; Jaafarzadeh, N. Removal of vanadium and palladium ions by adsorption onto magnetic chitosan nanoparticles. Environ. Sci. Pollut. Res. 2018, 25, 34262–34276. [Google Scholar] [CrossRef]
- Naeem, A.; Westerhoff, P.; Mustafa, S. Vanadium removal by metal (hydr)oxide adsorbents. Water Res. 2007, 41, 1596–1602. [Google Scholar] [CrossRef]
- Larsson, M.A.; Hadialhejazi, G.; Petter, J. Vanadium sorption by mineral soils: Development of a predictive model. Chemosphere 2017, 168, 925–932. [Google Scholar] [CrossRef]
- Tracey, A.S.; Galeffi, B.; Mahjour, S. Vanadium (V) oxyanions. The dependence of vanadate alkyl ester formation on the p K a of the parent alcohols. Can. J. Chem. 1988, 66, 2294–2298. [Google Scholar] [CrossRef]
- Vega, E.D.; Pedregosa, J.C.; Narda, G.E.; Morando, P.J. Removal of oxovanadium(IV) from aqueous solutions by using commercial crystalline calcium hydroxyapatite. Water Res. 2003, 37, 1776–1782. [Google Scholar] [CrossRef]
- Leiviskä, T.; Khalid, M.K.; Sarpola, A.; Tanskanen, J. Removal of vanadium from industrial wastewater using iron sorbents in batch and continuous flow pilot systems. J. Environ. Manag. 2017, 190, 231–242. [Google Scholar] [CrossRef]
- Ghazvini, M.P.T.; Ghorbanzadeh, S.G. Bioresource technology effect of salinity on vanadate biosorption by Halomonas sp. GT-83: Preliminary investigation on biosorption by micro-PIXE technique. Bioresour. Technol. 2009, 100, 2361–2368. [Google Scholar] [CrossRef]
- Huang, J.; Huang, F.; Evans, L.; Glasauer, S. Vanadium: Global (bio) geochemistry. Chem. Geol. 2015, 417, 68–89. [Google Scholar] [CrossRef]
- Baes, C.F.; Mesmer, R.S. The Hydrolysis of Cations; John Wiley & Sons: New York, NY, USA, 1976. [Google Scholar]
- Kunz, R.G.; Giannelli, J.F.; Stensel, H.D. Vanadium removal from industrial wastewaters. J. Water Pollut. Control Fed. 2016, 48, 762–770. [Google Scholar]
- Salvestrini, S. Analysis of the Langmuir rate equation in its differential and integrated form for adsorption processes and a comparison with the pseudo first and pseudo second order models. React. Kinet. Mech. Catal. 2018, 123, 455–472. [Google Scholar] [CrossRef]
- Su, T.; Guan, X.; Gu, G.; Wang, J. Adsorption characteristics of As(V), Se(IV), and V(V) onto activated alumina: Effects of pH, surface loading, and ionic strength. J. Colloid Interface Sci. 2008, 326, 347–353. [Google Scholar] [CrossRef]
- Golob, J.; Kosta, L.; Modic, R. Űber die adsorptive trennung des vanadiums von arsen, phosphor und fluor mit aktiven aluminiumoxyd. Vestn. Slov. Kem. Društva 1971, 18, 21–25. [Google Scholar]
- Agnoli, S.; Castellarin-cudia, C.; Sambi, M.; Surnev, S.; Ramsey, M.G.; Granozzi, G.; Netzer, F.P. Vanadium on TiO2 (110): Adsorption site and sub-surface migration. Surf. Sci. 2003, 546, 117–126. [Google Scholar] [CrossRef]
