1. Introduction
In orthopedic surgery, bone defects occur after fractures or in tumor operations. To fill up major bone defects, bone substitutes are often needed, especially in cases when autologous bone grafting is insufficiently available. Tibial head depression fractures, typical fractures of older patients, are common examples of daily clinical practice in which a metaphyseal bone defect regularly remains after fracture reduction [
1]. The availability and application of autologous bone grafts as the only osteogenic, osteoinductive, and osteoconductive material is limited [
2,
3]. Allogenous or xenogenous grafts have reduced osteogenic and osteoinductive properties as well as a reduced mechanical strength due to sterilization [
3,
4]. Thus, currently used alternatives are based on synthetic bone substitutes, such as self-setting calcium phosphate cements (CPCs). Though CPCs possess only osteoconductive properties [
5], they avoid the downsides associated with conventional bone grafts and provide injectability ensuring a proper filling of bone defects. Depending on the end product of the setting reaction, two major CPC types are differentiated. If the pH value during setting is below 4.2, brushite, a metastable calcium orthophosphate is formed, whereas a pH value above 4.2 produces hydroxyapatite, which has a similar composition and crystallinity to the inorganic phase of the mineral bone matrix [
5]. While brushite cements are known to be degradable in the human organism within months by both passive dissolution and acid producing osteoclasts [
6], hydroxyapatite cement (HA) is presumably remodeled similar to human bone, only within years, by solely osteoclastic activity due to the thermodynamic stable character of HA under physiological conditions [
7,
8].
Comparative biomechanical studies and adequate treatment recommendations for the clinical application of the available bone cements are missing. Moreover, no drillable bone substitute which would ensure a complete filling of the defect is currently available on the market [
9]. In a clinical context, drillability of mineral bone cements is defined as the possibility to drill a hole and insert a bone screw into the partially or fully set cement without fracturing the cement implant during processing. Additionally, if an apatite cement is applied, the resorbing and reorganization back to trabecular bone may not take place, thereby affecting long-term stability [
10].
To improve the drillability and biodegradability of bone substitutes, two new approaches have been developed in the last years. CPCs gained drillable characteristics either by modification with fibers, as it was realized in the former CPC Norian
® Drillable, or through the incorporation of reactive monomers, a concept of dual setting cement systems firstly described by Dos Santos et al. in 1999 [
11]. The in situ polymerization of those monomers forming an interpenetrating network of ceramic matrix and hydrogel is known to introduce a pseudoplastic mechanical fracture behavior [
12]. Moreover, biodegradability of cements can be obtained by using struvite-forming magnesium phosphate cements (MPCs), with an assumed degradation within 10–12 months in vivo [
13] as a result of their high solubility (pKs = 12–14) [
14]. Also, MPCs exhibit high initial compressive strength values above 50 MPa [
15,
16,
17,
18,
19].
So far, however, a bone substitute combining drillability, high biomechanical strength, and resorption in vivo is not commercially available. Therefore, this study analyzed two new promising bone substitutes: An in-house-developed dual setting calcium-deficient hydroxyapatite (Ca
9HPO
4(PO
4)
5OH) cement [
20] gaining the clinical desired drillability from an incorporated [
21] poly-2-hydroxyethyl methacrylate (HEMA)-hydrogel and an in-house-developed struvite (NH
4MgPO
4·6H
2O)-forming MPC [
17]. Cements often used in clinical contexts are the brushite cement (CaHPO
4·2H
2O) ChronOS
TM Inject and the apatite cement Graftys
® Quickset. ChronOS
TM inject is known to have a good degradation rate (within 18 months, manufacturer information) without a lack of strength in this time [
6] and the struvite cement has been demonstrated to show quantitative bone remodeling in an large animal model within ten months for both unloaded and load-bearing defects [
22,
23]. Such struvite cements provide a high initial strength of up to 80 MPa under compression [
22], while ChronOS™ inject is mechanically much weaker with a compressive strength of <1 MPa. In contrast, both the in-house-developed hydroxyapatite cement and Graftys
® Quickset are likely only slow-degrading materials due to their low soluble hydroxyapatite matrix. The latter offers a good primary stability (24 MPa after 24 h, manufacturer information) and the HEMA-modified hydroxyapatite cement shows compressive strengths of ~30 MPa after 7 days setting [
20].
