Accelerated Microbial Reduction of Azo Dye by Using Biochar from Iron-Rich-Biomass Pyrolysis
Abstract
:1. Introduction
2. Materials and Methods
2.1. Peanut Stalks Used for the Production of Biochars
2.2. Production and Chemical Analyses of Biochars
2.3. Microbial Reduction Capacities of Biochars
2.4. Treatment Design and Incubation Experiments for the Removal of Orange G
2.5. Sample Collection and Analysis
2.6. Calculations and Statistical Analysis
3. Results and Discussion
3.1. Physicochemical Characterization of Biochars
3.2. Adsorbing Removal of Orange G with Biochar as the Adsorbent
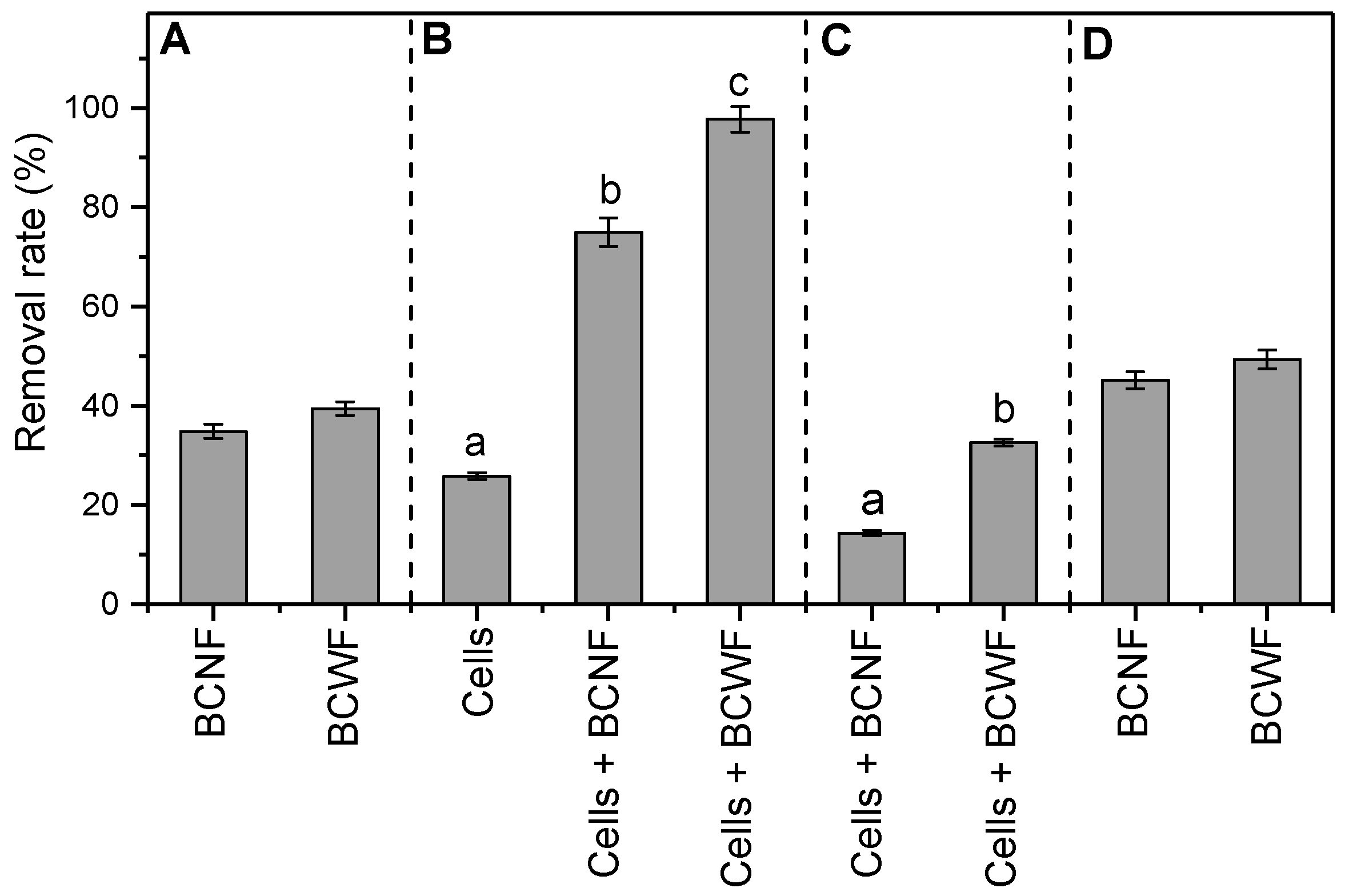
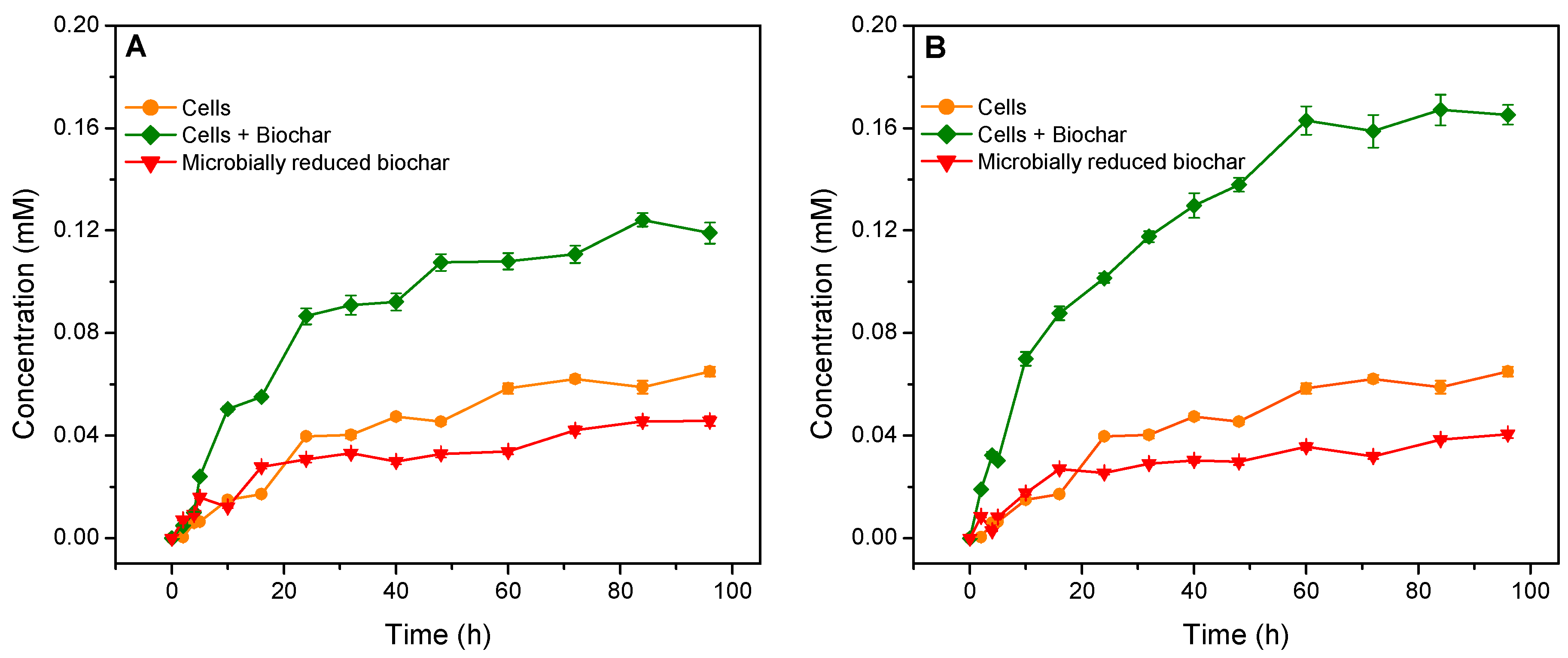
3.3. Stimulated Microbial Reduction of Orange G by Biochars as an Extracellular Electron Shuttle
3.4. Role of Biochar as the Battery and Conductor in Stimulating the Microbial Reduction of Orange G
3.5. Implications for the Future
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Tony, B.D.; Goyal, D.; Khanna, S. Decolorization of textile azo dyes by aerobic bacterial consortium. Int. Biodeterior. Biodegrad. 2009, 63, 462–469. [Google Scholar] [CrossRef]
- Ambashta, R.D.; Sillanpää, M. Water purification using magnetic assistance: A review. J. Hazard. Mater. 2010, 180, 38–49. [Google Scholar] [CrossRef] [PubMed]
- Crini, G. Non-conventional low-cost adsorbents for dye removal: A review. Bioresour. Technol. 2006, 97, 1061–1085. [Google Scholar] [CrossRef]
- Rafatullah, M.; Sulaiman, O.; Hashim, R.; Ahmad, A. Adsorption of methylene blue on low-cost adsorbents: A review. J. Hazard. Mater. 2010, 177, 70–80. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.; Chu, S.; Chen, J.; Xie, Z. Enhanced reduction of an azo dye using henna plant biomass as a solid-phase electron donor, carbon source, and redox mediator. Bioresour. Technol. 2014, 161, 465–468. [Google Scholar] [CrossRef] [PubMed]
- Stylidi, M.; Kondarides, D.I.; Verykios, X.E. Visible light-induced photocatalytic degradation of Acid Orange 7 in aqueous TiO2 suspensions. Appl. Catal. B 2004, 47, 189–201. [Google Scholar] [CrossRef]