- Kantcheva, M.M.; Hadjiivanov, K.I. Adsorption of vanadium-oxo species on pure and peroxide-treated TiO2 (anatase). J. Chem. Soc. Chem. Commun. 1991, 02, 1057–1058. [Google Scholar] [CrossRef]
- Davydov, S.Y.; Pavlyk, A.V. Adsorption of vanadium on rutile: A change in the electron work function. Tech. Phys. Lett. 2003, 29, 500–501. [Google Scholar] [CrossRef]
- Zhang, S.; Liu, S.; Hu, W.; Zhu, X.; Qu, R.; Wu, W.; Zheng, C.; Gao, X. New insight into alkali resistance and low temperature activation on vanadia-titania catalysts for selective catalytic reduction of NO. Appl. Surf. Sci. 2018, 466, 99–109. [Google Scholar] [CrossRef]
- Erto, A.; Chianese, S.; Lancia, A.; Musmarra, D. On the mechanism of benzene and toluene adsorption in single-compound and binary systems: Energetic interactions and competitive effects. Desalin. Water Treat. 2017, 86, 259–265. [Google Scholar] [CrossRef]
- Li, X.; Deng, G.; Zhang, Y.; Wang, J. Rapid removal of copper ions from aqueous media by hollow polymer nanoparticles. Colloids Surf. A 2019, 568, 345–355. [Google Scholar] [CrossRef]
- Kołodyńska, D.; Bąk, J.; Kozioł, M.; Pylychuk, L.V. Investigations of heavy metal ion sorption using nanocomposites of iron-modified biochar. Nanoscale Res. Lett. 2017, 12, 1–13. [Google Scholar] [CrossRef]
- Jansson-Charrier, M.; Guibal, E.; Roussy, J.; Delanghe, B.; Le Cloirec, P. Vanadium(IV) sorption by chitosan: Kinetics and equilibrium. Water Res. 1996, 30, 465–475. [Google Scholar] [CrossRef]
- Guzma, J.; Saucedo, I.; Navarro, R.; Revilla, J.; Guibal, E. Vanadium interactions with chitosan: Influence of polymer protonation and metal speciation. Langmuir 2018, 18, 1567–1573. [Google Scholar] [CrossRef]
- Navarro, R.; Guzmán, J.; Saucedo, I.; Revilla, J.; Guibal, E. Recovery of metal ions by chitosan: Sorption mechanisms and influence of metal speciation. Macromol. Biosci. 2003, 3, 552–561. [Google Scholar] [CrossRef]
- Padilla-Rodríguez, A.; Hernández-Viezcas, J.A.; Peralta-Videa, J.R.; Gardea-Torresdey, J.L.; Perales-Pérez, O.; Román-Velázquez, F.R. Synthesis of protonated chitosan flakes for the removal of vanadium(III, IV and V) oxyanions from aqueous solutions. Microchem. J. 2015, 118, 1–11. [Google Scholar] [CrossRef]
- Guibal, E.; Saucedo, I.; Jansson-Charrier, M.; Delanghe, B.; Le Cloirec, P. Uranium and vanadium sorption by chitosan derivatives. Water Sci. Technol. 1994, 30, 183–190. [Google Scholar] [CrossRef]
- Zdarta, J.; Antecka, K.; Jędrzak, A.; Synoradzki, K.; Łuczak, M.; Jesionowski, T. Biopolymers conjugated with magnetite as support materials for trypsin immobilization and protein digestion. Colloids Surf. B Biointerfaces 2018, 169, 118–125. [Google Scholar] [CrossRef]
