Applications of Copolymers Consisting of 2,6-di(9H-carbazol-9-yl)pyridine and 3,6-di(2-thienyl)carbazole Units as Electrodes in Electrochromic Devices
Abstract
:1. Introduction
2. Experimental
2.1. Materials
2.2. Electrochemical Preparation of PdCz, P(dCz2-co-dTC1), P(dCz2-co-dTC2), P(dCz1-co-dTC2), PdTC, and PProdot-Me2 Films
2.3. Fabrication of Electrochromic Devices
2.4. Characterizations of Polymer Films and ECDs
3. Results and Discussion
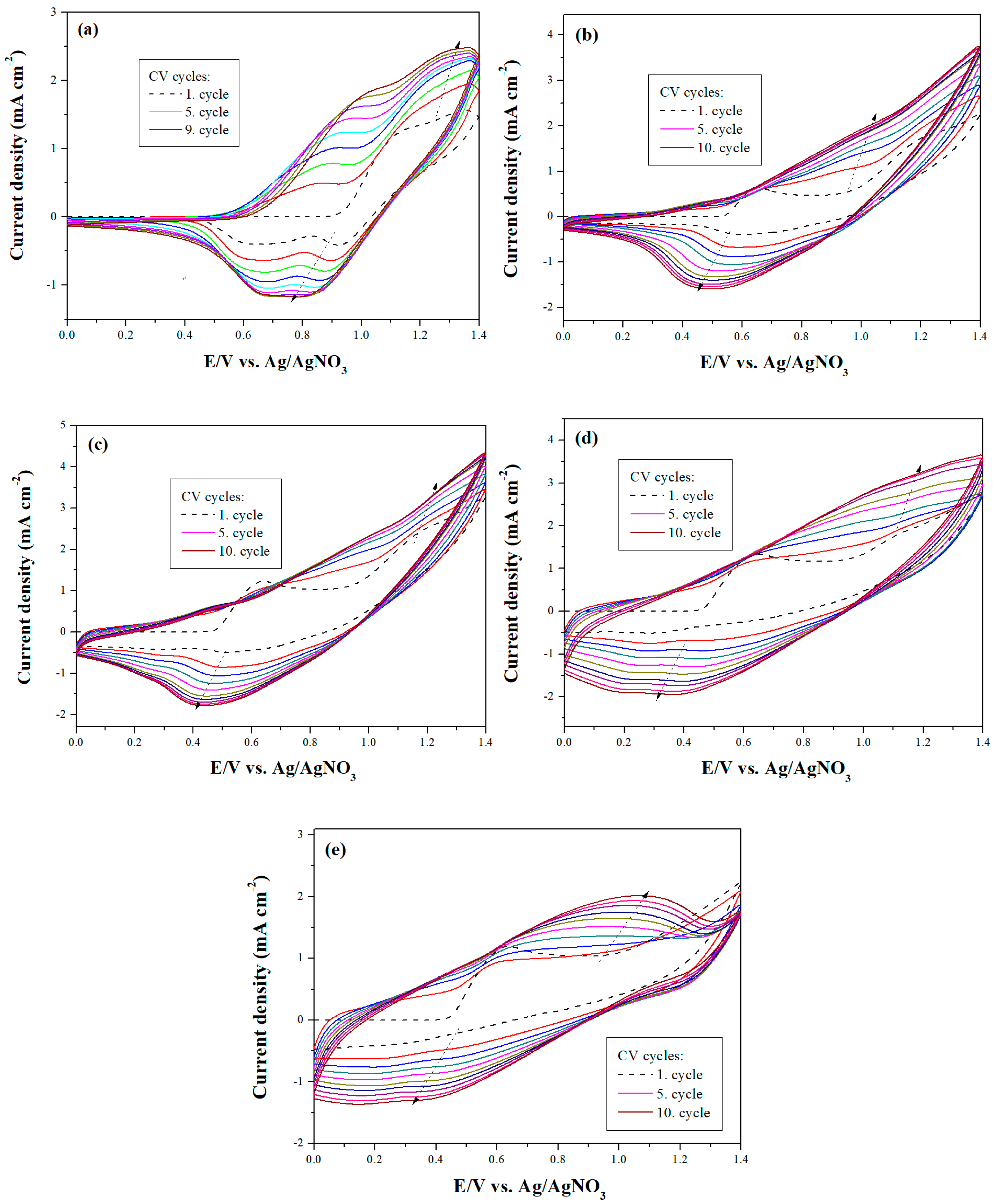
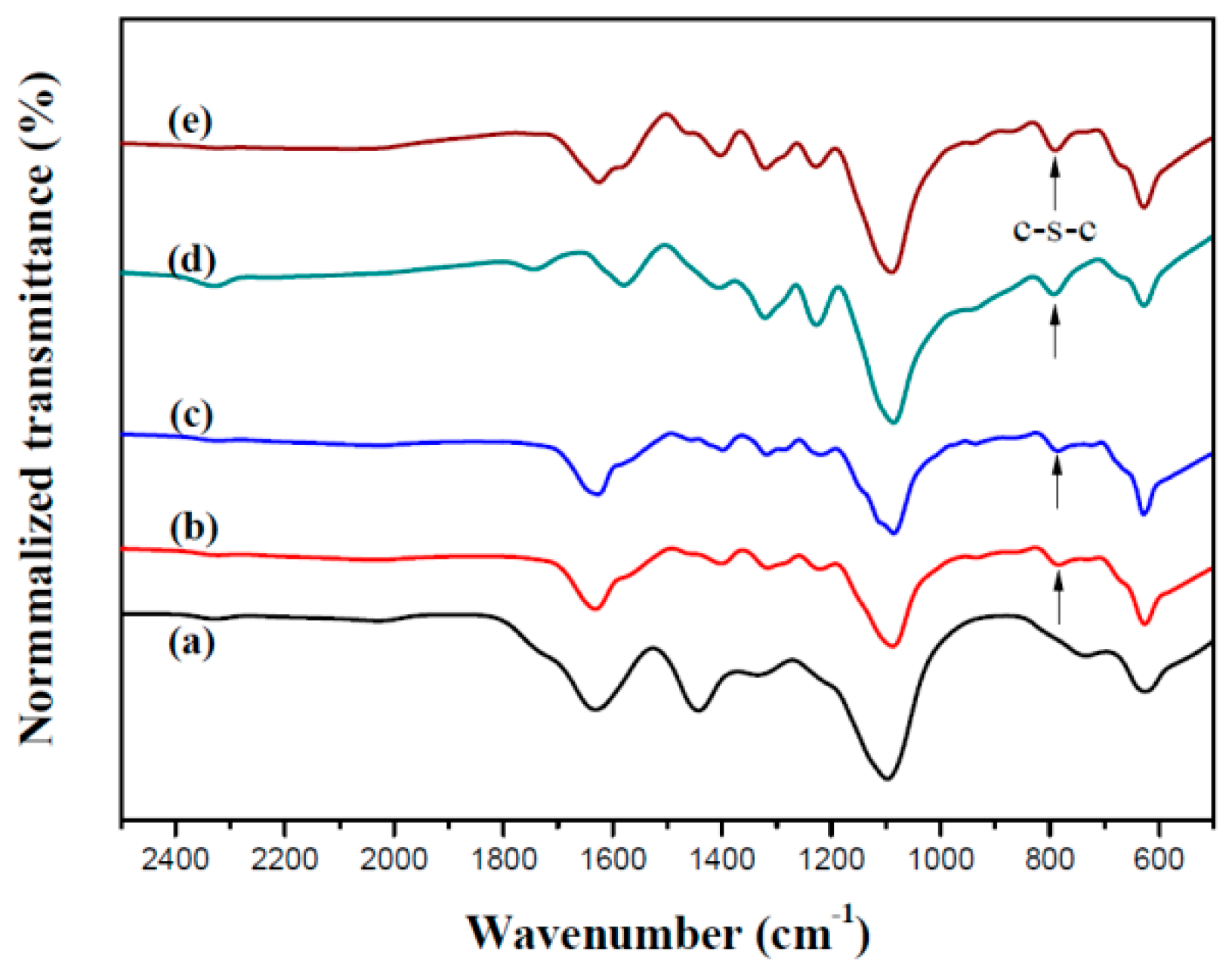
3.1. Electrochemical Polymerization and FT-IR Characterization
3.2. Electrochemical Properties of Polymer Films
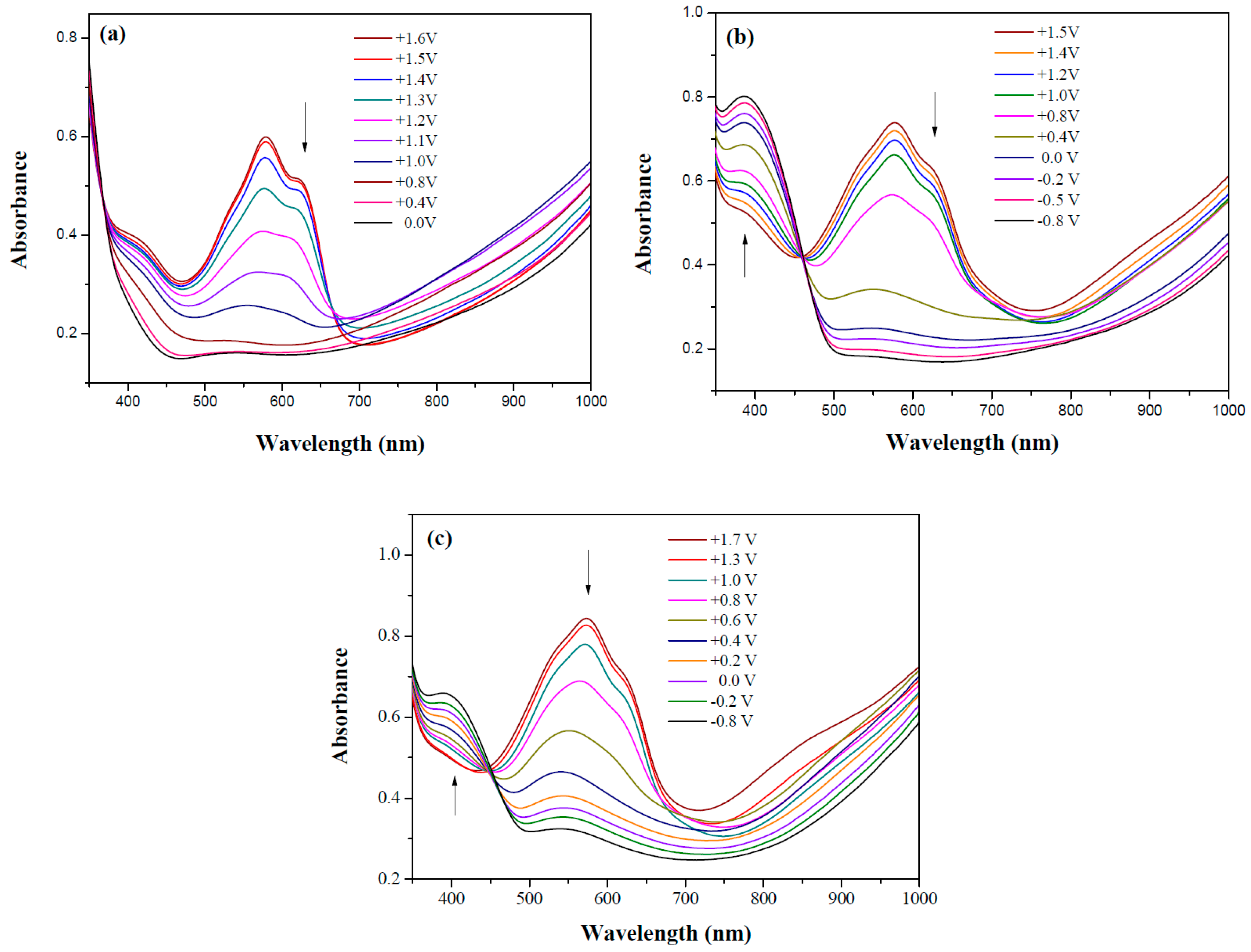
3.3. Spectroelectrochemical Investigation of Polymer Films
3.4. Electrochromic Switching of Polymer Electrodes
3.5. Spectroelectrochemical Investigation of ECDs
3.6. Electrochromic Switching of ECDs
3.7. Open-Circuit Memory of ECDs
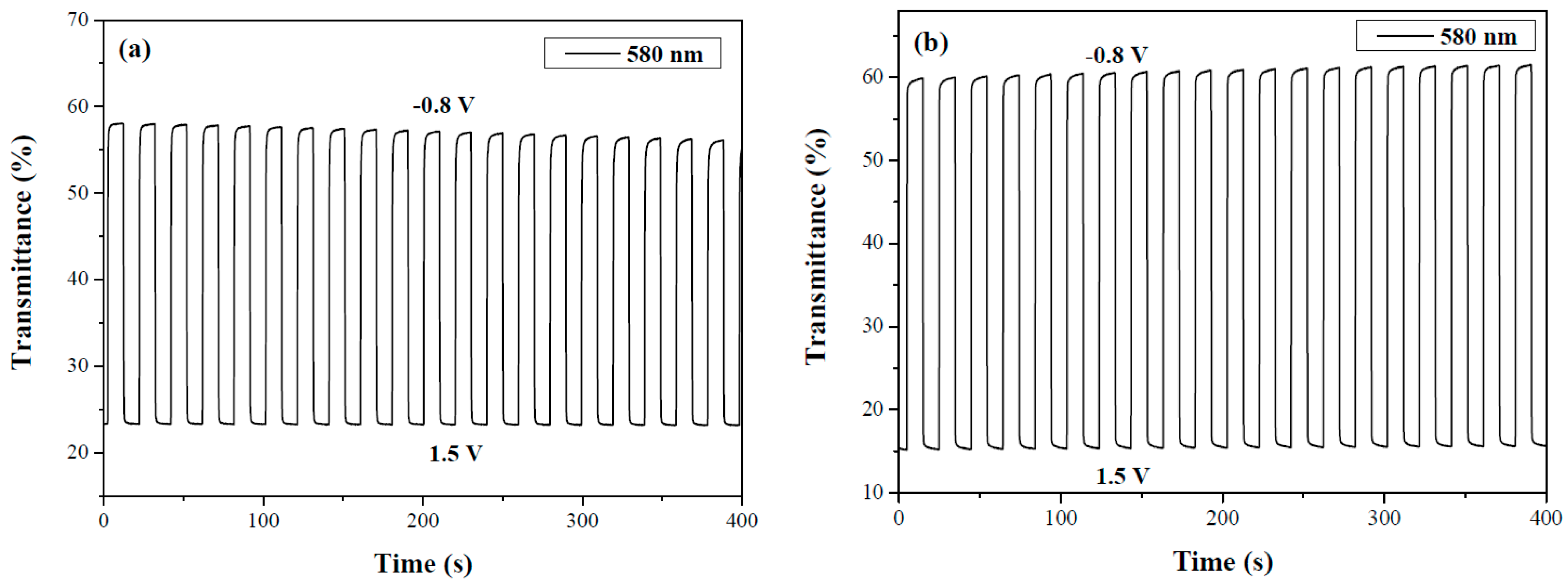
3.8. Electrochemical Stability of ECDs
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Mortimer, R.J. Electrochromic materials. Annu. Rev. Mater. Res. 2011, 41, 241–268. [Google Scholar] [CrossRef]
- Baetens, R.; Jelle, B.P.; Gustavsen, A. Properties, requirements and possibilities of smart windows for dynamic daylight and solar energy control in buildings: A state-of-the-art review. Sol. Energy Mater. Sol. Cells 2016, 94, 87–105. [Google Scholar] [CrossRef]
- Camurlu, P.; Gültekin, C.; Bicil, Z. Fast switching, high contrast multichromic polymers from alkyl-derivatized dithienylpyrrole and 3,4-ethylenedioxythiophene. Electrochim. Acta 2012, 61, 50–56. [Google Scholar] [CrossRef]
- Kuo, C.-W.; Wu, B.-W.; Chang, J.-K.; Chang, J.-C.; Lee, L.-T.; Wu, T.-Y.; Ho, T.-H. Electrochromic devices based on poly(2,6-di(9H-carbazol-9-yl)pyridine)-type polymer films and PEDOT-PSS. Polymers 2018, 10, 604. [Google Scholar] [CrossRef]
- Turkarslan, O.; Ak, M.; Tanyeli, C.; Toppare, L. Enhancing electrochromic properties of conducting polymers via copolymerization: Copolymer of 1-(4-fluorophenyl)-2,5-di(thiophen-2-yl)-1H-pyrrole with 3,4-ethylene dioxythiophene. J. Polym. Sci. Polym. Chem. 2007, 45, 4496. [Google Scholar] [CrossRef]