- Peternel, I.T.; Koprivanac, N.; Božić, A.M.L.; Kušić, H.M. Comparative study of UV/TiO2, UV/ZnO and photo-Fenton processes for the organic reactive dye degradation in aqueous solution. J. Hazard. Mater. 2007, 148, 477–484. [Google Scholar] [CrossRef]
- Abramian, L.; El-Rassy, H. Adsorption kinetics and thermodynamics of azo-dye Orange II onto highly porous titania aerogel. Chem. Eng. J. 2009, 150, 403–410. [Google Scholar] [CrossRef]
- Goudarzi, M.; Bazarganipour, M.; Salavati-Niasari, M. Synthesis, characterization and degradation of organic dye over Co3O4 nanoparticles prepared from new binuclear complex precursors. RSC Adv. 2014, 4, 46517–46520. [Google Scholar] [CrossRef]
- Lingamdinne, L.P.; Roh, H.; Choi, Y.; Koduru, J.R.; Yang, J.; Chang, Y. Influencing factors on sorption of TNT and RDX using rice husk biochar. J. Ind. Eng. Chem. 2015, 32, 178–186. [Google Scholar] [CrossRef]
- Mezzanotte, V.; Fornaroli, R.; Cannobbio, S.; Zoia, L.; Orlandi, M. Colour removal and carbonyl by-production in high dose ozonation for effluent polishing. Chemosphere 2013, 91, 629–634. [Google Scholar] [CrossRef] [PubMed]
- Nidheesh, P.; Gandhimathi, R.; Ramesh, S. Degradation of dyes from aqueous solution by Fenton processes: A review. Environ. Sci. Pollut. Res. 2013, 20, 2099–2132. [Google Scholar] [CrossRef] [PubMed]
- Blanco, M.; Martinez, A.; Marcaide, A.; Aranzabe, E. Heterogeneous Fenton catalysts for the efficient removal of azo dyes in water. Am. J. Anal. Chem. 2014, 5, 490–499. [Google Scholar] [CrossRef]
- Konstantinou, I.K.; Albanis, T.A. TiO2-assisted photocatalytic degradation of azo dyes in aqueous solution: Kinetic and mechanistic investigations: A review. Appl. Catal. B 2004, 49, 1–14. [Google Scholar] [CrossRef]
- Rajabi, H.R.; Khani, O.; Shamsipur, M.; Vatanpour, V. High-performance pure and Fe3+-ion doped ZnS quantum dots as green nanophotocatalysts for the removal of malachite green under UV-light irradiation. J. Hazard. Mater. 2013, 250–251, 370–378. [Google Scholar] [CrossRef]