- Jędrzak, A.; Grześkowiak, B.F.; Coy, E.; Wojnarowicz, J.; Szutkowski, K.; Jurga, S.; Jesionowski, T.; Mrówczyński, R. Dendrimer based theranostic nanostructures for combined chemo- and photothermal therapy of liver cancer cells in vitro. Colloids Surf. B Biointerfaces 2019, 173, 698–708. [Google Scholar] [CrossRef]
- Talebzadeh, F.; Zandipak, R.; Sobhanardakani, S. CeO2 nanoparticles supported on CuFe2O4 nanofibers as novel adsorbent for removal of Pb(II), Ni(II), and V(V) ions from petrochemical wastewater. Desalin. Water Treat. 2016, 57, 28363–28377. [Google Scholar] [CrossRef]
- Demetriou, A.; Pashalidis, I. Adsorption of boron on iron-oxide in aqueous solutions. Desalin. Water Treat. 2012, 37, 37–41. [Google Scholar] [CrossRef]
- Yamahira, M.; Kikawada, Y.; Oi, T. Boron isotope fractionation accompanying formation of potassium, sodium and lithium borates from boron-bearing solutions. Geochem. J. 2007, 41, 149–163. [Google Scholar] [CrossRef] [Green Version]
- De la Fuente García-Soto, M.M.; Muñoz Camacho, E. Boron removal by means of adsorption processes with magnesium oxide—Modelization and mechanism. Desalination 2009, 249, 626–634. [Google Scholar] [CrossRef]
- Liu, H.; Qing, B.; Ye, X.; Li, Q. Boron adsorption by composite magnetic particles. Chem. Eng. J. 2009, 151, 235–240. [Google Scholar] [CrossRef]
- World Health Organization. Boron in drinking-water: Background document for development of WHO guidelines for drinking-water quality. Guidel. Drink. Water Qual. 1998, 2, 1–12. [Google Scholar]
- World Health Organization. Boron in Drinking-Water; World Health Organization: Geneva, Switzerland, 2009. [Google Scholar]
- Hinz, K.; Altmaier, M.; Gaona, X.; Rabung, T.; Schild, D.; Richmann, M.; Reed, D.T.; Alekseev, E.V.; Geckeis, H. Interaction of Nd(III) and Cm(III) with borate in dilute to concentrated alkaline NaCl, MgCl2 and CaCl2 solutions: Solubility and TRLFS studies. New J. Chem. 2015, 39, 849–859. [Google Scholar] [CrossRef]
- Kameda, T.; Oba, J.; Yoshioka, T. Use of Mg-Al oxide for boron removal from an aqueous solution in rotation: Kinetics and equilibrium studies. J. Environ. Manag. 2016, 165, 280–285. [Google Scholar] [CrossRef]
- Wang, H.; Yang, Y.; Guo, L. Renewable-biomolecule-based electrochemical energy-storage materials. Adv. Energy Mater. 2017, 7, 1700663. [Google Scholar] [CrossRef]
- Peak, D.E.P.; Luther, G.E.W.L.; Sparks, D.O. ATR-FTIR spectroscopic studies of boric acid adsorption on hydrous ferric oxide. Geochim. Cosmochim. Acta 2003, 67, 2551–2560. [Google Scholar] [CrossRef]
- Pylypchuk, I.V.; Kołodyńska, D.; Gorbyk, P.P. Gd(III) adsorption on the DTPA-functionalized chitosan/magnetite nanocomposites. Sep. Sci. Technol. 2018, 53, 1006–1016. [Google Scholar] [CrossRef]
- Xu, H.; Yuan, H.; Yu, J.; Lin, S. Study on the competitive adsorption and correlational mechanism for heavy metal ions using the carboxylated magnetic iron oxide nanoparticles (MNPs-COOH) as efficient adsorbents. Appl. Surf. Sci. 2019, 473, 960–966. [Google Scholar] [CrossRef]