- Wu, T.Y.; Li, W.B.; Kuo, C.W.; Chou, C.F.; Liao, J.W.; Chen, H.R.; Tseng, C.G. Study of poly(methyl methacrylate)-based gel electrolyte for electrochromic device. Int. J. Electrochem. Sci. 2013, 8, 10720–10732. [Google Scholar]
- Hsiao, S.-H.; Liao, Y.-C. Facile synthesis of electroactive and electrochromic triptycene poly(ether-imide)s containing triarylamine units via oxidative electro-coupling. Polymers 2017, 9, 497. [Google Scholar] [CrossRef]
- Nie, G.M.; Zhou, L.J.; Yang, H.J. Electrosynthesis of a new polyindole derivative obtained from 5-formylindole and its electrochromic properties. J. Mater. Chem. 2011, 21, 13873–13880. [Google Scholar] [CrossRef]
- Hsiao, S.-H.; Lu, H.-Y. Electrosynthesis of aromatic poly(amide-amine) films from triphenylamine-based electroactive compounds for electrochromic applications. Polymers 2017, 9, 708. [Google Scholar] [CrossRef] [PubMed]
- Camurlu, P. Polypyrrole derivatives for electrochromic applications. RSC Adv. 2014, 4, 55832–55845. [Google Scholar] [CrossRef]
- Liu, J.; Mi, S.; Xu, Z.; Wu, J.; Zheng, J.; Xu, C. Solution-processable thiophene-based electrochromic polymers bearing trifluoromethyl rather than long side chains. Org. Electron. 2016, 37, 169–177. [Google Scholar] [CrossRef]
- Çetin, G.A.; Balan, A.; Durmus, A.; Günbas, G.; Toppare, L. A new p- and n-dopable selenophene derivative and its electrochromic properties. Org. Electron. 2009, 10, 34–41. [Google Scholar] [CrossRef]
- Yu, W.; Chen, J.; Fu, Y.; Xu, J.; Nie, G. Electrochromic property of a copolymer based on 5-cyanoindole and 3,4-ethylenedioxythiophene and its application in electrochromic devices. J. Electroanal. Chem. 2013, 700, 17–23. [Google Scholar] [CrossRef]
- Tao, Y.; Zhang, K.; Zhang, Z.; Cheng, H.; Jiao, C.; Zhao, Y. Synthesis, characterizations and electrochromic properties of polymers based on functionalized anthracene. Chem. Eng. J. 2016, 293, 34–43. [Google Scholar] [CrossRef]
- Hsiao, S.-H.; Liao, W.-K.; Liou, G.-S. Synthesis and electrochromism of highly organosoluble polyamides and polyimides with bulky trityl-substituted triphenylamine units. Polymers 2017, 9, 511. [Google Scholar] [CrossRef]
