Preparation of Lignin-Based Carbon Materials and Its Application as a Sorbent
Abstract
:1. Introduction
2. Experimental Section
2.1. Materials
2.2. Synthetic Process of Lignin-Based Carbon Materials
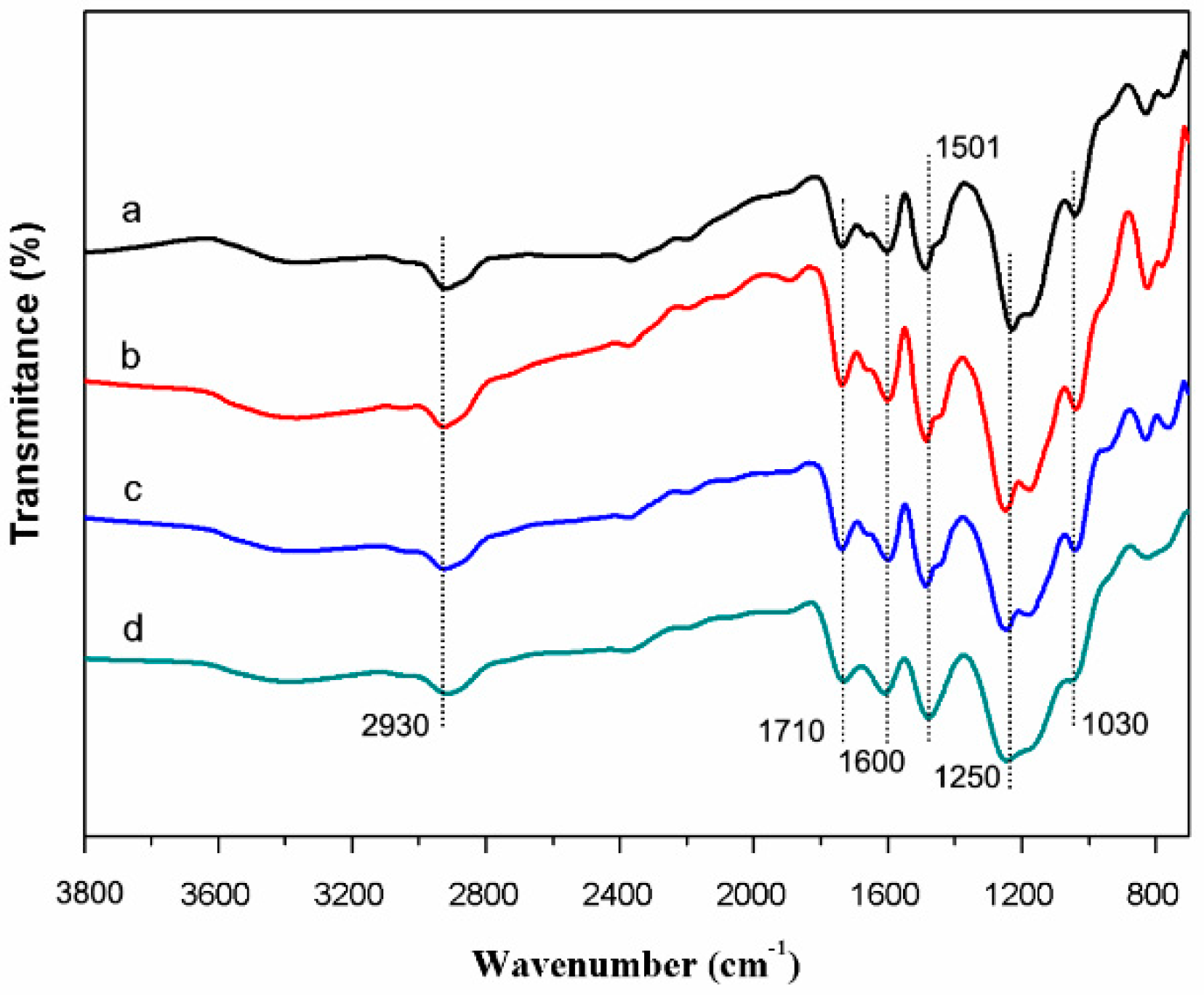
2.3. Characterization
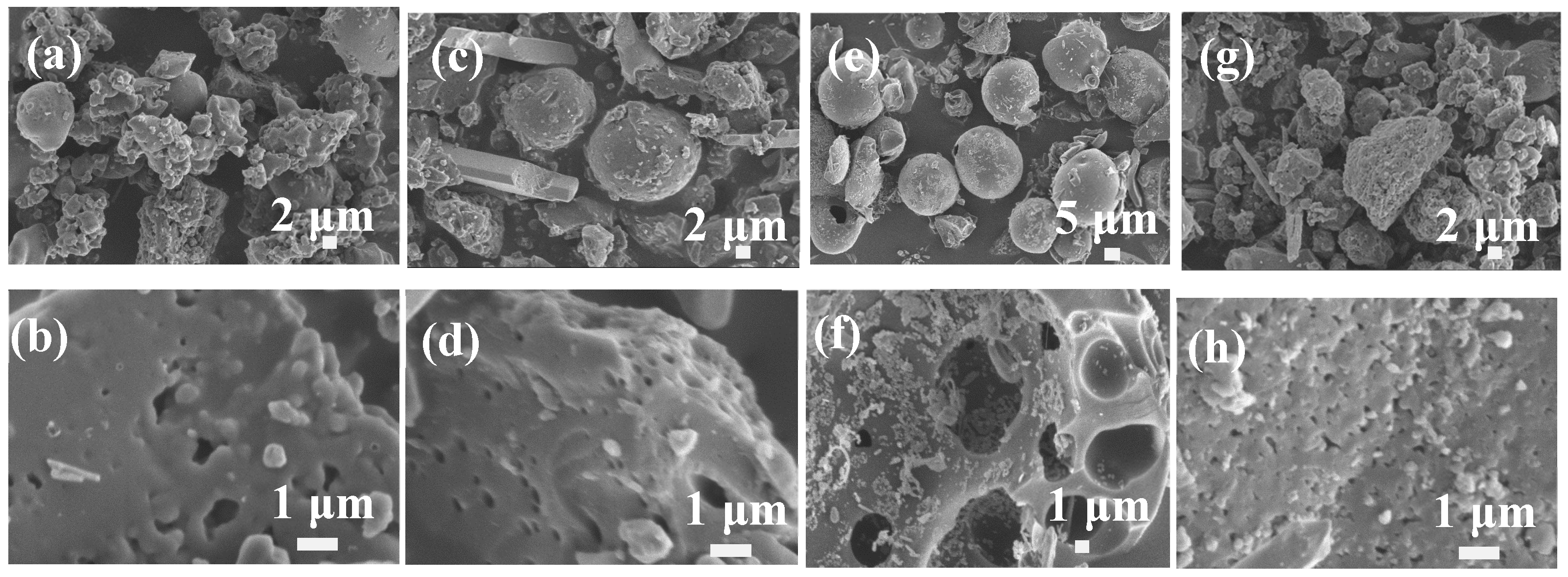
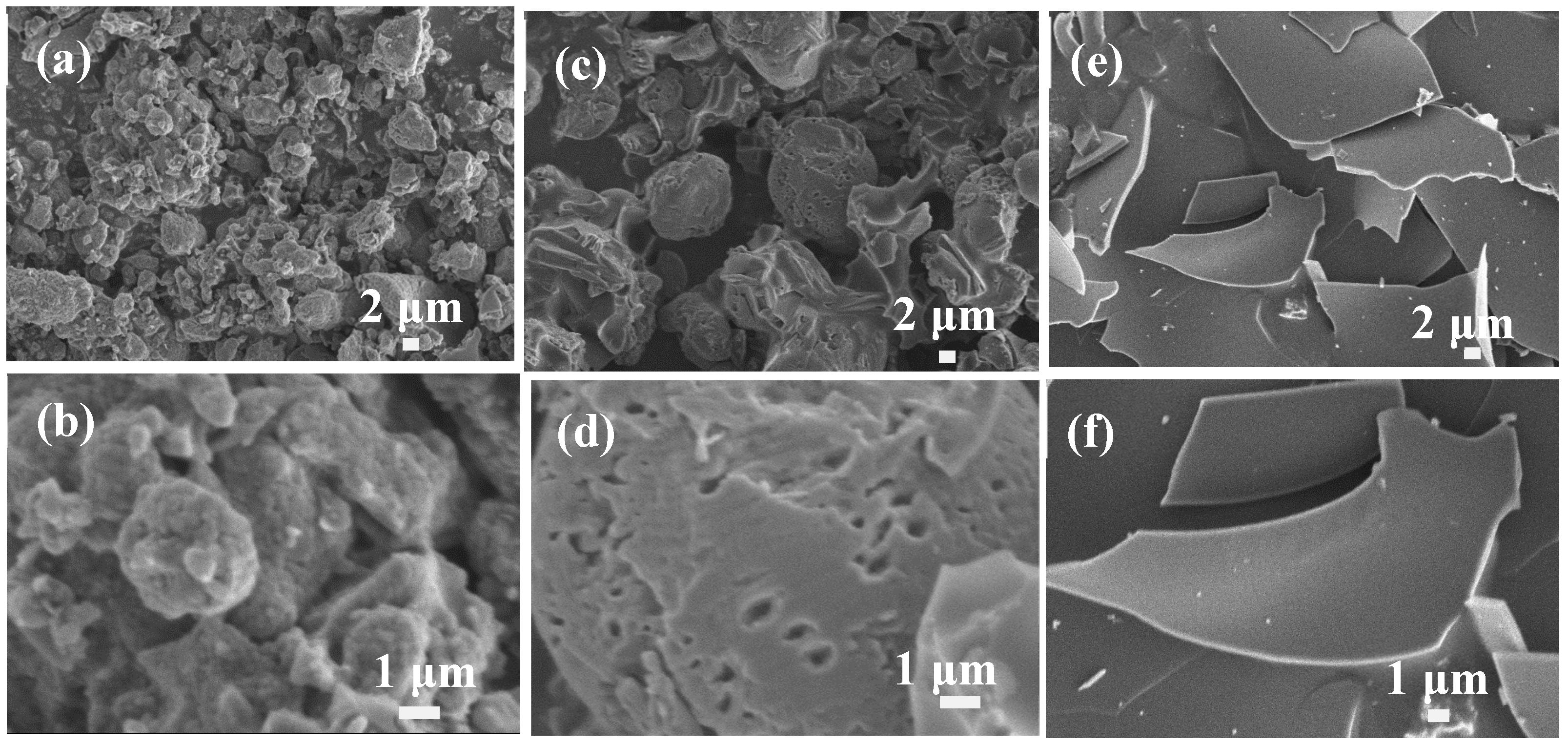
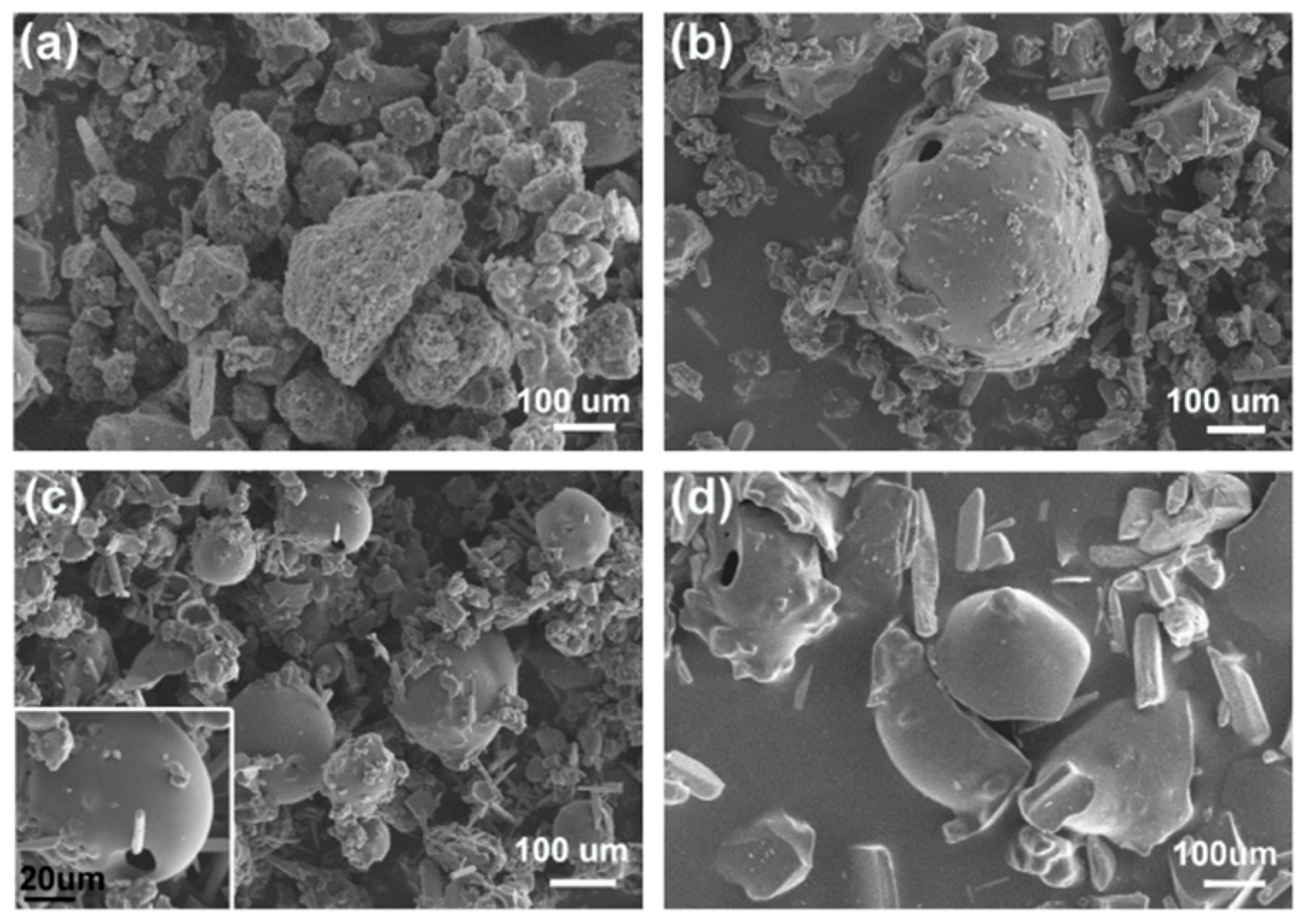
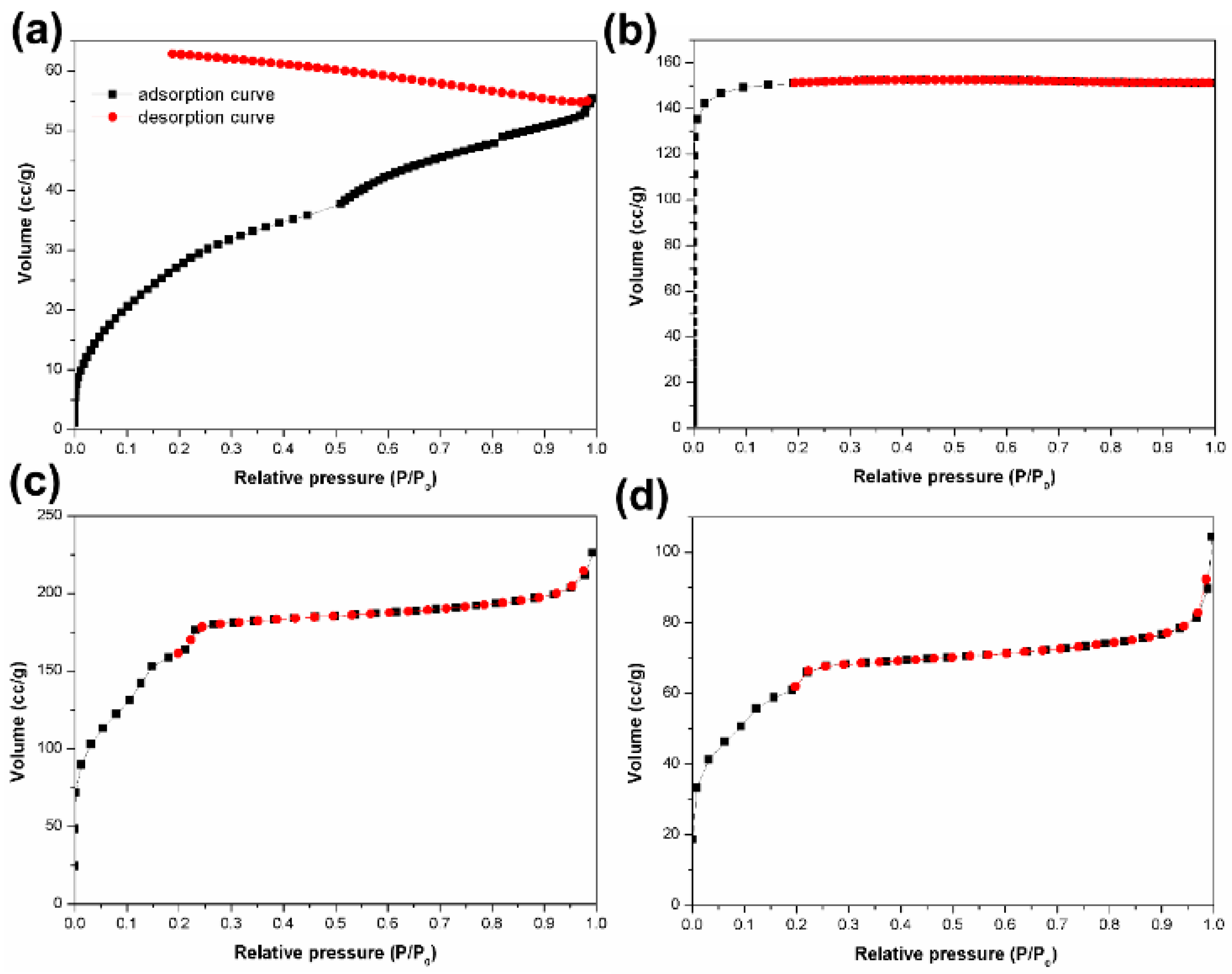
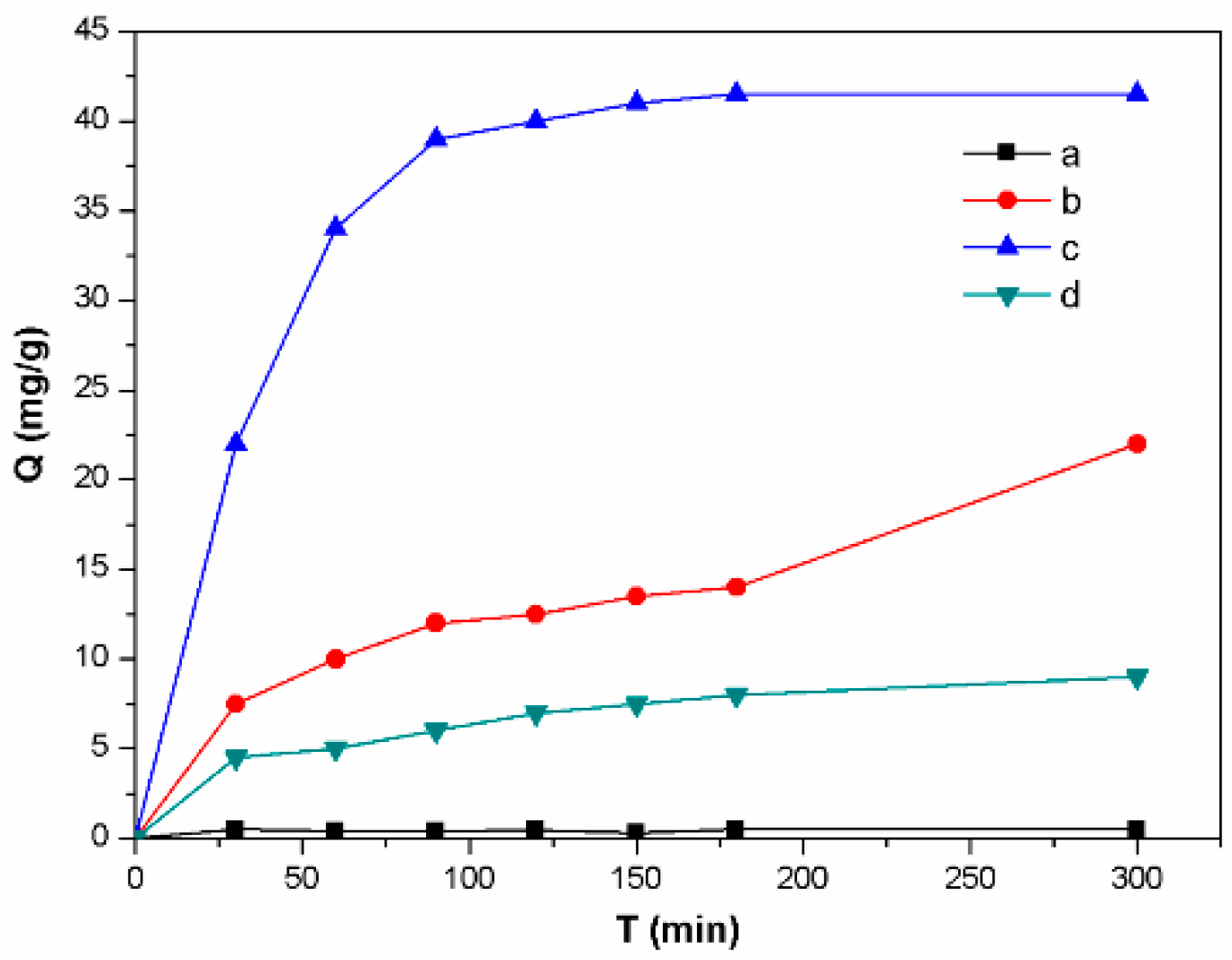
3. Results and Discussion
3.1. The Influences of Four Types of Lignin
3.2. The Influences of The Activated Solution Concentration
3.3. The Influences of The Types of Activated Solution
3.4. The Influences of Synthetic Methods
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Cao, S.W.; Yu, J.G. Carbon-based H2-production photocatalytic materials. J. Photochem. Photobiol. C Photochem. Rev. 2016, 27, 72–99. [Google Scholar] [CrossRef]
- Hou, H.S.; Qiu, X.Q.; Wei, W.F.; Zhang, Y.; Ji, X.B. Carbon anode materials for advanced sodium-ion batteries. Adv. Energy Mater. 2017, 7, 1602898. [Google Scholar] [CrossRef]
- Wang, J.; Xu, F.; Jin, H.Y.; Chen, Y.Q.; Wang, Y. Non-noble metal-based carbon composites in hydrogen evolution reaction: Fundamentals to applications. Adv. Mater. 2017, 29, 1605838. [Google Scholar] [CrossRef] [PubMed]
