Bentonites Modified with Phosphomolybdic Heteropolyacid (HPMo) for Biowaste to Biofuel Production
Abstract
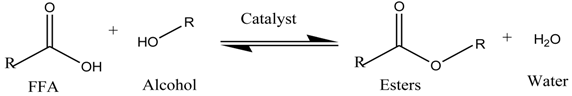
:1. Introduction
2. Materials and Methods
2.1. Raw Material and Chemicals
2.2. Preparation of the Supports and Impregnation with HPMo
Washing of Bentonite Impregnated with HPMo
2.3. Characterization
2.4. Reaction Procedure
2.5. Leaching Tests
3. Results
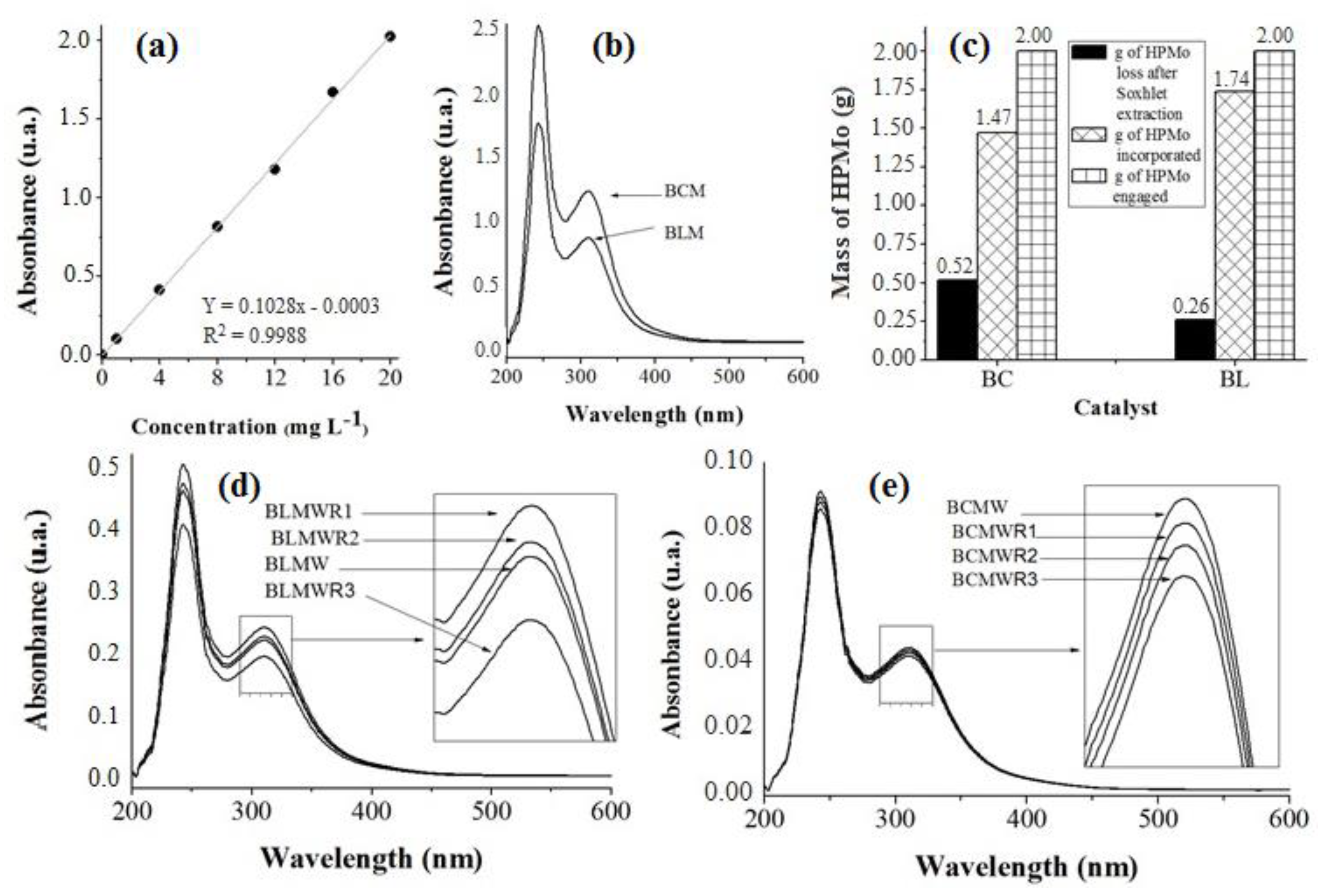
3.1. Chemical Composition of Samples by EDS and Heteropolyacids Immobilization inside the Matrix
3.2. Heteropolyacids Immobilization on BC and BL
