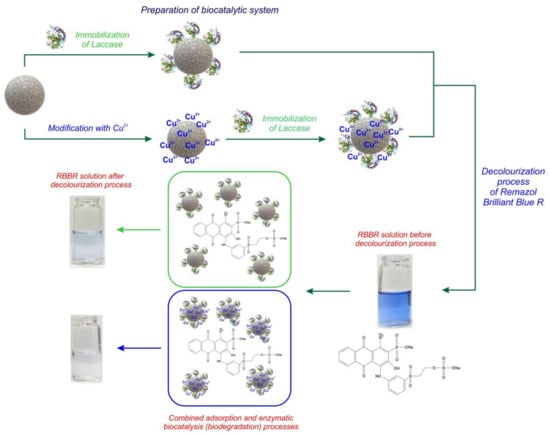
Laccase Immobilized onto Zirconia–Silica Hybrid Doped with Cu2+ as an Effective Biocatalytic System for Decolorization of Dyes
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals and Materials
2.2. Synthesis of ZrO2–SiO2 and ZrO2–SiO2/Cu2+ Oxide Systems
2.3. Immobilization of Laccase
2.4. Storage Stability and Kinetic Measurements of Free and Immobilized Laccase
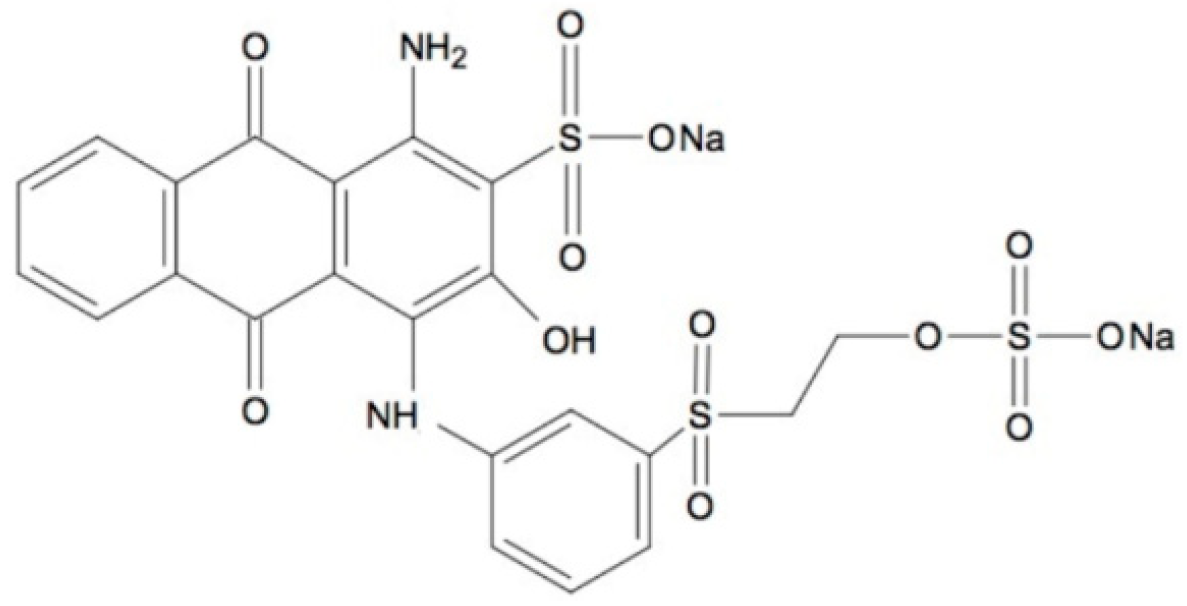
2.5. Decolorization of Remazol Brilliant Blue R Dye
2.6. Analytical Techniques
3. Results and Discussion
3.1. Characterization of the Oxide Materials before and after Immobilization of Laccase
3.2. Decolorization of Dye
3.2.1. Decolorization of Dye Using Oxide Materials with Inactivated Enzyme
3.2.2. Effect of Process Duration on Decolorization Efficiency
3.2.3. Effect of pH and Temperature on Decolorization Efficiency
3.2.4. Reusability of the Biocatalytic Systems
3.3. Toxicity Study
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Fatarella, E.; Spinelli, D.; Ruzzante, M.; Pogni, R. Nylon 6 film and nanofiber carriers: Preparation and laccase immobilization performance. J. Mol. Catal. B–Enzym. 2014, 102, 41–47. [Google Scholar] [CrossRef]
- Geng, A.; Wu, J.; Xie, R.; Li, X.; Chang, F.; Sun, J. Characterization of a laccase from a wood-feeding termite, Coptotermes formosanus. Insect Sci. 2018, 25, 251–258. [Google Scholar] [CrossRef]
- Mayer, A.; Staples, R. Laccase: New functions for an old enzyme. Phytochemistry 2002, 60, 551–565. [Google Scholar] [CrossRef]
- Cristovao, R.O.; Silverio, S.C.; Tavares, A.P.M.; Brigida, A.I.S.; Loureiro, J.M.; Boaventura, R.A.R.; Macedo, E.A.; Coelho, M.A.Z. Green coconut fiber: A novel carrier for immobilization of commercial laccase by covalent attachment for textile dyes decolorization. World J. Microbiol. Biotechnol. 2012, 28, 2827–2838. [Google Scholar] [CrossRef]
- Robinson, T.; McMullan, G.; Marchant, R.; Nigam, P. Remediation of dyes in textile effluent: A critical review on current treatment technologies with a proposed alternative. Bioresour. Technol. 2001, 77, 247–255. [Google Scholar] [CrossRef]
- Goscianska, J.; Ciesielczyk, F. Lanthanum enriched aminosilane-grafted mesoporous carbon material for efficient adsorption of tartrazine azo dye. Microporous Mesoporous Mater. 2019, 280, 7–19. [Google Scholar] [CrossRef]
- Jesionowski, T.; Zdarta, J.; Krajewska, B. Enzyme immobilization by adsorption: A review. Adsorption 2014, 20, 801–821. [Google Scholar] [CrossRef]
- Bolivar, J.M.; Rocha-Martin, J.; Mateo, C.; Guisan, J.M. Stabilization of a highly active but unstable alcohol dehydrogenase from yeast using immobilization and post-immobilization techniques. Process Biochem. 2012, 47, 679–686. [Google Scholar] [CrossRef]