- Biswal, M.; Banerjee, A.; Deo, M.; Ogale, S. From dead leaves to high energy density supercapacitors. Energy Environ. Sci. 2016, 6, 1249–1259. [Google Scholar] [CrossRef]
- He, X.J.; Li, R.C.; Qiu, J.S.; Xie, K.; Ling, P.H.; Yu, M.X.; Zhang, X.Y.; Zheng, M.D. Synthesis of mesoporous carbons for supercapacitors from coal tar pitch by coupling microwave-assisted KOH activation with a MgO template. Carbon 2012, 50, 4911–4921. [Google Scholar] [CrossRef]
- Zhai, Y.P.; Dou, Y.Q.; Zhao, D.Y.; Fulvio, P.F.; Mayes, R.T.; Dai, S. Carbon materials for chemical capacitive energy storage. Adv. Mater. 2011, 23, 4828–4850. [Google Scholar] [CrossRef]
- Qu, W.H.; Xu, Y.Y.; Lu, A.H.; Zhang, X.Q.; Li, W.C. Converting biowaste corncob residue into high value added porous carbon for supercapacitor electrodes. Bioresour. Technol. 2015, 189, 285–291. [Google Scholar] [CrossRef]
- Luan, Y.T.; Wang, L.; Guo, S.E.; Jiang, B.J.; Zhao, D.D.; Yan, H.J.; Tian, C.G.; Fu, H.G. A hierarchical porous carbon material from a loofah sponge network for high performance supercapacitors. RSC Adv. 2015, 5, 42430–42437. [Google Scholar] [CrossRef]