The aim of the study was to detect biomechanical differences in stability and handling for clinical use of four injectable bone substitutes in a clinically relevant test set-up. Thus, in addition to a basic biomechanical investigation, this study newly analyzed the interaction of the bone substitutes in a fracture model in tibial head depression fractures. It was hypothesized that the in-house-prepared bone cements would provide an equivalent biomechanical stability as the commercial counterparts combined with additional advantages for the clinical application, such as the ability to be drilled after a short period of setting due to their pseudo-plastic behavior and their high strength.
2. Materials and Methods
2.1. Raw Powder and Cement Preparation of In-House-Developed Bone Cements
α-tricalcium phosphate (α-TCP, Ca3(PO4)2) was synthesized by sintering a 2.15:1 molar ratio of CaHPO4 (J.T. Baker, Griesheim, Germany) and CaCO3 (Merck, Darmstadt, Germany) for 5 h at 1400 °C in a sintering furnace (Oyten Thermotechnic, Oyten, Germany). Farringtonite (Mg3(PO4)2) was synthesized sintering 0.6 mol MgHPO4∙3H2O (Sigma Aldrich, Steinheim, Germany) with 0.3 mol Mg(OH)2 (Sigma Aldrich, Steinheim, Germany) for 5 h at 1050 °C. The sintering cakes of both systems were crushed and sieved <355 µm and milled for 1 h (farringtonite) or 4 h (α-TCP) in a planetary ball mill (PM400, Retsch, Haan, Germany).
In case of the dual setting apatite formulation, α-TCP was mixed with 0.5% ammoniumpersulfate (Sigma Aldrich, Steinheim, Germany). The liquid phase of the cement paste was composed of 50% HEMA (Sigma Aldrich, Steinheim, Germany), 2.5% Na2HPO4 (Merck, Darmstadt, Germany), and 0.25% N,N,N′,N′ tetramethylethylene diamine (Sigma Aldrich, Steinheim, Germany). Both the solid and the liquid phase of the cement paste were homogenously mixed by means of a spatula and glass slab for 30 s at a powder-to-liquid ratio (PLR) of 1.6 g/mL. For the in-house-prepared struvite cement system, the farringtonite raw powder was equally mixed homogeneously for 30 s with an aqueous 3.5 M (NH4)2HPO4 (Sigma Aldrich, Steinheim, Germany) solution at a PLR of 2.0 g/mL.
The commercially available cement compounds, ChronOS
TM Inject and Graftys
® Quickset, form brushite and calcium-deficient hydroxyapatite, respectively, and were purchased from Depuy Synthes (Umkirch, Germany) and Graftys (Aix-en-Provence, France), respectively. They were prepared according to the manufacturers’ instructions. All used cement compositions are summarized in
Table 1.
2.2. X-ray Diffraction and Mercury Porosity Analysis
X-ray diffraction patterns of the in-house-prepared cement raw powders and the set cements were recorded with a Siemens D5005 diffractometer (Siemens, Karlsruhe, Germany) in a 2θ range from 20°–40° with a step size of 0.01°. The qualitative phase composition was evaluated by comparing the diffraction patterns with reference patterns from the PDF-database. Mercury porosity analysis (PASCAL 140/440, Porotec GmbH, Hofheim, Germany) was applied to compare relative and cumulative pore volume, average and median pore diameter, and visualize pore size distribution. Therefore 250–350 mg of each sample was placed in a dilatometer and filled with mercury. At an applied pressure of up to 400 MPa, the pore size distribution in the range of macropores and mesopores (0.01–10 μm) was determined. For evaluation, the software “solid” was used.
2.3. Static Mechanical Testing: Compressive Strength and Screw Pullout Test Setup
The as-prepared cement pastes were filled in cuboid silicone rubber molds with an aspect ratio of 2:1 (12 mm × 6 mm × 6 mm) and the test blocks (at least n = 10 per group) were stored in distilled water at 37 °C for 24 h before testing. Wet samples were tested in axial compression at a crosshead speed of 1 mm/min by means of the universal testing machine Z020 (Zwick, Ulm, Germany). The compressive strength was then calculated by dividing the maximum load at failure by the cross-sectional area of the test blocks.