- Martins, M.; Cardoso, M.; Queiroz, M.; Ramalho, M.; Campus, A. Biodegradation of azo dyes by the yeast Candida zeylanoides in batch aerated cultures. Chemosphere 1999, 38, 2455–2460. [Google Scholar] [CrossRef]
- McMullan, G.; Meehan, C.; Conneely, A.; Kirby, N.; Robinson, T.; Nigam, P.; Banat, I.; Marchant, R.; Smyth, W. Microbial decolourisation and degradation of textile dyes. Appl. Microbiol. Biotechnol. 2001, 56, 81–87. [Google Scholar] [CrossRef]
- Cao, X.; Ma, L.; Liang, Y.; Gao, B.; Willie, H. Simultaneous immobilization of lead and atrazine in contaminated soils using dairy-manure biochar. Environ. Sci. Technol. 2011, 45, 4884–4889. [Google Scholar] [CrossRef]
- Liu, W.; Jiang, H.; Yu, H. Development of biochar-based functional materials: Toward a sustainable platform carbon material. Chem. Rev. 2015, 115, 12251–12285. [Google Scholar] [CrossRef]
- Yu, L.; Yuan, Y.; Tang, J.; Wang, Y.; Zhou, S. Biochar as an electron shuttle for reductive dechlorination of pentachlorophenol by Geobacter sulfurreducens. Sci. Rep. 2015, 5, 16221. [Google Scholar] [CrossRef] [PubMed]
- Saquing, J.M.; Yu, Y.; Chiu, P.C. Wood-derived black carbon (biochar) as a microbial electron donor and acceptor. Environ. Sci. Technol. 2016, 3, 62–66. [Google Scholar] [CrossRef]
- Sun, T.; Levin, B.D.A.; Guzman, J.J.L.; Enders, A.; Muller, D.A.; Angenent, L.T.; Lehmann, J. Rapid electron transfer by the carbon matrix in natural pyrogenic carbon. Nat. Commun. 2017, 8, 14873. [Google Scholar] [CrossRef] [Green Version]
- Huggins, T.; Wang, H.M.; Kearns, J.; Jenkins, P.; Ren, Z.J. Biochar as a sustainable electrode material for electricity production in microbial fuel cells. Bioresour. Technol. 2014, 157, 114–119. [Google Scholar] [CrossRef] [PubMed]
- Lei, C.; Sun, Y.; Tsang, D.C.W.; Lin, D. Environmental transformations and ecological effects of iron-based nanoparticles. Environ. Pollut. 2018, 232, 10–30. [Google Scholar] [CrossRef]
- Wiedner, K.; Fischer, D.; Walther, S.; Criscuoli, I.; Favilli, F.; Nelle, O.; Glaser, B. Acceleration of biochar surface oxidation during composting? J. Agric. Food. Chem. 2015, 63, 3830–3837. [Google Scholar] [CrossRef]