- Lu, Q.; Cai, W.; Niu, H.; Wang, W.; Bai, X.; Hou, Y. Novel polyamides with 5H-dibenzo[b,f]azepin-5-yl-substituted triphenylamine: Synthesis and visible-NIR electrochromic properties. Polymers 2017, 9, 542. [Google Scholar] [CrossRef]
- Guzela, M.; Karatasbz, E.; Ak, M. Synthesis and fluorescence properties of carbazole based asymmetric functionalized star shaped polymer. J. Electrochem. Soc. 2017, 164, H49–H55. [Google Scholar] [CrossRef]
- Ouyang, M.; Fu, Z.; Lv, X.; Hu, B.; Wang, P.; Huang, S.; Dai, Y.; Zhang, C. A multichromic copolymer based on 4-(9H-carbazol-9-yl)-N,N-diphenylaniline and 3,4-ethylenedioxythiophene prepared via electrocopolymerization. J. Electrochem. Soc. 2013, 160, H787–H792. [Google Scholar] [CrossRef]
- Feng, F.; Kong, L.; Du, H.; Zhao, J.; Zhang, J. Donor-acceptor-type copolymers based on 3,4-propylenedioxy-thiophene and 5,6-difluorobenzotriazole: Synthesis and electrochromic properties. Polymers 2018, 10, 427. [Google Scholar] [CrossRef]
- Alkan, S.; Cutler, C.A.; Reynolds, J.R. High-quality electrochromic polythiophenes via BF3·Et2O electropolymerization. Adv. Funct. Mater. 2003, 13, 331–336. [Google Scholar] [CrossRef]
- Welsh, D.M.; Kumar, A.; Morvant, M.C.; Reynolds, J.R. Fast electrochromic polymers based on new poly(3,4-alkylenedioxythiophene) derivatives. Synth. Met. 1999, 102, 967–968. [Google Scholar] [CrossRef]
- Heydari Gharahcheshmeh, M.; Gleason, K.K. Device fabrication based on oxidative chemical vapor deposition (oCVD): Synthesis of conducting polymers and related conjugated organic materials. Adv. Mater. Interfaces 2019, 6, 1801564. [Google Scholar] [CrossRef]
- Brooke, R.; Cottis, P.; Talemi, P.; Fabretto, M.; Murphy, P.; Evans, D. Recent advances in the synthesis of conducting polymers from the vapour phase. Prog. Mater. Sci. 2017, 86, 127. [Google Scholar] [CrossRef]
- Su, Y.-S.; Wu, T.-Y. Three carbazole-based polymers as potential anodically coloring materials for high-contrast electrochromic devices. Polymers 2017, 9, 284. [Google Scholar] [CrossRef]