For testing the screw pullout force, a similar test set-up to former biomechanical studies was used [
24,
25]: Silicone rubber molds were used to prepare cylindrical cement samples of 20 mm in length and 15 mm in diameter. Through a hole in the bottom, cortical screws (25 mm × 3.5 mm, DePuy Synthes, Umkirch, Germany) were put in the molds before cement was filled in, enabling a standardized screw embedding depth of 15 mm. For drilling screws into the samples, we used molds with a central notch at the bottom, serving as a mark on the pre-set cement for the subsequent drilling, tapping, and screwing (
Figure 1a). To ensure the same depth of penetration, a 2.5 mm drill and a 3.5 mm tap and screw were marked 15 mm from the apex. Before testing, the samples (n = 10 per group) were placed for 24 h in distilled water at 37 °C. Every sample was examined by X-ray to detect air bubbles, ensure straight screw placement and equal embedment depth (
Figure 1b). For every bone substitute, one group with embedded screws was tested. In addition, one group with manually placed screws was tested for the drillable dual setting CPC. An axial tensile test was performed at a speed of 1 mm/min through the traverse and tubular holder moving upwards to determine the screw pullout force (
Figure 1c).
2.4. Cyclic Biomechanical Testing within a Tibial Depression Fracture Model
To generate pure depression fractures of the lateral tibial plateau (AO 41-B2.2, Schatzker III), a validated fracture model in synthetic bones (Synbone
® 1110, Synbone, Malans, Switzerland) [
9,
26], was chosen. Consequently, tibiae were cut at mid-diaphysis 20 cm below the tibial plateau and embedded with gypsum at 5° valgus in a custom-made device [
27]. Five predetermined breaking points were set with a 2 mm drill in a 12 mm circle centrally on the lateral plateau (
Figure 2a). Subsequently, the apparatus was mounted on the universal testing machine, the 12 mm indenter was positioned exactly over the breaking points, and an axial load was applied with 500 mm/min to produce a 15 mm-deep pure depression fracture (
Figure 2b,c).
Fractures were reduced indirectly by the often-used clinical technique ARIF (arthroscopically supported reduction and internal fixation) [
9,
26,
28,
29]. Through a metaphyseal cortical window, a K-wire was placed under the depressed articular fracture fragment (
Figure 2d), a tunnel was drilled (
Figure 2e) and a K-wire-guided cannulated ram (
Figure 2f) was then inserted to compact the subchondral spongiosa and thus restore the plateau anatomically to a plane joint surface (
Figure 2g). In groups 1 to 3 the metaphyseal void remaining after reduction was filled with bone substitute only (
Figure 3a,b). Specimens were stored dry at 37 °C for 24 h in an incubator to allow comparison with previous studies [
9,
26,
28]. In contrast, all residual groups were stored with distilled water-soaked gauzes wrapped around the bone at 37 °C for 24 h [
27,
30] (
Table 2). This storage takes the high water demand of the HEMA-hydrogel of the CPC into account and enables post-curing of the ceramic constituent [
20], allowing further comparability. In groups 5 and 6, the fracture was stabilized with a four screw “jail technique” after reduction [
9,
26,
31]. Two 3.5 mm cortical bone screws were inserted by two anterior small incisions and two 6.5 mm cancellous bone screws by lateral incisions, supporting the reduced fragment like a grid (
Figure 2h and
Figure 3d). The remaining metaphyseal void was then filled up retrogradely with bone substitute injected through a cannula (
Figure 3c,
Table 2). This procedure was done backwards for group 7, in which the defect was filled up first with drillable CPC and then drilled after 10 min of pre-setting (
Table 2). To assess the filling of the metaphyseal defect and the position of the screw osteosynthesis, specimens were examined by X-rays before storage (
Figure 3b,d).
For testing, the specimens were fixed in the universal testing machine Z020 (Zwick Roell, Ulm, Germany), the indenter was positioned exactly over the reduced fracture fragment, and an axial load was applied (
Figure 3e). The testing protocol included a cyclic loading phase followed by maximal loading and has been validated in previous studies [
9,
26,
28]. Ten settling cycles from 20 to 125 N were followed by 3000 measuring cycles from 20 to 250 N with 25 mm/min [
27]. The force levels in the protocol were chosen in compliance with the postoperative partial weight bearing conditions of around 20 kg of the operated limb [
29] and the number of cycles (3000) has been found to be sufficient for detecting differences in displacement [
9,
26,
28]. After the last measuring cycle, axial load was steadily increased with a constant speed of 100 mm/min until failure (
Figure 4).