- Subramanian, V.; Luo, C.; Stephan, C.; Nahm, A.M.; Thomas, K.S.; Wei, B.Q. Supercapacitors from activated carbon derived from banana fibers. J. Phys. Chem. C 2007, 111, 7527–7531. [Google Scholar] [CrossRef]
- Du, X.; Zhao, W.; Wang, Y.; Wang, C.Y.; Chen, M.M.; Qi, T.; Hua, C.; Ma, M.G. Preparation of activated carbon hollow fibers from ramie at low temperature for electric double-layer capacitor applications. Bioresour. Technol. 2013, 149, 31–37. [Google Scholar] [CrossRef] [PubMed]
- Du, X.; Zhao, W.; Ma, S.H.; Ma, M.G.; Qi, T.; Wang, Y.; Hua, C. Effect of ZnCl2 impregnation concentration on the microstructure and electrical performance of ramie-based activated carbon hollow fiber. Ionics 2016, 22, 545–553. [Google Scholar] [CrossRef]
- Liu, S.; Liu, Y.J.; Deng, F.; Ma, M.G.; Bian, J. Comparison of the effects of microcrystalline cellulose and cellulose nanocrystals on the Fe3O4/C nanocomposites. RSC Adv. 2015, 5, 74198–74205. [Google Scholar] [CrossRef]
- Wang, B.; Gao, B.; Fang, J. Recent advances in engineered biochar productions and applications. Crit. Rev. Environ. Sci. Technol. 2017, 47, 2158–2207. [Google Scholar] [CrossRef]
- Rinaldi, R.; Jastrzebski, R.; Clough, M.T.; Ralph, J.; Kennema, M.; Bruijnincx, P.C.A.; Weckhuysen, B.M. Paving the way for lignin valorisation: Recent advances in bioengineering, biorefining and catalysis. Angew. Chem.Int. Ed. 2016, 55, 8164–8215. [Google Scholar] [CrossRef] [PubMed]