- Welsh, D.M.; Kumar, A.; Meijer, E.W.; Reynolds, J.R. Enhanced contrast ratio and rapid switching in electrochromics based on poly(3,4-propylenedioxythiophene) derivatives. Adv. Mater. 1999, 11, 1379–1382. [Google Scholar] [CrossRef]
- Kuo, C.W.; Chen, B.K.; Li, W.B.; Tseng, L.Y.; Wu, T.Y.; Tseng, C.G.; Chen, H.R.; Huang, Y.C. Effects of supporting electrolytes on spectroelectrochemical and electrochromic properties of polyaniline-poly(styrene sulfonic acid) and poly(ethylenedioxythiophene)-poly(styrene sulfonic acid)-based electrochromic device. J. Chin. Chem. Soc. 2014, 61, 563–570. [Google Scholar] [CrossRef]
- Kuo, C.-W.; Wu, T.-Y.; Fan, S.-C. Applications of poly(indole-6-carboxylic acid-co-2,2′-bithiophene) films in high-contrast electrochromic devices. Coatings 2018, 8, 102. [Google Scholar] [CrossRef]
- Ates, M.; Özyılmaz, A.T. The application of polycarbazole, polycarbazole/nanoclay and polycarbazole/Zn-nanoparticles as a corrosion inhibition for SS304 in saltwater. Prog. Org. Coat. 2015, 84, 50–58. [Google Scholar] [CrossRef]
- Wu, T.Y.; Li, J.L. Electrochemical synthesis, optical, electrochemical and electrochromic characterizations of indene and 1,2,5-thiadiazole-based poly(2,5-dithienylpyrrole) derivatives. RSC Adv. 2016, 6, 15988–15998. [Google Scholar] [CrossRef]
- Soganci, T.; Soyleyici, H.C.; Ak, M.; Cetisli, H. An amide substituted dithienylpyrrole based copolymer: Its electrochromic properties, physical and analytical electrochemistry, electrocatalysis, and photoelectrochemistry. J. Electrochem. Soc. 2016, 163, H59–H66. [Google Scholar] [CrossRef]
- Wu, T.-Y.; Su, Y.-S.; Chang, J.-C. Dithienylpyrrole- and tris[4-(2-thienyl)phenyl]amine-containing copolymers as promising anodic layers in high-contrast electrochromic devices. Coatings 2018, 8, 164. [Google Scholar] [CrossRef]
- Kuo, C.W.; Wu, T.Y.; Huang, M.W. Electrochromic characterizations of copolymers based on 4,4′-bis(N-carbazolyl)-1,1′-biphenyl and indole-6-carboxylic acid and their applications in electrochromic devices. J. Taiwan Inst. Chem. Eng. 2016, 68, 481–488. [Google Scholar] [CrossRef]