- Öztürk, N.; Kavak, D. Boron removal from aqueous solutions by batch adsorption onto cerium oxide using full factorial design. Desalination 2008, 223, 106–112. [Google Scholar] [CrossRef]
- Irawan, C.; Liu, J.C.; Wu, C. Removal of boron using aluminum-based water treatment residuals (Al-WTRs). Desalination 2011, 276, 322–327. [Google Scholar] [CrossRef]
- Afkhami, A.; Aghajani, S.; Mohseni, M.; Madrakian, T. Effectiveness of Ni0.5Zn0.5Fe2O4 for the removal and preconcentration of Cr(VI), Mo(VI), V(V) and W(VI) oxyanions from water and wastewater samples. J. Iran. Chem. Soc. 2015, 12, 1–7. [Google Scholar] [CrossRef]
- Rakshit, S.; Sallman, B.; Davantes, A.; Lefevre, G. Tungstate(VI) sorption on hematite: An in situ ATR-FTIR probe on the mechanism. Chemosphere 2017, 168, 685–691. [Google Scholar] [CrossRef]
- Ding, S.; Xu, D.; Wang, Y.; Wang, Y.; Li, Y.; Gong, M.; Zhang, C. Simultaneous measurements of eight oxyanions using high-capacity diffusive gradients in thin films (Zr-Oxide DGT) with a high-efficiency elution procedure. Environ. Sci. Technol. 2016, 50, 7572–7580. [Google Scholar] [CrossRef]
- Strigul, N.; Koutsospyros, A.; Arienti, P.; Christodoulatos, C.; Dermatas, D.; Braida, W. Effects of tungsten on environmental systems. Chemosphere 2005, 61, 248–258. [Google Scholar] [CrossRef]
- Kashiwabara, T.; Kubo, S.; Tanaka, M.; Senda, R.; Iizuka, T.; Tanimizu, M.; Takahashi, Y. Stable isotope fractionation of tungsten during adsorption on Fe and Mn (oxyhydr)oxides. Geochim. Cosmochim. Acta 2017, 204, 52–67. [Google Scholar] [CrossRef]
- Hur, H.; Reeder, R.J. Tungstate sorption mechanisms on boehmite: Systematic uptake studies and X-ray absorption spectroscopy analysis. J. Colloid Interface Sci. 2016, 461, 249–260. [Google Scholar] [CrossRef] [Green Version]
- Plattes, M.; Bertrand, A.; Schmitt, B.; Sinner, J.; Verstraeten, F.; Welfring, J. Removal of tungsten oxyanions from industrial wastewater by precipitation, coagulation and flocculation processes. J. Hazard. Mater. 2007, 148, 613–615. [Google Scholar] [CrossRef]
- Mikko, D.U.S. Military “green bullet”. Assoc. Firearm Tool Mark Exam. J. 1999, 31, 1–4. [Google Scholar]
- Gustafsson, J.P. Modelling molybdate and tungstate adsorption to ferrihydrite. Chem. Geol. 2003, 200, 105–115. [Google Scholar] [CrossRef] [Green Version]
- Halmi, M.I.E.; Ahmad, S.A. Chemistry, biochemistry, toxicity and pollution of molybdenum: A mini review. J. Biochem. Microbiol. Biotechnol. 2014, 2, 1–6. [Google Scholar]
- Verbinnen, B.; Block, C.; Hannes, D.; Lievens, P.; Vaclavikova, M.; Stefusova, K.; Gallios, G.; Vandecasteele, C. Removal of molybdate anions from water by adsorption on zeolite-supported magnetite. Water Environ. Res. 2012, 84, 753–760. [Google Scholar] [CrossRef]