- Bilal, M.; Cui, J.; Iqbal, H.M.N. Tailoring enzyme microenvironment: State-of-art strategy to fulfil the quest for efficient bio-catalysis. Int. J. Biol. Macromol. 2019, 130, 186–196. [Google Scholar] [CrossRef] [PubMed]
- Datta, S.; Christena, L.R.; Rajaram, Y.R.S. Enzyme immobilization: An overview on techniques and support materials. 3 Biotech 2013, 3, 1–9. [Google Scholar] [CrossRef] [PubMed]
- D’Souza, S.F. Immobilized enzymes in bioprocess. Curr. Sci. 1999, 77, 69–79. [Google Scholar]
- Barbosa, O.; Ortiz, C.; Berenguer-Murcia, A.; Torres, R.; Rodrigues, R.C.; Fernandez-Lafuente, R. Strategies for the one-step immobilization–purification of enzymes as industrial biocatalysts. Biotechnol. Adv. 2015, 33, 435–456. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gaberc-Porekar, V.; Menart, V. Perspectives of immobilized-metal affinity chromatography. J. Biochem. Biophys. Methods 2001, 49, 335–360. [Google Scholar] [CrossRef]
- Porath, J. Immobilized metal ion affinity chromatography. Protein Expr. Purif. 1992, 3, 263–281. [Google Scholar] [CrossRef]
- Bilal, M.; Asgher, M.; Cheng, H.; Yan, Y.; Iqbal, H.M.N. Multi-point enzyme immobilization, surface chemistry, and novel platforms: A paradigm shift in biocatalyst design. Crit. Rev. Biotechnol. 2019, 39, 202–219. [Google Scholar] [CrossRef] [PubMed]
- Bilal, M.; Iqbal, H.M.N. Chemical, physical and biological coordination: An interplay between materials and enzymes as potential platforms for immobilization. Coord. Chem. Rev. 2019, 388, 1–23. [Google Scholar] [CrossRef]
- Bilal, M.; Asgher, M.; Iqbal, H.M.; Hu, H.; Zhang, X. Gelatin-immobilized manganese peroxidase with novel catalytic characteristics and its industrial exploitation for fruit juice clarification purposes. Catal. Lett. 2016, 146, 2221–2228. [Google Scholar] [CrossRef]
- Sheldon, R.A.; Pereira, P.C. Biocatalysis engineering: The big picture. Chem. Soc. Rev. 2017, 46, 2678–2691. [Google Scholar] [CrossRef] [PubMed]
- Bilal, M.; Iqbal, H.M.N. Naturally-derived biopolymer: Potential platforms for enzyme immobilization. Int. J. Biol. Macromol. 2019, 130, 462–482. [Google Scholar] [CrossRef]
- Koloti, L.E.; Gule, N.P.; Arotiba, O.A.; Malinga, S.P. Laccase-immobilized dendritic nanofibrous membranes as a novel approach towards the removal of bisphenol A. Environ. Technol. 2018, 39, 392–404. [Google Scholar] [CrossRef] [PubMed]
- Zdarta, J.; Antecka, K.; Frankowski, R.; Zgoła-Grześkowiak, A.; Ehrilch, H.; Jesionowski, T. The effect of operational parameters on the biodegradation of bisphenols by Trametes versicolor laccase immobilized on Hippospongia communis spongin scaffolds. Sci. Total Environ. 2018, 615, 784–795. [Google Scholar] [CrossRef] [PubMed]