- Udum, Y.A.; Hızlıateş, C.G.; Ergün, Y.; Toppare, L. Electrosynthesis and characterization of an electrochromic material containing biscarbazole–oxadiazole units and its application in an electrochromic device. Thin Solid Films 2015, 595, 61–67. [Google Scholar] [CrossRef]
- Koyuncu, S.; Gultekin, B.; Zafer, C.; Bilgili, H.; Can, M.; Demic, S.; Kaya, I.; Icli, S. Electrochemical and optical properties of biphenyl bridged-dicarbazole oligomer films: Electropolymerization and electrochromism. Electrochim. Acta 2009, 54, 5694–5702. [Google Scholar] [CrossRef]
- Kuo, C.W.; Lee, P.Y. Electrosynthesis of copolymers based on 1,3,5-tris(N-carbazolyl)benzene and 2,2′-bithiophene and their applications in electrochromic devices. Polymers 2017, 9, 518. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.; Zhao, J.; Liu, R.; Liu, J.; He, Q. Electrosyntheses, characterizations and electrochromic properties of a copolymer based on 4,4′-di(N-carbazoyl)biphenyl and 2,2′-bithiophene. Sol. Energy Mater. Sol. Cells 2011, 95, 1867–1874. [Google Scholar] [CrossRef]
- Kuo, C.W.; Chang, J.K.; Lin, Y.C.; Wu, T.Y.; Lee, P.Y.; Ho, T.H. Poly(tris(4-carbazoyl-9-ylphenyl)amine)/three poly(3,4-ethylenedioxythiophene) derivatives complementary high-contrast electrochromic devices. Polymers 2017, 9, 543. [Google Scholar] [CrossRef]
- Kuo, C.W.; Wu, T.L.; Lin, Y.C.; Chang, J.K.; Chen, H.R.; Wu, T.Y. Copolymers based on 1,3-bis(carbazol-9-yl)benzene and three 3,4-ethylenedioxythiophene derivatives as potential anodically coloring copolymers in high-contrast electrochromic devices. Polymers 2016, 8, 368. [Google Scholar] [CrossRef] [PubMed]
- Kuo, C.-W.; Chang, J.-C.; Lee, P.-Y.; Wu, T.-Y.; Huang, Y.-C. Applications of electrochromic copolymers based on tris(4-carbazoyl-9-ylphenyl)amine and bithiophene derivatives in electrochromic devices. Materials 2018, 11, 1895. [Google Scholar] [CrossRef]
- Wu, T.Y.; Su, Y.S. Electrochemical synthesis and characterization of 1,4-benzodioxan-based electrochromic polymer and its application in electrochromic devices. J. Electrochem. Soc. 2015, 162, G103–G112. [Google Scholar] [CrossRef]