The parameters of interest were the displacement of the reduced fracture fragment in the cyclic loading phase, the maximum load, and the stiffness of the load-to-failure tests.
Compressive strength and maximum pullout force (
Section 2.3), as well as the displacement and maximum load in the fracture model biomechanical test set-up (
Section 2.4), were recorded by a 20 kN load cell at the traverse of the universal material testing machine, Zwick Roell Z020, Ulm, Germany. Additionally, the pullout stiffness was calculated as the slope during the elastic deformation curve in the load-displacement diagrams, either in the screw pullout tests (
Section 2.3) or in the load-to-failure tests after the cyclic loading phase (
Section 2.4).
2.5. Stereomicroscopic and Scanning Electron Microscopy Images
To analyze the interdigitation of the bone cement into the adjacent spongiosa, specimens of the dry and humid CPC and MPC were prepared and cut sagittally at the lateral tibial plateau through the filled-up bone defect. Subsequently, images of the exposed surface were taken with a stereomicroscope (Carl Zeiss, Oberkochen, Germany). The aforementioned specimens were then further prepared with a diamond saw and dried for 6 days in a desiccator (Pfeiffer Vacuum, Aßlar, Germany). After sputter coating with a 4 nm layer of platinum (Leica EM ACE 600, Leica Microsystems, Wetzlar, Germany) the interface between bone cement and spongiosa was analyzed by scanning electron microscopy (Crossbeam 340, Carl Zeiss, Oberkochen, Germany) with an acceleration voltage of 3.0 kV via detection of secondary electrons.
2.6. Statistical Analysis
The number of specimens for the experimental groups (n = 9) was estimated by power analysis using a significance level of 5% and a power of 80%. The calculation of effect size d was based on the results of a comparable pilot study. Descriptive statistics (means and standard deviations) for the outcome variables were initially calculated for each of the experimental groups (expert’s report by the statistical institute of the mathematical department, University of Wuerzburg, Germany).
Normal distribution was confirmed and significant differences were calculated by one-way ANOVA. Non-normally distributed data were analyzed by a Kruskal–Wallis test, followed by a Mann–Whitney U-test to find significant differences between groups. The statistical analyses were conducted using IBM® SPSS® Statistics 21, with the level of significance set at p < 0.05.
4. Discussion
Difficulties with conventional bone cements, like a lack of drillability, uncertain resorbability [
10], and mechanical weakness compared to human bone [
32], emphasize the need for new alternative cements for clinical application. Therefore, two new concepts, i.e., a drillable apatite cement and a high strength, supposedly resorbable MPC, were mechanically and biomechanically evaluated against the clinically used formulations Graftys
® Quickset and ChronOS
TM Inject. Up until the date of the tests, both cements were often used in our clinic, although the brushite cement is actually not available anymore.
Throughout static compressive strength testing, the in-house-prepared magnesium phosphate revealed the significant highest compressive strength compared to all other bone substitutes (
Figure 7). Our findings correspond well with previously published studies, demonstrating early strength acquisition and high strength values (above 60 MPa) for MPCs [
15,
16,
17]. The low porosity of the MPC (
Figure 6a) might also play a role, as porosity and mechanical strength are inversely and exponentially linked [
5]. In contrast to the results of this study, Christel et al. [
20] demonstrated a greater than 4-fold higher compressive strength of 30 MPa for a similarly composed apatite cement, which is likely due to the differences in the PLR. Whereas Christel et al. [
20] used a PLR of 3.0 g/mL, the lower PLR of 1.6 g/mL used here should ensure injectability of the cement paste. It is well described in literature that a higher PLR results in a higher compressive strength [
5]. Moreover, the drillable apatite cement showed a pseudoplastic mechanical behavior, a phenomenon which is also described by the aforementioned publication, as the addition of HEMA resulted in a decrease of the bending modulus and a simultaneous increase of the work of fracture [
20]. Both parameters enabled a similar cement formulation to be drilled after short pre-setting, which was shown here for the first time. The compressive strength of Graftys
® Quickset is well in accordance with the manufacturer’s data of 24 MPa and of a micro-, meso-, and macroporous structure, which could be confirmed by the mercury porosity analysis (
Figure 6d) and SEM images (
Figure 11d). ChronOS
TM Inject demonstrated a very low mechanical stability (
Figure 7 and
Figure 8), which is due to the fact that this cement was not cohesive when stored in distilled water. This observation has previously been reported by Luo et al. [
33]. In addition, the porosity analysis showed the highest cumulative porosity for the brushite cement (
Figure 6c).