3.3. XRD Analysis
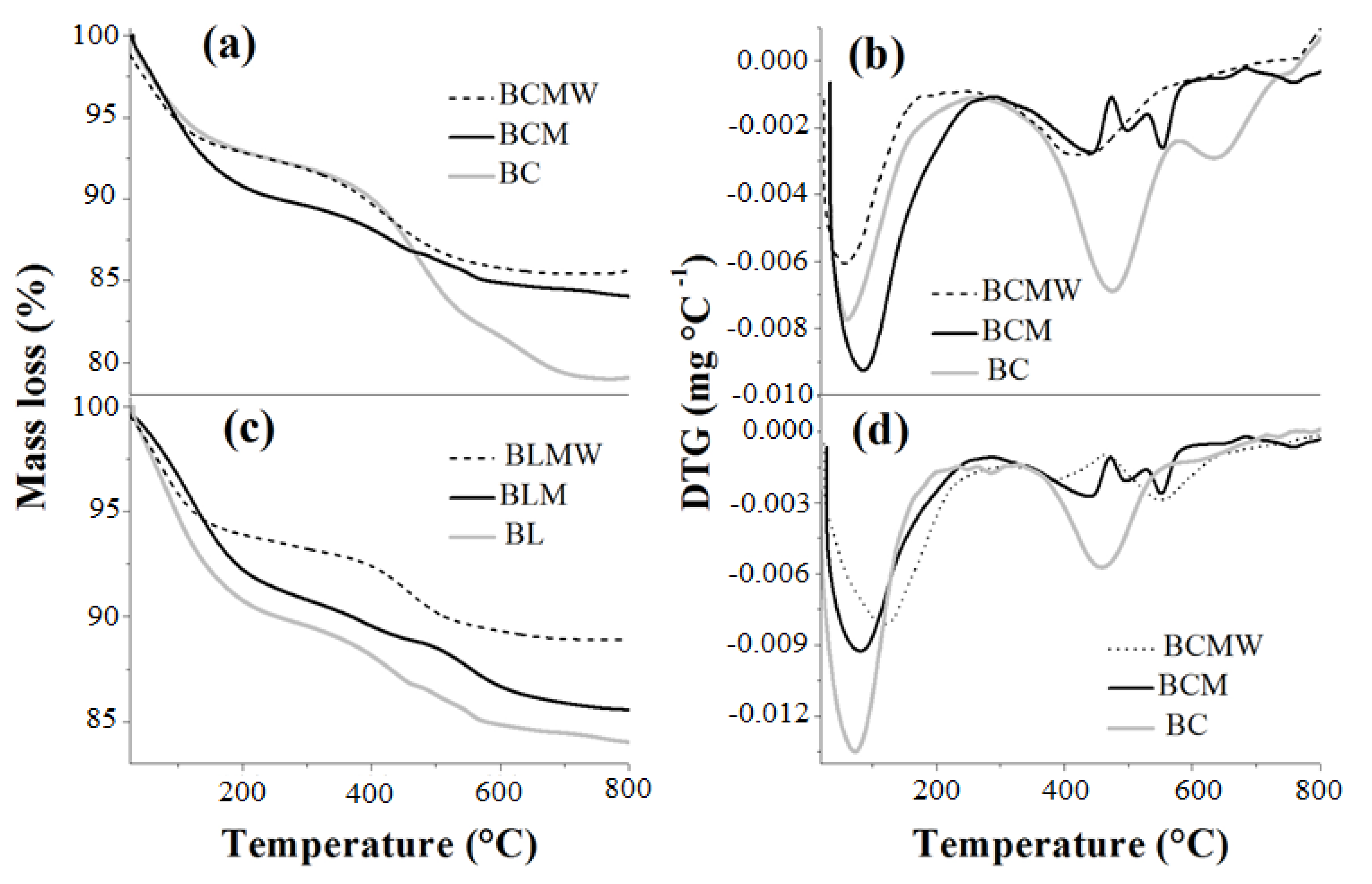
3.4. Thermogravimetric Analysis (TGA/DTG)
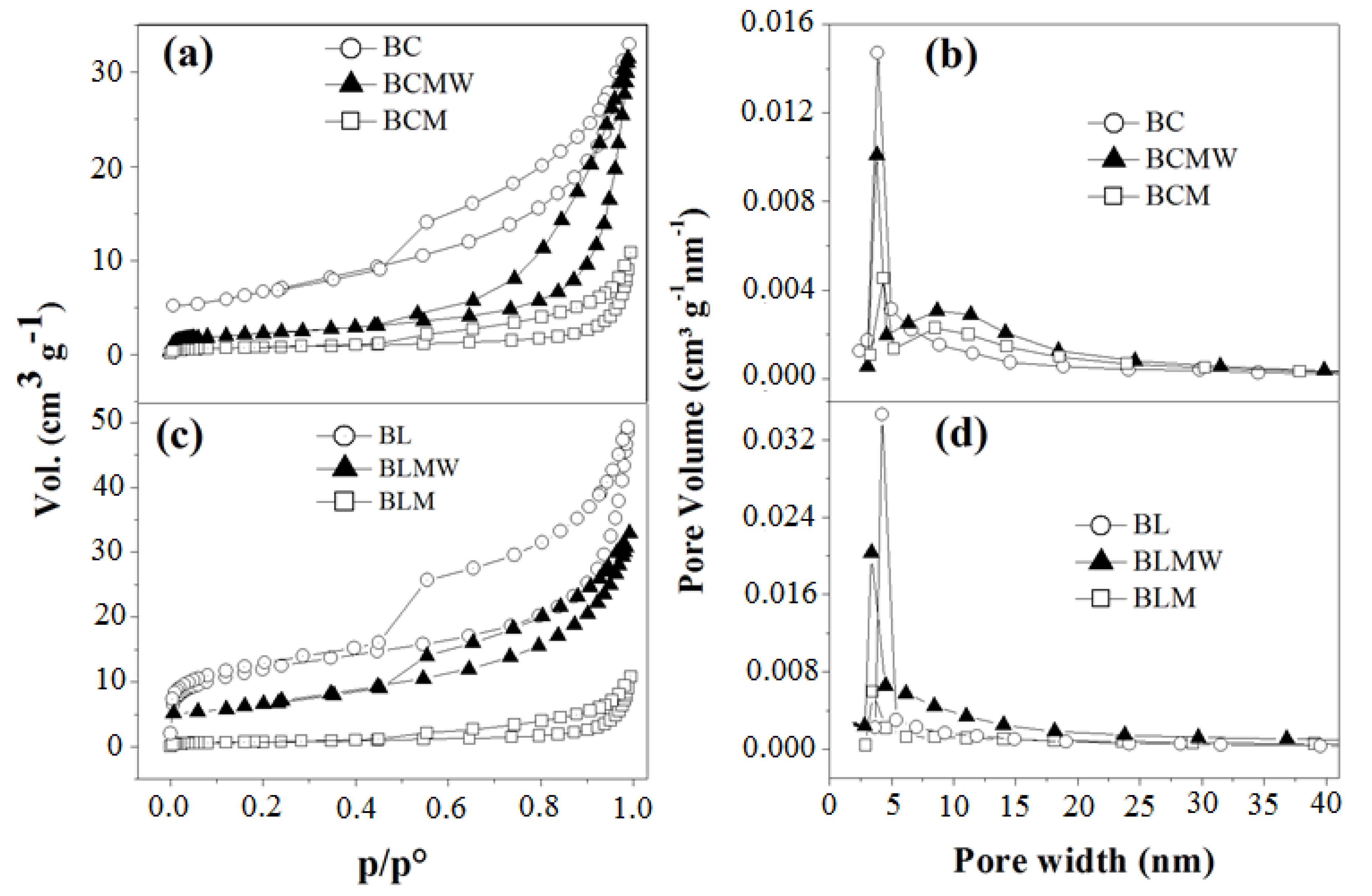
3.5. Textural Properties
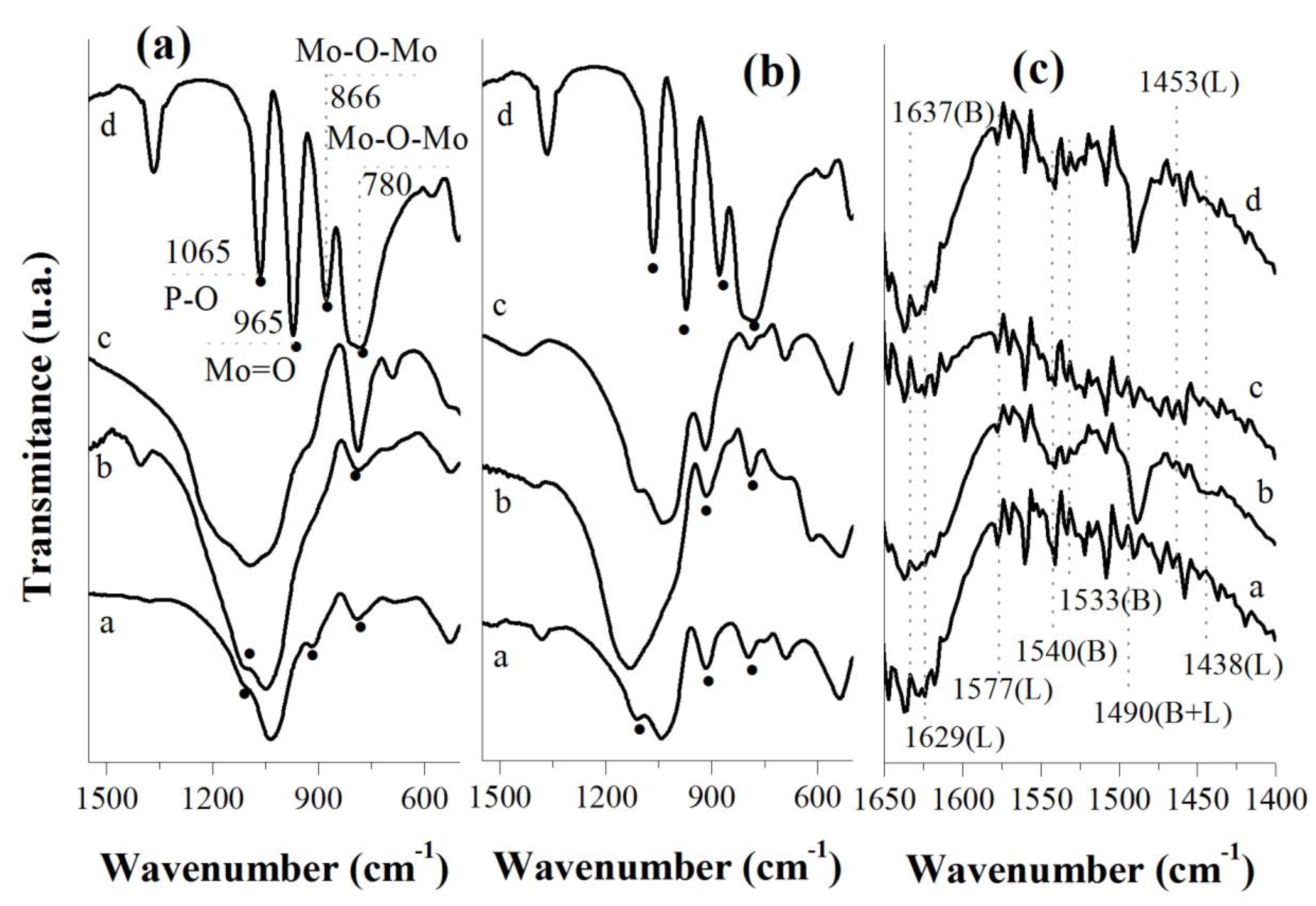