- Kuo, C.W.; Hsieh, T.H.; Hsieh, C.K.; Liao, J.W.; Wu, T.Y. Electrosynthesis and characterization of four electrochromic polymers based on carbazole and indole-6-carboxylic acid and their applications in high-contrast electrochromic devices. J. Electrochem. Soc. 2014, 161, D782–D790. [Google Scholar] [CrossRef]
- Su, Y.S.; Chang, J.C.; Wu, T.Y. Applications of three dithienylpyrroles-based electrochromic polymers in high-contrast electrochromic devices. Polymers 2017, 9, 114. [Google Scholar] [CrossRef] [PubMed]







| Electrodes | Anodic Polymer | Feed Species of Anodic Polymer | Feed Molar Ratio of Anodic Polymer |
|---|---|---|---|
| (a) | PdCz | 2 mM dCz | Neat dCz |
| (b) | P(dCz2-co-dTC1) | 2 mM dCz + 1 mM dTC | 2:1 |
| (c) | P(dCz2-co-dTC2) | 2 mM dCz + 2 mM dTC | 2:2 |
| (d) | P(dCz1-co-dTC2) | 1 mM dCz + 2 mM dTC | 1:2 |
| (e) | PdTC | 2 mM dTC | Neat dTC |
| (a) | ||||||
| Potential (V) | L* | a* | b* | x | y | Diagram |
| 0.0 | 95.12 | −0.34 | 2.95 | 0.4500 | 0.4106 | 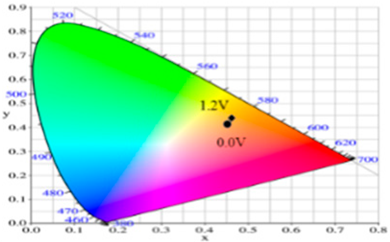 |
| 0.8 | 92.64 | −1.33 | 18.74 | 0.4638 | 0.4258 | |
| 0.9 | 90.35 | −1.99 | 25.66 | 0.4692 | 0.4328 | |
| 1.0 | 87.76 | −3.07 | 23.96 | 0.4664 | 0.4334 | |
| 1.2 | 84.27 | −4.52 | 18.11 | 0.4588 | 0.4311 | |
| (b) | ||||||
| Potential (V) | L* | a* | b* | x | y | Diagram |
| −0.5 | 90.36 | −2.48 | 41.83 | 0.4815 | 0.4456 | 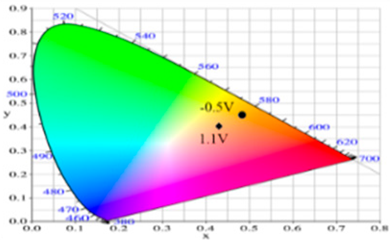 |
| 0.2 | 78.82 | 1.71 | 24.79 | 0.4775 | 0.4293 | |
| 0.4 | 64.49 | 5.65 | 1.11 | 0.4612 | 0.3999 | |
| 0.8 | 56.45 | 6.50 | −7.41 | 0.4509 | 0.3859 | |
| 1.1 | 58.22 | −2.34 | −8.72 | 0.4284 | 0.3987 | |
| (c) | ||||||
| Potential (V) | L* | a* | b* | x | y | Diagram |
| −0.2 | 87.82 | −1.08 | 35.11 | 0.4793 | 0.4395 | 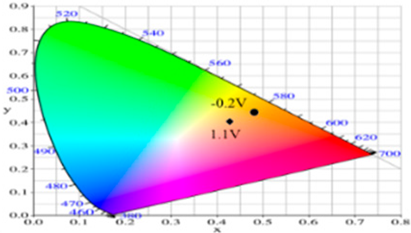 |
| 0.2 | 65.75 | 4.92 | 1.02 | 0.4593 | 0.4011 | |
| 0.8 | 57.30 | 5.37 | −6.45 | 0.4498 | 0.3893 | |
| 0.9 | 56.84 | 3.41 | −6.98 | 0.4444 | 0.3916 | |
| 1.1 | 57.99 | −3.38 | −8.82 | 0.4258 | 0.4002 | |
| (d) | ||||||
| Potential (V) | L* | a* | b* | x | y | Diagram |
| −0.2 | 85.42 | 0.44 | 42.42 | 0.4883 | 0.4432 | 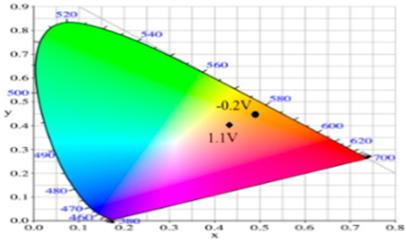 |