Analogously, during screw pullout testing, the same tendencies were detected between the different bone substitutes. Further, it seemed that manually drilling and inserting the screw significantly weakened the screw–cement interface in comparison to that of embedded samples (
Figure 8a). Chapman et al. [
34] demonstrated that tapping a soft polyurethane foam increased the drill hole diameter by 27% and significantly reduced screw pullout strength. As the drillable apatite cement had a soft consistency after 10 min of pre-setting, the findings are in strong agreement with other authors, stating that tapping a soft material reduces pullout strength by setting a larger defect than the original drill hole [
35,
36]. This sounds especially conclusive regarding corresponding pullout stiffnesses of the dual setting bone substitutes. Those were comparable to specimens with ChronOS
TM Inject, which presumably softened in the aqueous environment due to its poor cohesiveness. Interestingly, samples with screw–cement combinations from Graftys
® Quickset revealed a three times higher stiffness than magnesium phosphate (
Figure 8b), which may be explained by the fact that cements can behave different under compressive and tensile tests and, as a consequence, Graftys
® Quickset did not show stiffness values as anticipated from the tendencies of the compressive strength tests.
With respect to the settling and measuring cycles during the dynamical tests with bone substitute-treated tibial head depression fractures, the combination with MPC exhibited the significant lowest displacement in the groups without screws (
Figure 9a). This might be explained by taking into consideration that complete filling of the proximate cancellous bone cavities was verified via stereomicroscopy (
Figure 10) as well as via SEM (
Figure 11a) and that the tested cement showed high primary stability in the pure material tests as well. Results proposed by Jordan et al. [
28] support this explanation by finding a similar connection of significant lower displacement and higher stiffness with a better integration in the nearby synthetic spongiosa. The in-house-prepared drillable CPC revealed a high displacement when used as a bone substitute alone (
Figure 9a), which was likely due to the aforementioned pseudoplastic characteristics. Such cement formulations enable the drilling of the screws after injection of the bone cement in order to optimize the filling precision of the paste also inside irregularly shaped defects. Therefore, the displacement of the drillable CPC could be significantly reduced in combination with the jail technique (
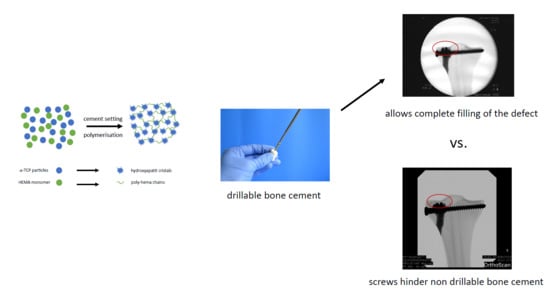
Figure 9a). The issue with incomplete filling of non-drillable formulations could exemplarily be illustrated in the case of Graftys
® Quickset, where radiographs taken after fracture stabilization with screws disclosed that, in four of nine specimens, the screws worked detrimentally (data not shown). They hindered the complete filling of the defect to the subchondral area, which might account for the corresponding large displacement and high standard deviation (
Figure 9a). Equally, Hoelscher-Doht et al. [
9] previously demonstrated that using the jail technique with screw placement after replenishment resulted in a significant lower displacement and higher stiffness of the fixed tibial plateau depression fractures, as if the procedure was done in reverse. Overall, the displacement results of this study are in the range of the displacements measured in other studies for the same fracture type [
9,
26,
28].
It is a consensus in the literature that there is a correlation between a remaining step after fracture reduction and post-traumatic arthritis [
37,
38]. Brown et al. [
39] showed in an animal study that a fracture step in the cartilage of more than 1.5 mm leads to significantly higher stress than under physiological conditions. The obtained displacement of the reduced tibial fractures filled up with magnesium phosphate lies close to this value (
Figure 9a). Honkonen et al. [
40] demonstrated that the functional and clinical outcome was significantly deteriorated if a step-off of more than 3 mm was the case in tibial plateau fractures. What can be positively mentioned is that the displacements of all bone substitutes in this study were below 3 mm (
Figure 9a).