- Dai, Z.; Meng, J.; Shi, Q.; Xu, B.; Lian, Z.; Brookes, P.C.; Xu, J. Effects of manure- and lignocellulose-derived biochars on adsorption and desorption of zinc by acidic types of soil with different properties. Eur. J. Soil Sci. 2016, 67, 40–50. [Google Scholar] [CrossRef]
- Amstaetter, K.; Borch, T.; Kappler, A. Influence of humic acid imposed changes of ferrihydrite aggregation on microbial Fe(III) reduction. Geochim. Cosmochim. Acta 2012, 85, 326–341. [Google Scholar] [CrossRef]
- Kappler, A.; Wuestner, M.L.; Ruecker, A.; Harter, J.; Halama, M.; Behrens, S. Biochar as an electron shuttle between bacteria and Fe(III) minerals. Environ. Sci. Technol. Lett. 2014, 1, 339–344. [Google Scholar] [CrossRef]
- Cao, D.; Xiao, X.; Wu, Y.; Ma, X.; Wang, M.; Wu, Y.; Du, D. Role of electricity production in the anaerobic decolorization of dye mixture by exoelectrogenic bacterium Shewanella oneidensis MR-1. Bioresour. Technol. 2013, 136, 176–181. [Google Scholar] [CrossRef] [PubMed]
- Cao, X.; Qi, Y.; Xu, C.; Yang, Y.; Wang, J. Transcriptome and metabolome responses of Shewanella oneidensis MR-1 to methyl orange under microaerophilic and aerobic conditions. Appl. Microbiol. Biotechnol. 2017, 101, 3463–3472. [Google Scholar] [CrossRef]
- Tong, H.; Hu, M.; Li, F.B.; Liu, C.S.; Chen, M.J. Biochar enhances the microbial and chemical transformation of pentachlorophenol in paddy soil. Soil Biol. Biochem. 2014, 70, 142–150. [Google Scholar] [CrossRef]
- Okamoto, A.; Hashimoto, K.; Nealson, K.H.; Nakamura, R. Rate enhancement of bacterial extracellular electron transport involves bound flavin semiquinones. Proc. Natl. Acad. Sci. USA 2013, 110, 7856–7861. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Richardson, D.J.; Butt, J.N.; Clarke, T.A. Controlling electron transfer at the microbe-mineral interface. Proc. Natl. Acad. Sci. USA 2013, 110, 7537–7538. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Timpe, O.; Hamid, S.B.A.; Schlogl, R.; Su, D.S. Direct synthesis of carbon nanofibers on modified biomass-derived activated carbon. Carbon 2009, 47, 340–343. [Google Scholar] [CrossRef]