- Baker, D.A.; Rials, T.G. Recent advances in low-cost carbon fiber manufacture from lignin. J. Appl. Polym. Sci. 2013, 130, 713–728. [Google Scholar] [CrossRef] [Green Version]
- Fu, K.F.; Yue, Q.Y.; Gao, B.Y.; Sun, Y.Y.; Zhu, L.J. Preparation, characterization and application of lignin-based activated carbon from black liquor lignin by steam activation. Chem. Eng. J. 2013, 228, 1074–1082. [Google Scholar] [CrossRef]
- Saha, D.; Li, Y.C.; Bi, Z.H.; Chen, J.H.; Keum, J.K.; Hensley, D.K.; Grappe, H.A.; Meyer, H.M.; Dai, S.; Paranthaman, M.P. Studies on supercapacitor electrode material from activated lignin-derived mesoporous carbon. Langmuir 2014, 30, 900–910. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.M.; Hu, Y.S.; Li, H.; Chen, L.Q.; Huang, X.J. A superior low-cost amorphous carbon anode made from pitch and lignin for sodium-ion batteries. J. Mater. Chem. A 2016, 4, 96–104. [Google Scholar] [CrossRef]
- Zhang, L.M.; You, T.T.; Zhou, T.; Zhou, X.; Xu, F. Interconnected hierarchical porous carbon from lignin-derived byproducts of bioethanol production for ultra-high performance supercapacitors. ACS Appl. Mater. Interfaces 2016, 8, 13918–13925. [Google Scholar] [CrossRef]