- Bayramoglu, G.; Salih, B.; Akbulut, A.; Arica, M.Y. Biodegradation of Cibacron Blue 3GA by insolubilized laccase and identification of enzymatic byproducts using MALDI-ToF-MS: Toxity assessment studies by Daphnia magna and Chlorella vulgaris. Ecotoxicol. Environ. Saf. 2019, 170, 453–460. [Google Scholar] [CrossRef]
- Gioia, L.; Ovsejevi, K.; Manta, C.; Miguez, D.; Menendez, P. Biodegradation of acid dyes by an immobilized laccase: An ecotoxicological approach. Environ. Sci. Water Res. Technol. 2018, 4, 2125–2135. [Google Scholar] [CrossRef]
- Iyer, P.V.; Ananthanarayan, L. Enzyme stability and stabilization–Aqueous and non-aqueous environment. Process Biochem. 2008, 43, 1019–1032. [Google Scholar] [CrossRef]
- Fernandez-Lafuente, R. Stabilization of multimeric enzymes: Strategies to prevent subunit dissociation. Enzym. Microb. Technol. 2009, 45, 405–418. [Google Scholar] [CrossRef]
- Barbosa, O.; Torres, R.; Ortiz, C.; Berenguer-Murcia, A.; Rodrigues, R.C.; Fernadez-Lafuente, R. Heterofunctional supports in enzyme immobilization: From traditional immobilization protocols to opportunities in tuning enzyme properties. Biomacromolecules 2013, 14, 2433–2462. [Google Scholar] [CrossRef]
- Pezzella, C.; Russo, M.E.; Marzocchella, A.; Salatino, P.; Sannia, G. Immobilization of a Pleurotus ostreatus laccase mixture on perlite and its application to dye decolorisation. BioMed Res. Int. 2014, 2014, 308613. [Google Scholar] [CrossRef]
- Amin, R.; Khorshidi, A.; Shojaei, A.F.; Rezaai, S.; Faramarzi, M.A. Immobilization of laccase on modified Fe3O4@SiO2@Kit-6 magnetite nanoparticles for enhanced delignification of olive pomace bio-waste. Int. J. Biol. Macromol. 2018, 114, 106–113. [Google Scholar] [CrossRef]
- Li, Q.Y.; Wang, P.Y.; Zhou, Y.L.; Nie, Z.R.; Wei, Q. A magnetic mesoporous SiO2/Fe3O4 hollow microsphere with a novel network-like composite shell: Synthesis and application on laccase immobilization. J. Sol-Gel Sci. Technol. 2016, 78, 523–530. [Google Scholar] [CrossRef]
- Antecka, K.; Zdarta, J.; Siwińska-Stefańska, K.; Sztuk, G.; Jankowska, E.; Oleskowicz-Popiel, P.; Jesionowski, T. Synergistic degradation of dye wastewaters using binary or ternary oxide systems with immobilized laccase. Catalysts 2018, 8, 402. [Google Scholar] [CrossRef]
- Kashefi, S.; Borghei, S.M.; Mahmoodi, N.M. Covalently immobilized laccase onto graphene oxide nanosheets: Preparation, characterization, and biodegradation of azo dyes in colored wastewater. J. Mol. Liq. 2019, 276, 153–162. [Google Scholar] [CrossRef]
- Kashefi, S.; Borghei, S.M.; Mahmoodi, N.M. Superparamagnetic enzyme-graphene oxide magnetic nanocomposite as an environmentally friendly biocatalyst: Synthesis and biodegradation of dye using response surface methodology. Microchem. J. 2019, 145, 547–558. [Google Scholar] [CrossRef]