- Keskİn, C.S. Arsenic and molybdenum ions removal by Fe3O4 embedded acrylamide-yirazine hybrid-polymer. Rev. Roum. Chim. 2017, 62, 139–148. [Google Scholar]
- Torres, J.; Gonzatto, L.; Peinado, G.; Kremer, C.; Kremer, E. Interaction of molybdenum(VI) oxyanions with +2 metal cations. J. Solut. Chem. 2014, 43, 1687–1700. [Google Scholar] [CrossRef]
- Oyerinde, O.F.; Weeks, C.L.; Anbar, A.D.; Spiro, T.G. Solution structure of molybdic acid from Raman spectroscopy and DFT analysis. Inorg. Chim. Acta 2008, 361, 1000–1007. [Google Scholar] [CrossRef]
- Xu, N.; Christodoulatos, C.; Braida, W. Adsorption of molybdate and tetrathiomolybdate onto pyrite and goethite: Effect of pH and competitive anions. Chemosphere 2006, 62, 1726–1735. [Google Scholar] [CrossRef]
- Goldberg, S.; Forster, H.S.; Godfrey, C.L. Molybdenum adsorption on oxides, clay minerals, and soils. Soil Sci. Soc. Am. J. 1996, 60, 425–432. [Google Scholar] [CrossRef]
- Aulmann, M.A.; Siri, G.J.; Blanco, M.N.; Caceres, C.V.; Thomas, H.J. Molybdenum adsorption isotherms on γ-alumina. Appl. Catal. 1983, 7, 139–149. [Google Scholar] [CrossRef]
- Luthraand, N.P.; Cheng, W.C. Molybdenum-95 NMR study of the adsorption molybdates on alumina. J. Catal. 1987, 107, 154–160. [Google Scholar] [CrossRef]
- Spanos, N.; Vordonis, L.; Kordulis, C.; Koutsoukos, P.G.; Lycourghiotis, A. Molybdenum-oxo species deposited on alumina by adsorption: II. regulation of the surface Mo(VI) concentration by control of the protonated surface hydroxyls. J. Catal. 1990, 124, 315–323. [Google Scholar] [CrossRef]
- Wu, C.; Lo, S.; Lin, C. Competitive adsorption of molybdate, chromate, sulfate, selenate, and selenite on γ-Al2O3. Colloids Surf. A Physicochem. Eng. Asp. 2000, 166, 251–259. [Google Scholar] [CrossRef]
- Sbai, S.; Elyahyaoui, A.; Sbai, Y.; Bentayeb, F.; Bricha, M.R. Study of adsorption of molybdate ion by alumina. J. Environ. Res. Develop. 2017, 11, 452–460. [Google Scholar]
- Verbinnen, B.; Block, C.; Lievens, P.; Van Brecht, A.; Vandecasteele, C. Simultaneous removal of molybdenum, antimony and selenium oxyanions from wastewater by adsorption on supported magnetite. Waste Biomass Valor 2013, 4, 635–645. [Google Scholar] [CrossRef]
- Swedlund, P.J.; Webster, J.G. Adsorption and polymerisation of silicic acid on ferrihydrite, and its effect on arsenic adsorption. Water Res. 1999, 33, 3413–3422. [Google Scholar] [CrossRef]










| Adsorbent | Surface Area (m2/g) | As Concentration (mg/L) | Adsorption Capacity (mg/g) | Temperature (°C) | Contact Time (h) | pH | Ref. | |
|---|---|---|---|---|---|---|---|---|
| As3+ | As5+ | |||||||
| Al2O3 | - | 100 | 16.0 ± 0.9 | 24.5 ± 1.6 | 25 | 12 | 7.0 | [56] |
| 0.55 | 0.6 | - | 0.14 | 23 ± 0.5 | 2 | 7.0 ± 0.1 | [45] | |
| 0.55 | 0.2 | - | 0.098 | 25 ± 0.5 | 1–2 | 6 ± 0.1 | [63] | |