- Zhao, Y.; Liu, Y.; Tong, C.C.; Ru, J.; Geng, B.Y.; Ma, Z.Q.; Liu, H.Z.; Wang, L.K. Flexible lignin-derived electrospun carbon nanofiber mats as a highly efficient and binder-free counter electrode for dye-sensitized solar cells. J. Mater. Sci. 2018, 53, 7637–7647. [Google Scholar] [CrossRef]
- Garcia-Mateos, F.J.; Berenguer, R.; Valero-Romero, M.J.; Rodriguez-Mirasol, J.; Cordero, T. Phosphorus functionalization for the rapid preparation of highly nanoporous submicron-diameter carbon fibers by electrospinning of lignin solutions. J. Mater. Chem. A 2018, 6, 1219–1233. [Google Scholar] [CrossRef]
- Zeng, Z.H.; Wang, C.X.; Zhang, Y.F.; Wang, P.Y.; Seyed, S.; Seyed, I.; Pei, Y.M.; Chen, M.J.; Lu, X.H. Ultralight and highly elastic graphene/lignin-derived carbon nanocomposite aerogels with ultrahigh electromagnetic interference shielding performance. ACS Appl. Mater. Interfaces 2018, 10, 8205–8213. [Google Scholar] [CrossRef] [PubMed]
- Correa, C.R.; Stollovsky, M.; Hehr, T.; Rauscher, Y.; Rolli, B.; Kruse, A. Influence of the carbonization process on activated carbon properties from lignin and lignin-rich biomasses. ACS Sustain. Chem. Eng. 2017, 5, 8222–8233. [Google Scholar] [CrossRef]