- Rani, M.; Shanker, U.; Chaurasia, A.K. Catalytic potential of laccase immobilized on transition metal oxides nanomaterials: Degradation of Alizarin Red S dye. J. Environ. Chem. Eng. 2017, 5, 2730–2739. [Google Scholar] [CrossRef]
- Zdarta, J.; Meyer, A.S.; Jesionowski, T.; Pinelo, M. Developments in support materials for immobilization of oxidoreductases: A comprehensive review. Adv. Colloids Interf. 2018, 258, 1–20. [Google Scholar] [CrossRef] [PubMed]
- Dos Santos, J.C.; Barbosa, O.B.; Ortiz, C.; Berenguer-Murcia, A.; Rodrigues, R.C.; Fernadez-Lafuente, R. Importance of the suport properties for immobilization or purification of enzymes. ChemCatChem 2015, 7, 2413–2432. [Google Scholar] [CrossRef]
- Gomma, O.M.; Momtaz, O.A. Copper induction and differential expression of laccase in Aspergillus flavus. Braz. J. Microbiol. 2015, 46, 285–292. [Google Scholar] [CrossRef] [PubMed]
- Yaseen, D.A.; Scholz, M. Treatment of synthetic textile wastewater containing dye mixtures with microcosms. Environ. Sci. Pollut. Res. 2018, 25, 1980–1997. [Google Scholar] [CrossRef]
- Ciesielczyk, F.; Goscianska, J.; Zdarta, J.; Jesionowski, T. The development of zirconia/silica hybrids for the adsorption and controlled release of active pharmaceutical ingredients. Colloids Surf. A 2018, 545, 39–50. [Google Scholar] [CrossRef]
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Lu, J.; Zhu, X.; Tian, S.; Lv, X.; Chen, Z.; Jiang, Y.; Liao, X.; Cai, Z.; Chen, B. Graphene oxide in the marine environment: Toxicity to Artemia salina with and without the presence of Phe and Cd2+. Chemosphere 2018, 211, 390–396. [Google Scholar] [CrossRef]
- Sidhu, G.K.; Kaushik, A.K.; Rana, S.; Bhansali, S.; Kumar, R. Photoluminescence quenching of zirconia nanoparticle by surface modification. Appl. Surf. Sci. 2015, 334, 216–221. [Google Scholar] [CrossRef]
- Radhakrishnan, A.; Beena, B. Structural and optical absorption analysis of CuO nanoparticles. Indian J. Adv. Chem. Sci. 2014, 2, 158–161. [Google Scholar]
- Kadhom, M.; Deng, B. Thin film nanocomposite membranes filled with bentonite nanoparticles for brackish water desalination: A novel water uptake concept. Microporous Mesoporous Mater. 2019, 279, 82–91. [Google Scholar] [CrossRef]
- Alves, J.A., Jr.; Baldo, J.B. The behaviour of zeta potential of silica suspensions. New J. Glass Ceram. 2014, 4, 29–37. [Google Scholar] [CrossRef]
- Bousse, L.; Mostarshed, S.; van der Shoot, B.; de Rooij, N.F.; Gimmel, P.; Gopel, W. Zeta potential measurements of Ta2O5 and SiO2 thin films. J. Colloid Interf. Sci. 1991, 147, 22–32. [Google Scholar] [CrossRef]