| Al2O3 (granular) | 115–118 | 0.79–4.90 | 1.69 | - | 25 ± 0.5 | 40 | 6.1 (±0.1) | [46] |
| 115–118 | 2.85–11.50 | - | 15.90 | 170 | 5.2 (±0.1) | [46] | ||
| Al2O3-La2O3 | - | 0.51 | - | 0.050 | 21 | 48 | 7.8–9.3 | [35] |
| - | 3.62 | - | 0.029 | 21 | 48 | 7.8–9.3 | [35] | |
| Fe2O3 | - | 100 | 60.9 ± 1.1 | 21.3 ± 0.1 | 25 | 12 | 7.0 | [56] |
| 5.05 | 0.6 | - | 0.56 | 23 ± 0.5 | 1 | 7 ± 0.1 | [45] | |
| 5.05 | 0.2 | - | 0.616 | 25 ± 0.5 | 1–2 | 6 ± 0.1 | [63] | |
| Crystalline hydrous ferric oxide | - | 50 | 66–68 | 55–58 | 30 ± 2 | 4 | 7.0 | [47] |
| Fe3O4 (magnetite) | 2.43–16.5 | 2 | 0.65 | - | - | 24 | 7.0 | [51] |
| 2.43–16.5 | 2 | - | 0.7 | - | 24 | 2.5–4.0 | [51] | |
| TiO2 | - | 100 | 0.0001 | - | 40 | 10 | 3.0 | [53] |
| Slag-Fe2O3-TiO2 | 163 | 100 | 0.0047 | - | 40 | 10 | 3.0 | [53] |
| Fe2O3-TiO2 | 77.8 ± 0.2 | 5–10 | 85.0 | 14.3 | 30 ± 2 | 3.5/6 | 7.0 ± 0.1 | [54] |
| MnO2 | 77 | 60 | 2.55 (As3+ + As5+) | 22 | 1/6 | 4.0 | [57] | |
| Fe2O3-MnO2 | 123 | 60 | 9.89 (As3+ + As5+) | 22 | 1/6 | 4.0 | [57] | |
| Fe2O3-ZrO2 | 339 | 5–40 | 120.0 | 46.1 | 25 ± 1 | 36 | 7.0 ± 0.1 | [49] |
| - | 10 | 66.5 ± 1.8 | - | 30 ± 1.6 | 2 | 7.0 ± 0.2 | [58] | |
| 263 | 10 | - | 9.36 | 30 ± 1.6 | 1.6 | 7.0 ± 0.2 | [59] | |
| Nano ZrO2-B2O3 | - | 5–300 | - | 98.04 | room | 2 | 3.0 | [60] |
| ZrO2-alginate beads (ZOAB) | 13.2 | 32.9 | 32.3 | - | 25 | 240 | ~5.0 | [62] |
| 13.2 | 35.2 | - | 28.5 | 25 | 240 | ~5.0 | [62] | |
| Material | XRF Results | XRD Results |
|---|---|---|
| CFH-12 | 83% FeO, 6.1% S, 4.2% MgO, 1.4% SiO2, 1.1% CaO | Gypsum (CaSO4·2H2O) mostly amorphous iron material |
| AQM | 40.1% SiO2, 24.8% Al2O3, 18.3% FeO, 3.4% MgO, 2.9% K2O | Quartz (SiO2) Muscovite (KAl2(Si3Al)O10(OH, F)2) Kaolinite (Al2Si2O5(OH)4) |
| BFS | 63.2% FeO, 12.5% CaO, 11.0% SiO2, 2.9% Al2O3, 2.2% MgO, 1.0% K2O | Hematite (Fe2O3) Calcite (CaCO3) Quartz (SiO2) |
| SCS | 90.3% FeO, 5.0% CaO, 1.4% SiO2 56.3% | Magnetite (Fe3O4) Hematite (Fe2O3) Cuspidine (Ca4(F1.5(OH)0.5)Si2O7) |
| OKTO | 56.3% CaO, 26.6% SiO2, 6.6% MgO, 3.1% F, 2.3% Al2O3, 1.3% Cr2O3 | Periclase (MgO) Calcium hydroxide (Ca(OH)2) Enstatite (Fe0.3Mg0.7SiO3) Calcium silicate (Ca2SiO4) |
| FeCr | 32.5% SiO2, 25.8% Al2O3, 24.1% MgO, 11.2% Cr2O3, 4.3% FeO, 1.4% CaO | Spinel magnesioferrite Aluminum iron oxide (AlFe2O4) Iron silicon oxide Chromium iron (Cr0.7Fe0.3) Magnesium aluminum chromium oxide (Mg(Al1.5Cr0.5)O4) |
| Adsorbent | Surface Area (m2/g) | V Concentration (mg/L) | Adsorption Capacity (mg/g) | Temperature (°C) | Contact Time (h) | pH | Ref. |
|---|---|---|---|---|---|---|---|
| GFH (584 mg Fe/g GFH) | 231 | 1–250 | 111.11 | 25 | 24 | 7.0 ± 0.1 | [68] |
| E-33 (574 mg Fe/g E-33) | 128 | 1–250 | 25.06 | 25 | 24 | 7.0 ± 0.1 | [68] |
| GTO (650 mg Ti/g TiO2) | 150 | 1–250 | 45.66 | 25 | 24 | 7.0 ± 0.1 | [68] |