- Guo, N.N.; Li, M.; Sun, X.K.; Wang, F.; Yang, R. Enzymatic hydrolysis lignin derived hierarchical porous carbon for supercapacitors in ionic liquids with high power and energy densities. Green Chem. 2017, 19, 2595–2602. [Google Scholar] [CrossRef]
- Liu, W.S.; Yao, Y.M.; Fu, O.L.; Jiang, S.H.; Fang, Y.C.; Wei, Y.; Lu, X.H. Lignin-derived carbon nanosheets for high-capacitance supercapacitors. RSC Adv. 2017, 7, 48537–48543. [Google Scholar] [CrossRef] [Green Version]
- Pan, Z.Z.; Dong, L.B.; Lv, W.; Zheng, D.Q.; Li, Z.J.; Luo, C.; Zheng, C.; Yang, Q.H.; Kang, F.Y. A hollow spherical carbon derived from the spray drying of corncob lignin for high-rate-performance supercapacitors. Chem. Asian J. 2017, 12, 503–506. [Google Scholar] [CrossRef]
- Zhu, Y.J.; Chen, F. Microwave-assisted preparation of inorganic nanostructures in liquid phase. Chem. Rev. 2014, 114, 6462–6555. [Google Scholar] [CrossRef]
- Meng, L.Y.; Wang, B.; Ma, M.G.; Lin, K.L. The progress of microwave-assisted hydrothermal method in the synthesis of functional nanomaterials. Mater. Today Chem. 2016, 1, 63–83. [Google Scholar] [CrossRef]
- Bang, J.H.; Suslick, K.S. Applications of ultrasound to the synthesis of nanostructured materials. Adv. Mater. 2010, 22, 1039–1059. [Google Scholar] [CrossRef]