| 0.2 | 75.20 | 3.48 | 26.48 | 0.4836 | 0.4287 | |
| 0.6 | 54.48 | 7.37 | −6.51 | 0.4546 | 0.3851 | |
| 0.9 | 53.03 | 4.21 | −6.75 | 0.4466 | 0.3898 | |
| 1.1 | 55.04 | −1.72 | −7.61 | 0.4309 | 0.3990 | |
| (e) | ||||||
| Potential (V) | L* | a* | b* | x | y | Diagram |
| −0.2 | 87.95 | −1.58 | 31.51 | 0.4754 | 0.4374 | 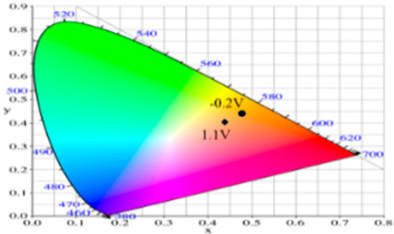 |
| 0.2 | 82.07 | 0.27 | 22.50 | 0.4719 | 0.4287 | |
| 0.6 | 65.32 | 5.12 | −2.87 | 0.4543 | 0.3960 | |
| 0.9 | 61.22 | 5.69 | −5.11 | 0.4525 | 0.3915 | |
| 1.1 | 61.51 | −0.69 | −6.32 | 0.4366 | 0.3999 | |
| Electrodes | λ (nm) | Tox | Tred | ΔT | ΔOD | Qd (mC cm−2) | η (cm2 C−1) | τc (s) | τb (s) |
|---|---|---|---|---|---|---|---|---|---|
| PdCz | 790 | 69.7 | 89.3 | 19.7 | 0.11 | 1.351 | 81.4 | 4.0 | 0.8 |
| P(dCz2-co-dTC1) | 790 | 18.5 | 73.9 | 55.7 | 0.60 | 6.043 | 99.3 | 2.7 | 2.7 |
| P(dCz2-co-dTC2) | 784 | 10.7 | 67.7 | 57.0 | 0.80 | 3.221 | 248.4 | 3.5 | 1.8 |
| P(dCz1-co-dTC2) | 790 | 10.9 | 61.4 | 50.6 | 0.75 | 5.163 | 145.3 | 4.2 | 5.6 |
| PdTC | 790 | 9.1 | 57.1 | 48.0 | 0.80 | 4.851 | 164.9 | 4.3 | 6.0 |
| ECDs | Potential (V) | Photographs | L* | a* | b* | x | y | Diagrams |
|---|---|---|---|---|---|---|---|---|
| PdCz/PProdot-Me2 | 0.0 |  | 86.69 | 0.03 | −0.06 | 0.4476 | 0.4073 |  |
| 0.8 |  | 84.93 | −0.01 | 3.78 | 0.4517 | 0.4113 | ||
| 1.0 |  | 75.69 | 1.54 | −3.55 | 0.4460 | 0.4013 | ||
| 1.2 |  | 67.20 | 0.73 | −14.09 | 0.4290 | 0.3881 | ||
| 1.3 |  | 63.53 | 1.25 | −18.88 | 0.4216 | 0.3796 | ||
| P(dCz2-co-dTC2)/PProdot-Me2 | −0.8 |  | 85.06 | −1.40 | 26.92 | 0.4724 | 0.4342 | 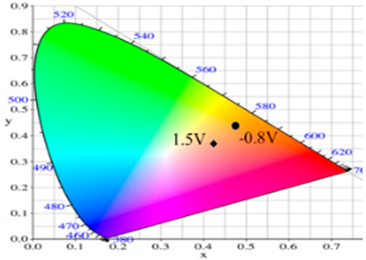 |
| 0.0 |  | 80.27 | −0.28 | 19.04 | 0.4679 | 0.4268 | ||
| 0.8 |  | 61.82 | 2.72 | −8.38 | 0.4410 | 0.3918 | ||
| 1.2 |  | 56.30 | 4.46 | −17.66 | 0.4284 | 0.3737 | ||
| 1.5 |  | 54.18 | 5.26 | −21.30 | 0.4229 | 0.3655 | ||
| PdTC/PProdot-Me2 | −0.8 |  | 75.67 | 2.71 | 13.63 | 0.4686 | 0.4179 |  |
| 0.0 |  | 72.35 | 3.54 | 8.02 | 0.4644 | 0.4112 | ||
| 0.8 |  | 55.56 | 8.38 | −14.34 | 0.4433 | 0.3724 | ||
| 1.3 |  | 50.05 | 9.67 | −22.05 | 0.4307 | 0.3547 | ||
| 1.7 |  | 49.19 | 9.66 | −22.34 | 0.4298 | 0.3535 |