- Liu, W.; Tian, K.; He, Y.; Jiang, H.; Yu, H. High-yield harvest of nanofibers/mesoporous carbon composite by pyrolysis of waste biomass and its application for high durability electrochemical energy storage. Environ. Sci. Technol. 2014, 48, 13951–13959. [Google Scholar] [CrossRef]
- Tang, J.; Zhu, W.; Kookana, R.; Katayama, A. Characteristics of biochar and its application in remediation of contaminated soil. J. Biosci. Bioeng. 2013, 16, 653–659. [Google Scholar] [CrossRef]
- Mohan, D.; Sarswat, A.; Ok, Y.S.; Pittman, C.U. Organic and inorganic contaminants removal from water with biochar, a renewable, low cost and sustainable adsorbent—A critical review. Bioresour. Technol. 2014, 160, 191–202. [Google Scholar] [CrossRef]
- Inyang, M.; Dickenson, E. The potential role of biochar in the removal of organic and microbial contaminants from potable and reuse water: A review. Chemosphere 2015, 134, 232–240. [Google Scholar] [CrossRef]
- Tan, X.; Liu, Y.; Zeng, G.; Wang, X.; Hu, X.; Gu, Y.; Yang, Z. Application of biochar for the removal of pollutants from aqueous solutions. Chemosphere 2015, 125, 70–85. [Google Scholar] [CrossRef]
- Cimò, G.; Kucerik, J.; Berns, A.E.; Schaumann, G.E.; Alonzo, G.; Conte, P. Effect of heating time and temperature on the chemical characteristics of biochar from poultry manure. J. Agric. Food Chem. 2014, 62, 1912–1918. [Google Scholar] [CrossRef]
- McBeath, A.V.; Smernik, R.J.; Krull, E.S.; Lehmann, J. The influence of feedstock and production temperature on biochar carbon chemistry: A solid-state 13C NMR study. Biomass Bioenerg. 2014, 60, 121–129. [Google Scholar] [CrossRef]
- Oliveira, F.R.; Patel, A.K.; Jaisi, D.P.; Adhikari, S.; Lu, H.; Khanal, S.K. Environmental application of biochar: Current status and perspectives. Bioresour. Technol. 2017, 246, 110–122. [Google Scholar] [CrossRef] [PubMed]
- Yuan, Y.; Bolan, N.; Prévoteau, A.; Vithanage, M.; Biswas, J.K.; Ok, Y.S.; Wang, H. Applications of biochar in redox-mediated reactions. Bioresour. Technol. 2017, 246, 271–281. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, C.; Sun, H.; Bai, J.; Li, L. Innovative methodology for comprehensive utilization of iron ore tailings: Part 1. The recovery of iron from iron ore tailings using magnetic separation after magnetizing roasting. J. Hazard. Mater. 2010, 174, 71–77. [Google Scholar] [CrossRef]