- Jolivalt, C.; Brenon, S.; Caminade, E.; Mougin, C.; Pontie, M. Immobilization of laccase from Trametes versicolor on a modified PVDF microfiltration membrane: Characterization of the grafted support and application in removing a phenylurea pesticide in wastewater. J. Membr. Sci. 2000, 180, 103–113. [Google Scholar] [CrossRef]
- Arica, M.Y.; Senel, S.; Alaeddinoglu, N.G.; Patir, S.; Denizli, A. Invertase immobilized on spacer-arm attached poly(hydroxyethyl metacrylate) membrane: Preparation and properties. J. Appl. Polym. Sci. 2000, 75, 1685–1692. [Google Scholar] [CrossRef]
- Xu, R.; Cui, J.; Tang, R.; Li, F.; Zhang, B. Removal of 2,4,6-trichlorophenol by laccase immobilized on nano-copper incorporated electrospun fibrous membrane-high efficiency, stability and reusability. Chem. Eng. J. 2017, 326, 647–655. [Google Scholar] [CrossRef]
- Fu, M.; Xing, J.; Ge, Z. Preparation of laccase-loaded magnetic nanoflowers and their recycling for efficient degradation of bisphenol A. Sci. Total Environ. 2019, 651, 2857–2865. [Google Scholar] [CrossRef]
- Batule, B.S.; Park, K.S.; Kim, M.I.; Park, H.G. Ultrafast sonochemical synthesis of protein-inorganic nanoflowers. Int. J. Nanomed. 2015, 10, 137–142. [Google Scholar] [CrossRef]
- Stajic, M.; Persky, L.; Hadar, Y.; Friesem, D.; Duletic-Lausevic, S.; Wasser, S.P.; Nevo, E. Effect of copper and manganese ions on activities of laccase and peroxidases in three Pleurotus species grown on agricultural wastes. Appl. Biochem. biotechnol. 2006, 128, 87–96. [Google Scholar] [CrossRef]
- Baldrian, P.; Gabriel, J. Copper and cadmium increase laccase activity in Pleurotus ostreatus. FEMS Microbiol. Lett. 2002, 206, 69–74. [Google Scholar] [CrossRef]
- Bayramoglu, G.; Yilmaz, M.; Arica, M.Y. Reversible immobilization of laccase to poly(4-vinylpyridine) grafted and Cu(II) chelated magnetic beads: Biodegradation of reactive dyes. Bioresour. Technol. 2010, 101, 6615–6621. [Google Scholar] [CrossRef]
- Wang, X.; Hu, J.; Liang, Y.; Zhan, H. Effects of metal ions on laccase activity. Asian J. Chem. 2011, 23, 5422–5424. [Google Scholar]
- Olajuyigbe, F.M.; Adetuyi, O.Y.; Fatokun, C.O. Characterization of free and immobilized laccase from Cyberlindnera fabianii and application in degradation of bisphenol A. Int. J. Biol. Macromol. 2019, 125, 856–864. [Google Scholar] [CrossRef]
- Tapia-Orozco, N.; Melendez-Saavedra, F.; Figueroa, M.; Gimeno, M.; Garcia-Arrazola, R. Removal of bisphenol A in canned liquid food by enzyme-based nanocomposites. Appl. Nanosci. 2018, 8, 427–434. [Google Scholar] [CrossRef]
- Zhang, X.; Wang, M.; Lin, L.; Xiao, G.; Tang, Z.; Zhu, X. Synthesis of novel laccase-biotitania biocatalysts for malachite green decolorization. J. Biosci. Bioeng. 2018, 126, 69–77. [Google Scholar] [CrossRef]