| Devices | λ (nm) | Tox (V a) | Tred (V a) | ΔT | ΔOD | Qd (mC cm−2) | η (cm2 C−1) | τc (s) | τb (s) |
|---|---|---|---|---|---|---|---|---|---|
| PdCz/PProdot-Me2 | 580 | 23.1 (1.5) | 57.5 (−0.8) | 34.4 | 0.396 | 0.781 | 507.0 | 0.2 | 0.2 |
| P(dCz2-co-dTC1)/PProdot-Me2 | 580 | 15.1 (1.5) | 55.2 (−0.8) | 40.1 | 0.563 | 1.001 | 562.4 | 0.3 | 0.2 |
| P(dCz2-co-dTC2)/PProdot-Me2 | 580 | 16.0 (1.5) | 61.8 (−0.8) | 45.8 | 0.587 | 1.110 | 528.8 | 0.2 | 0.3 |
| P(dCz1-co-dTC2)/PProdot-Me2 | 578 | 36.8 (1.5) | 69.0 (−0.8) | 32.2 | 0.273 | 0.622 | 438.9 | 0.9 | 0.2 |
| PdTC/PProdot-Me2 | 578 | 15.6 (1.5) | 46.8 (−0.8) | 31.2 | 0.477 | 1.083 | 440.4 | 0.2 | 0.2 |
| ECD Configuration | ΔTmax (%) | η (cm2 C−1) | Ref. |
|---|---|---|---|
| P(Bmco)/PEDOT | 35 (620 nm) | --- | [33] |
| P(dNcbph)/PEDOT | 19 (550 nm) | --- | [34] |
| P(tnCz1-bTp2)/PProdot-Me2 | 40 (630 nm) | 539 (630 nm) | [35] |
| p(dNcbph-co-bth)/PEDOT | 28.6 (700 nm) | 234 (700 nm) | [36] |
| PtCz/PProDOT-Me2 | 36 (572 nm) | 343.4 (572 nm) | [37] |
| P(BCz-co-ProDOT)/triple-layer PEDOT-PSS | 41 (642 nm) | 417 (642 nm) | [38] |
| P(dCz2-co-dTC2)/PProdot-Me2 | 45.8 (580 nm) | 528.8 (580 nm) | This work |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kuo, C.-W.; Chang, J.-C.; Huang, Y.-T.; Chang, J.-K.; Lee, L.-T.; Wu, T.-Y. Applications of Copolymers Consisting of 2,6-di(9H-carbazol-9-yl)pyridine and 3,6-di(2-thienyl)carbazole Units as Electrodes in Electrochromic Devices. Materials 2019, 12, 1251. https://doi.org/10.3390/ma12081251
Kuo C-W, Chang J-C, Huang Y-T, Chang J-K, Lee L-T, Wu T-Y. Applications of Copolymers Consisting of 2,6-di(9H-carbazol-9-yl)pyridine and 3,6-di(2-thienyl)carbazole Units as Electrodes in Electrochromic Devices. Materials. 2019; 12(8):1251. https://doi.org/10.3390/ma12081251
Chicago/Turabian StyleKuo, Chung-Wen, Jui-Cheng Chang, Yu-Ting Huang, Jeng-Kuei Chang, Li-Ting Lee, and Tzi-Yi Wu. 2019. "Applications of Copolymers Consisting of 2,6-di(9H-carbazol-9-yl)pyridine and 3,6-di(2-thienyl)carbazole Units as Electrodes in Electrochromic Devices" Materials 12, no. 8: 1251. https://doi.org/10.3390/ma12081251