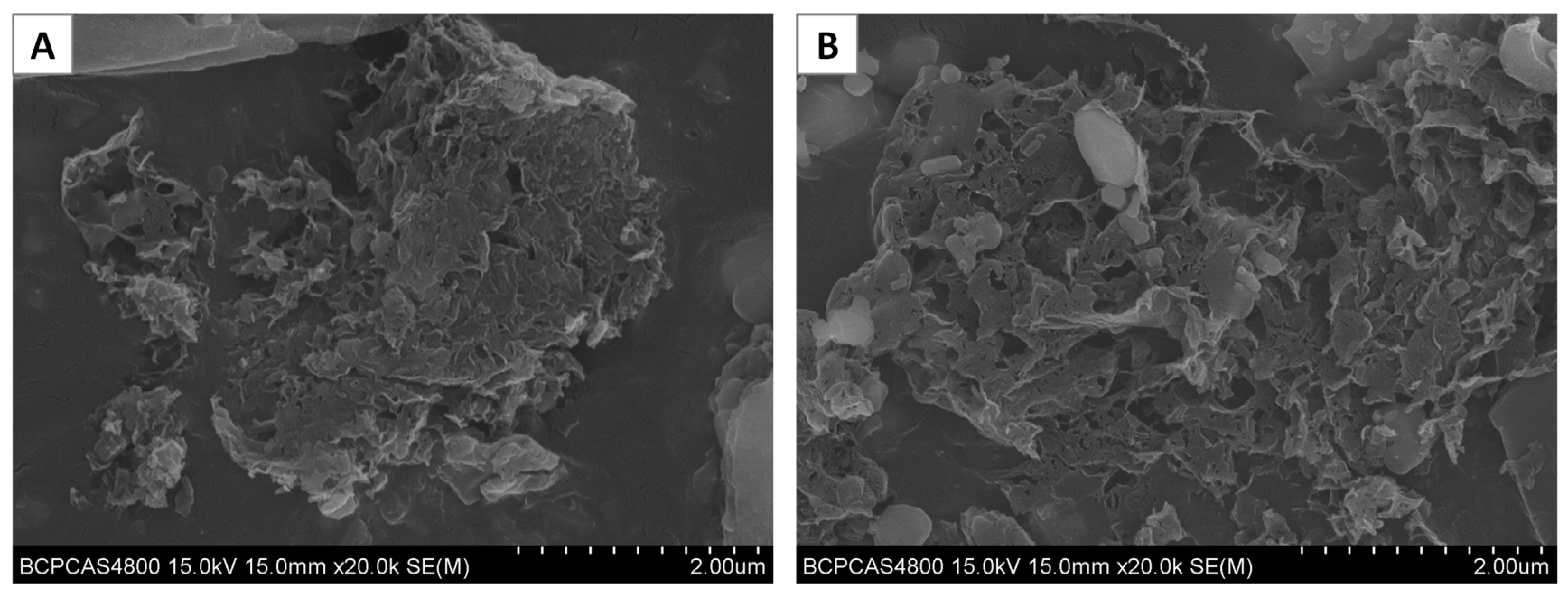



| Straw Type | Fe (mg kg−1) | C (g kg−1) | N (g kg−1) | H (g kg−1) | O (g kg−1) |
|---|---|---|---|---|---|
| PSNF | 18.2 ± 1.7 a | 482 ± 38 | 32.4 ± 3.0 | 43.2 ± 4.2 | 329 ± 29 |
| PSWF | 79.7 ± 6.0 b | 464 ± 41 | 35.2 ± 2.8 | 40.5 ± 3.4 | 310 ± 25 |
| Parameter | BCNF | BCWF |
|---|---|---|
| C (%) | 53.2 ± 3.7 | 56.7 ± 4.0 |
| H (%) | 3.67 ± 0.24 | 3.52 ± 0.29 |
| N (%) | 1.87 ± 0.16 | 1.98 ± 0.15 |
| O (%) | 23.4 ± 2.8 | 26.1 ± 2.1 |
| P (%) | 0.38 ± 0.05 | 0.31 ± 0.06 |
| K (%) | 1.12 ± 0.17 | 0.97 ± 0.11 |
| Ca (%) | 8.53 ± 0.61 | 8.11 ± 0.57 |
| Mg (%) | 6.12 ± 0.48 | 5.66 ± 0.52 |
| Fe (mg kg−1) | 548 ± 43a | 3578 ± 210b |
| Co (mg kg−1) | 1.17 ± 0.08 | 1.32 ± 0.18 |
| Cu (mg kg−1) | 27.3 ± 2.3 | 23.4 ± 3.5 |
| Zn (mg kg−1) | 17.5 ± 1.2 | 15.7 ± 1.5 |
| Mo (mg kg−1) | 0.34 ± 0.06 | 0.41 ± 0.04 |
| Ni (mg kg−1) | 3.11 ± 0.41 | 3.43 ± 0.34 |
| pH | 6.84 ± 0.11 | 7.21 ± 0.27 |
| Ash (%) | 19.3 ± 1.4 | 21.7 ± 2.0 |
| Specific surface area (m2 g−1) | 279 ± 23 | 305 ± 34 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tan, W.; Wang, L.; Yu, H.; Zhang, H.; Zhang, X.; Jia, Y.; Li, T.; Dang, Q.; Cui, D.; Xi, B. Accelerated Microbial Reduction of Azo Dye by Using Biochar from Iron-Rich-Biomass Pyrolysis. Materials 2019, 12, 1079. https://doi.org/10.3390/ma12071079
Tan W, Wang L, Yu H, Zhang H, Zhang X, Jia Y, Li T, Dang Q, Cui D, Xi B. Accelerated Microbial Reduction of Azo Dye by Using Biochar from Iron-Rich-Biomass Pyrolysis. Materials. 2019; 12(7):1079. https://doi.org/10.3390/ma12071079
Chicago/Turabian StyleTan, Wenbing, Lei Wang, Hanxia Yu, Hui Zhang, Xiaohui Zhang, Yufu Jia, Tongtong Li, Qiuling Dang, Dongyu Cui, and Beidou Xi. 2019. "Accelerated Microbial Reduction of Azo Dye by Using Biochar from Iron-Rich-Biomass Pyrolysis" Materials 12, no. 7: 1079. https://doi.org/10.3390/ma12071079