| CFH-12 | 173 | 58.2 | 5.71 | room | 24 | 5.8 | [72] |
| AQM | - | 58.2 | 1.72 | room | 24 | 5.8 | [72] |
| BFS | - | 58.2 | 1.93 | room | 24 | 5.8 | [72] |
| SCS | - | 58.2 | 2.62 | room | 24 | 5.8 | [72] |
| Fe-AC | 777 | 25–200 | 119.01 | 25 | 24 | 4.5 | [66] |
| CeO2/CuFe2O4 | 190.2 | 30–250 | 798.6 | 25 | 3 | 6.0 | [94] |
| Fe3O4-CSN | 35.6 | 16.37 | 186.6 | 19.85 | 1/6 | 5.0 | [67] |
| PdO-MWCNTs nanocomposites | 209.59 | 60 | 245.05 | 25 | 0.5 | 3.0 | [12] |
| Adsorbent | Surface Area (m2/g) | B Concentration (mg/L) | Adsorption Capacity (mg/g) | T (°C) | Contact Time (h) | pH | Ref. |
|---|---|---|---|---|---|---|---|
| MgO | - | 50 | 303.87 | room | 48 | 9.5–10.5 | [97] |
| - | 500 | 542.11 | room | 48 | 9.5–10.5 | [97] | |
| FeO(OH) | - | 55 | 0.324 | 22 ± 3 | - | 8 | [95] |
| Al2O3-Fe2O3-SiO2 (Al-WTR1) | 40.5 ± 5 | 5–100 | 0.980 | room | 24 | 8.3 ± 0.2 | [108] |
| Al2O3-Fe2O3-SiO2 (Al-WTR2) | 34.6 ± 3 | 5–100 | 0.700 | room | 24 | 8.3 ± 0.2 | [108] |
| Al2O3-Fe2O3-SiO2 (Al-WTR3) | 14.5 ± 1 | 5–100 | 0.190 | room | 24 | 8.3 ± 0.2 | [108] |
| MgO-Al2O3 | - | 108–648 | 80.00 | 30 | 168 | 10.5 | [102] |
| Adsorbent | Surface Area (m2/g) | W Concentration (mg/L) | Adsorption Capacity (mg/g) | Temperature (°C) | Contact Time (h) | pH | Ref. |
|---|---|---|---|---|---|---|---|
| Ni0.5Zn0.5Fe2O4 | - | 10–250 | 72 | 25 | 0.5 | 5 | [109] |
| Boehmite (γ-AlO(OH) | 136 | 1000 | 7.35–132.36 | room | 24 | 4 | [114] |
| Birnessite (MnO2) | - | 18–359 | 6.15 | 25 | 24 | 4 | [27] |
| Ferrihydrite (Fe2O3) | - | 18–359 | 30.24 | 25 | 24 | 4 | [27] |
| Gibbsite (Al(OH)3) | - | 18–359 | 49.82 | 25 | 24 | 4 | [27] |
| Goethite (α-FeO(OH)) | - | 18–359 | 43.12 | 25 | 24 | 4 | [27] |
| Adsorbent | Surface Area (m2/g) | Mo Concentration (mg/L) | Adsorption Capacity (mg/g) | Temperature (°C) | Contact Time (h) | pH | Ref. |
|---|---|---|---|---|---|---|---|
| Fe3O4 embedded hydrolyzed triazine polymer | - | 2.5 | 0.213 | 25 | 2.5 | 2.5 | [120] |
| zeolite-supported-Fe3O4 | 74.5 | 1 | 17.92 | 25 | 24 | 3 | [119] |
| Goethite | - | 1 | 1.76 | 25 | 24 | 3 | [119] |
| 43.96 | 0–32 | 25.9 | room | 17 | 4.0 ± 0.1 | [33,123] | |
| Hematite | - | 1 | 1.43 | 25 | 24 | 3 | [119] |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Weidner, E.; Ciesielczyk, F. Removal of Hazardous Oxyanions from the Environment Using Metal-Oxide-Based Materials. Materials 2019, 12, 927. https://doi.org/10.3390/ma12060927
Weidner E, Ciesielczyk F. Removal of Hazardous Oxyanions from the Environment Using Metal-Oxide-Based Materials. Materials. 2019; 12(6):927. https://doi.org/10.3390/ma12060927
Chicago/Turabian StyleWeidner, Ewelina, and Filip Ciesielczyk. 2019. "Removal of Hazardous Oxyanions from the Environment Using Metal-Oxide-Based Materials" Materials 12, no. 6: 927. https://doi.org/10.3390/ma12060927