As anticipated from the hygroscopic nature of the in-house-prepared drillable CPC, the storage conditions of the treated depression fracture models influenced the mechanical outcome such that samples with this cement exhibited a significantly higher load-bearing capacity when stored under humid conditions (
Figure 9c). As already described, incorporation of the HEMA-hydrogel in the CPC changes its characteristics from brittle to pseudoplastic, going along with a decrease of the bending modulus and an increase of the work of fracture when stored in water. This theory might be confirmed by corresponding stereomicroscopic and SEM images illustrating a swelling of the whole cement (
Figure 10a,b and
Figure 11b,c). This presumably leads to a better interdigitation with the spongiosa and results in a higher maximum load (
Figure 9c). Furthermore, such storage conditions allow for an additional post-hardening of the cement, which is not fully set even after 24 h, as demonstrated by X-ray diffraction analysis (
Figure 5D), which can explain the higher load due to a higher degree of cement conversion.
All groups with an additional osteosynthesis provided a significantly higher maximum load compared to the groups in which only cement was used (
Figure 9c). This again confirms the results of previous studies that display the mandatory nature of osteosynthesis for the maximum load-bearing capacity of the fixed fracture [
26,
28]. In line with the former results, tibial fracture models treated with MPC showed the significant highest stiffness both with and without screw osteosynthesis. Those findings might be explained with the high intrinsic mechanical performance of the cement in the pure material tests and the seamless interdigitation to the spongiosa (
Figure 10c and
Figure 11a). Accordingly, it can be hypothesized that under increasing axial loading the specimens with MPC immediately resisted the loading forces, whereas in groups with a poorer interdigitation the bone substitute was shifted to a certain degree out of the drill channel, resulting in the lower stiffness of other groups. This theory is supported by a former study, in which the whole bone substitute Norian Drillable was pressed out of the drill channel under maximal loading [
26]. Additionally, osteosynthesis significantly increased the stiffness of every group (
Figure 9d); it can be concluded, as already done from the maximum load values, that a combination of bone substitute and osteosynthesis should always be performed.
Beyond the in vitro tests in this study, stress distribution of fractures and their stabilization methods can be analyzed by three-dimensional virtual models like the finite element analysis or Von Mises analysis [
41,
42,
43]. Especially in dental surgery, computer-based simulation of prostheses and dental implants provide valuable information to guide the surgeon toward which implant position and size to choose [
41,
43]. In addition, those models can provide information about different mechanical properties of diverse materials of dental implants [
41] and could be an interesting addition in a further study to the in vitro tests of the bone substitutes investigated in this study.
Another crucial parameter in clinical scenarios like the treatment of tibial depression fractures is the viscosity of the applied cement system, as it might affect the interface between bone substitute and the adjacent spongiosa as well as the complete and precise filling of the defect. Thus, like the MPC demonstrated (
Figure 7,
Figure 8 and
Figure 9), there might be a correlation between pure material testing and testing in the bone compound when the viscosity of the cement is appropriate. Concerning the limitations of this study, blocks of bone substitutes were only tested under uniaxial compression, despite in vivo bone substitutes being additionally exposed to more complex forces which consist of tensile, bending, and torsional components. Further, it is assumed that a fracture model with synthetic bones is limited in simulating physiological conditions, as no efforts were taken to consider the influence of soft-tissue and ingrowing bone into the implanted bone cements. Also, a storage with water-soaked gauzes around the Synbones
® may not correctly reflect in vivo humidity conditions. Bone cement in vivo is surrounded by a moist or aqueous environment immediately after injection, whereas in Synbones
® the inner surface of the drill channel is dry and contact with water may be limited to the cranial and caudal end of the cement body.
The biomechanical test set-up is similar to former studies in literature with regard to loading level, number of cycles, separating load-to-failure and cyclic testing, and concentrating on the main axial forces on the tibial plateau [
28,
44,
45]. In contrast to separated load-to-failure and cyclic testing, McDonald et al. [
46] designed a testing protocol with continuously increasing loading levels over a higher number of cycles overall. Compared with the aforementioned publication, the main interest in this study was more on the secondary displacement of the fracture fragment under loading approximated to the typical clinical postoperative loading conditions. By loading the stabilized fracture fragment directly with an indenter and by creating a resulting contact stress slightly above the values during gait, a more rigorous test set-up was performed with the focus on the lateral tibial plateau and the depressed articular fracture fragment.