- Liu, Y.J.; Liu, S.; Li, Z.W.; Ma, M.G.; Wang, B. Microwave synthetic mesoporous carbon sponge as an efficient adsorbent for Cr(VI) removal. RSC Adv. 2018, 8, 7892–7898. [Google Scholar] [CrossRef]
- Yang, S.; Wen, J.L.; Yuan, T.Q.; Sun, R.C. Characterization and phenolation of biorefinery technical lignins for lignin–phenol–formaldehyde resin adhesive synthesis. RSC Adv. 2014, 4, 57996–58004. [Google Scholar] [CrossRef]
- Shi, X.J.; Wang, X.; Tang, B.; Dai, Z.; Chen, K.F.; Zhou, J.H. Impact of lignin extraction methods on microstructure and mechanical properties of lignin-based carbon fibers. J. Appl. Polym. Sci. 2018, 135, 45580. [Google Scholar] [CrossRef]
- Wang, J.; Shen, L.; Ding, B.; Nie, P.; Deng, H.F.; Dou, H.; Zhang, X.G. Fabrication of porous carbon spheres for high-performance electrochemical capacitors. RSC Adv. 2014, 4, 7538–7544. [Google Scholar] [CrossRef]
- Yuan, T.Q.; Sun, S.N.; Xu, F.; Sun, R.C. Isolation and physico-chemical characterization of lignins from ultrasound irradiated fast-growing poplar wood. BioResources 2011, 6, 414–433. [Google Scholar]
- Yang, H.P.; Yan, R.; Chen, H.P.; Zheng, C.G.; Lee, D.H.; Liang, D.T. In-depth investigation of biomass pyrolysis based on three major components: Hemicellulose, cellulose and lignin. Energy Fuels 2006, 20, 388–393. [Google Scholar] [CrossRef]