- Patel, S.K.S.; Kalia, V.C.; Choi, J.H.; Haw, J.R.; Kim, I.W.; Lee, J.K. Immobilization of laccase on SiO2 nanocarriers improves its stability and reusability. J. Microb. Biotechnol. 2014, 24, 639–647. [Google Scholar] [CrossRef]
- Chen, J.; Leng, J.; Yang, X.; Liao, L.; Liu, L.; Xiao, A. Enhanced performance of magnetic graphene oxide-immobilized laccase and its application for the decolorization of dyes. Molecules 2017, 22, 221. [Google Scholar] [CrossRef]
- Le, T.T.; Murugesan, K.; Lee, C.-S.; Vu, C.H.; Chang, Y.-S.; Jeon, J.-R. Degradation of synthetic pollutants in real wastewaters using laccase encapsulated in core-shell magnetic copper alginate beads. Bioresour. Technol. 2016, 216, 203–210. [Google Scholar] [CrossRef]
- Da Silva, M.R.; da Sa, L.R.V.; Russo, C.; Scio, E.; Ferreira-Leitao, V.S. The use of HRP in decolorization of reactive dyes and toxicological evaluation of their products. Enzym. Res. 2010, 2010, 703824. [Google Scholar] [CrossRef] [PubMed]
- Osma, J.F.; Toca-Herra, J.L.; Rodriguez-Couto, S. Transformation pathway of Remazol Brilliant Blue R by immobilised laccase. Bioresour. Technol. 2010, 101, 8509–8514. [Google Scholar] [CrossRef] [PubMed]










| Examined System | Parameter | ||
|---|---|---|---|
| ABET (m2/g) | Vp (cm3/g) | Sp (nm) | |
| ZrO2–SiO2 | 440.2 | 0.369 | 3.4 |
| ZrO2–SiO2–laccase | 419.9 | 0.354 | 3.4 |
| ZrO2–SiO2/Cu2+ | 498.3 | 0.376 | 3.0 |
| ZrO2–SiO2/Cu2+–laccase | 445.7 | 0.344 | 3.0 |
| Kinetic Parameters and Immobilization Data | Free Laccase | ZrO2–SiO2–Laccase | ZrO2–SiO2/Cu2+–Laccase |
|---|---|---|---|
| Km (mM) | 0.049 ± 0.002 | 0.132 ± 0.009 | 0.098 ± 0.008 |
| Vmax (U/mg) | 0.041 ± 0.006 | 0.029 ± 0.007 | 0.037 ± 0.009 |
| Amount of enzyme (mg/g) | - | 86 ± 3.8 | 94 ± 3.2 |
| Immobilization yield (%) | - | 86 ± 3.9 | 94 ± 3.1 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jankowska, K.; Ciesielczyk, F.; Bachosz, K.; Zdarta, J.; Kaczorek, E.; Jesionowski, T. Laccase Immobilized onto Zirconia–Silica Hybrid Doped with Cu2+ as an Effective Biocatalytic System for Decolorization of Dyes. Materials 2019, 12, 1252. https://doi.org/10.3390/ma12081252
Jankowska K, Ciesielczyk F, Bachosz K, Zdarta J, Kaczorek E, Jesionowski T. Laccase Immobilized onto Zirconia–Silica Hybrid Doped with Cu2+ as an Effective Biocatalytic System for Decolorization of Dyes. Materials. 2019; 12(8):1252. https://doi.org/10.3390/ma12081252
Chicago/Turabian StyleJankowska, Katarzyna, Filip Ciesielczyk, Karolina Bachosz, Jakub Zdarta, Ewa Kaczorek, and Teofil Jesionowski. 2019. "Laccase Immobilized onto Zirconia–Silica Hybrid Doped with Cu2+ as an Effective Biocatalytic System for Decolorization of Dyes" Materials 12, no. 8: 1252. https://doi.org/10.3390/ma12081252