- Frank, E.; Steudle, L.M.; Ingildeev, D.; Sporl, J.M.; Buchmeiser, M.R. Carbon fibers: Precursor systems, processing, structure, and properties. Angew. Chem.Int. Ed. 2014, 53, 5262–5298. [Google Scholar] [CrossRef] [PubMed]
- Sarkar, M.; Tian, C.; Jahan, M.S. Activated carbon from potassium hydroxide spent liquor lignin using phosphoric acid. TAPPI J. 2018, 17, 63–69. [Google Scholar] [CrossRef]
- Liu, S.; Yao, K.; Fu, L.H.; Ma, M.G. Selective synthesis of Fe3O4, γ-Fe2O3, and α-Fe2O3 using cellulose-based composites as precursors. RSC Adv. 2016, 6, 2135–2140. [Google Scholar] [CrossRef]



© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Meng, L.-Y.; Ma, M.-G.; Ji, X.-X. Preparation of Lignin-Based Carbon Materials and Its Application as a Sorbent. Materials 2019, 12, 1111. https://doi.org/10.3390/ma12071111
Meng L-Y, Ma M-G, Ji X-X. Preparation of Lignin-Based Carbon Materials and Its Application as a Sorbent. Materials. 2019; 12(7):1111. https://doi.org/10.3390/ma12071111
Chicago/Turabian StyleMeng, Ling-Yan, Ming-Guo Ma, and Xing-Xiang Ji. 2019. "Preparation of Lignin-Based Carbon Materials and Its Application as a Sorbent" Materials 12, no. 7: 1111. https://doi.org/10.3390/ma12071111