3.6. FTIR Spectroscopy Analysis
3.7. Acidity of the Modified Materials
3.8. Scanning Electron Microscopy (SEM) Results
3.9. Catalytic Activity Results
3.9.1. Influence of Reaction Temperature
3.9.2. Influence of Catalyst Amount
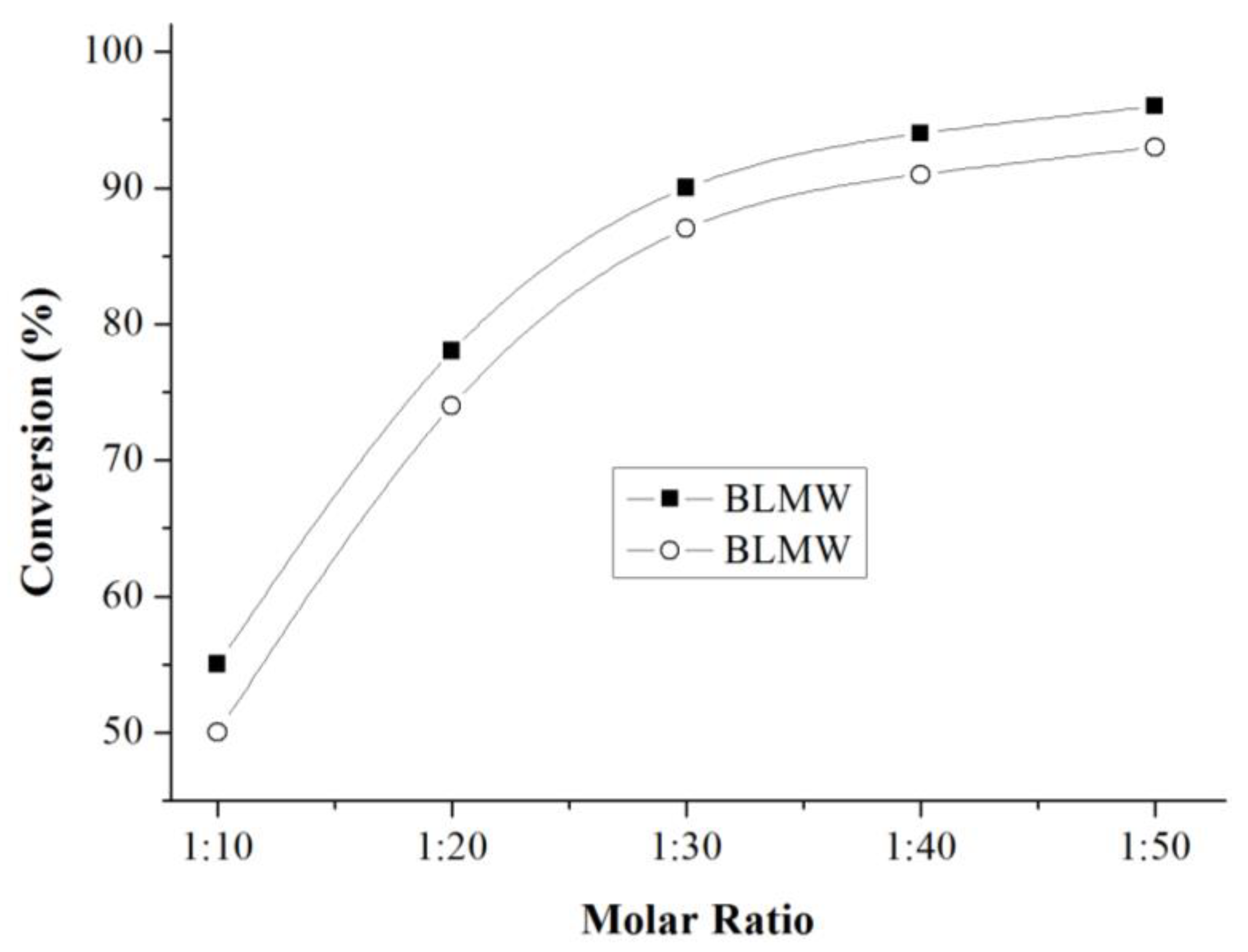
3.9.3. Effect of Molar Ratio
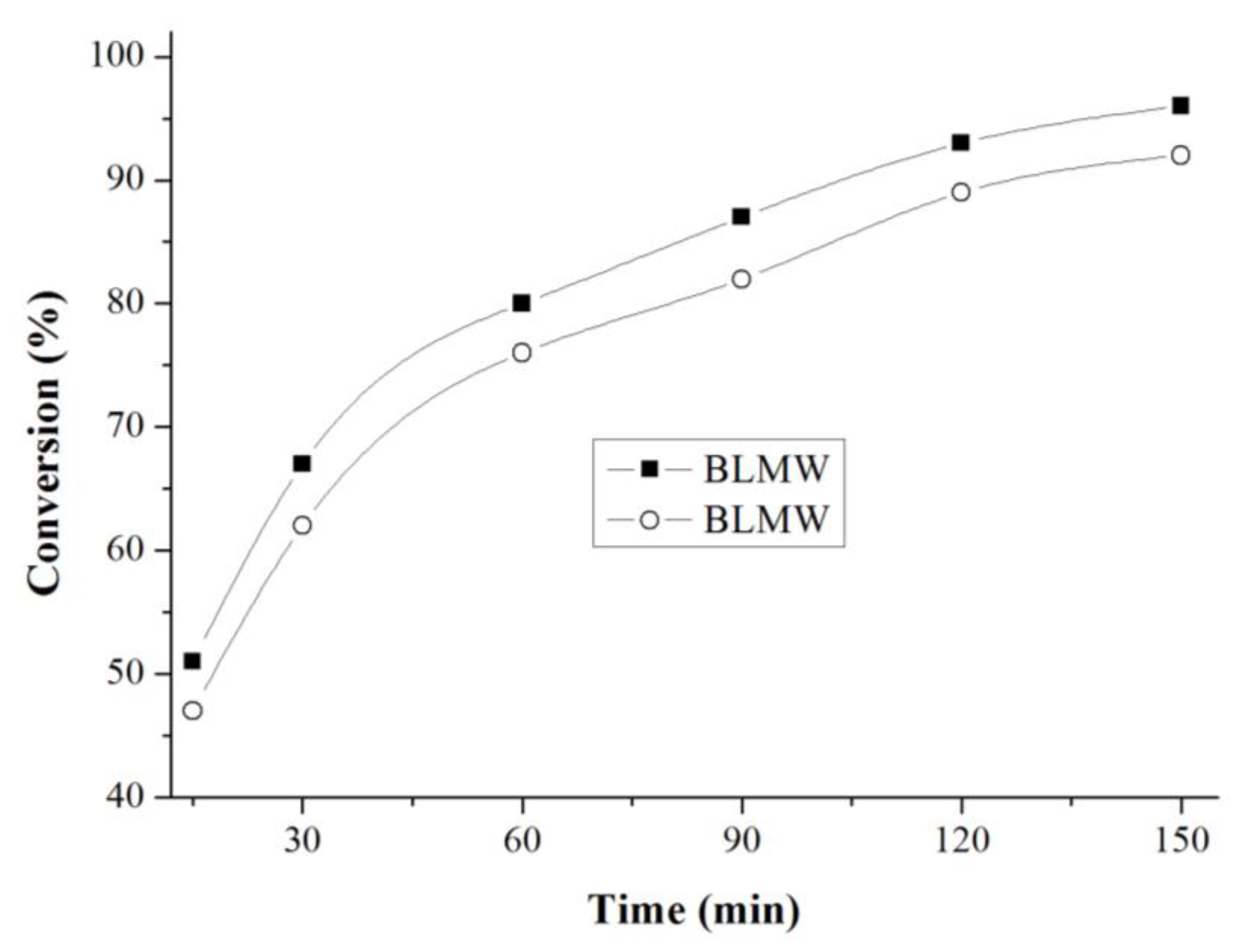
3.9.4. Effect of Reaction Time
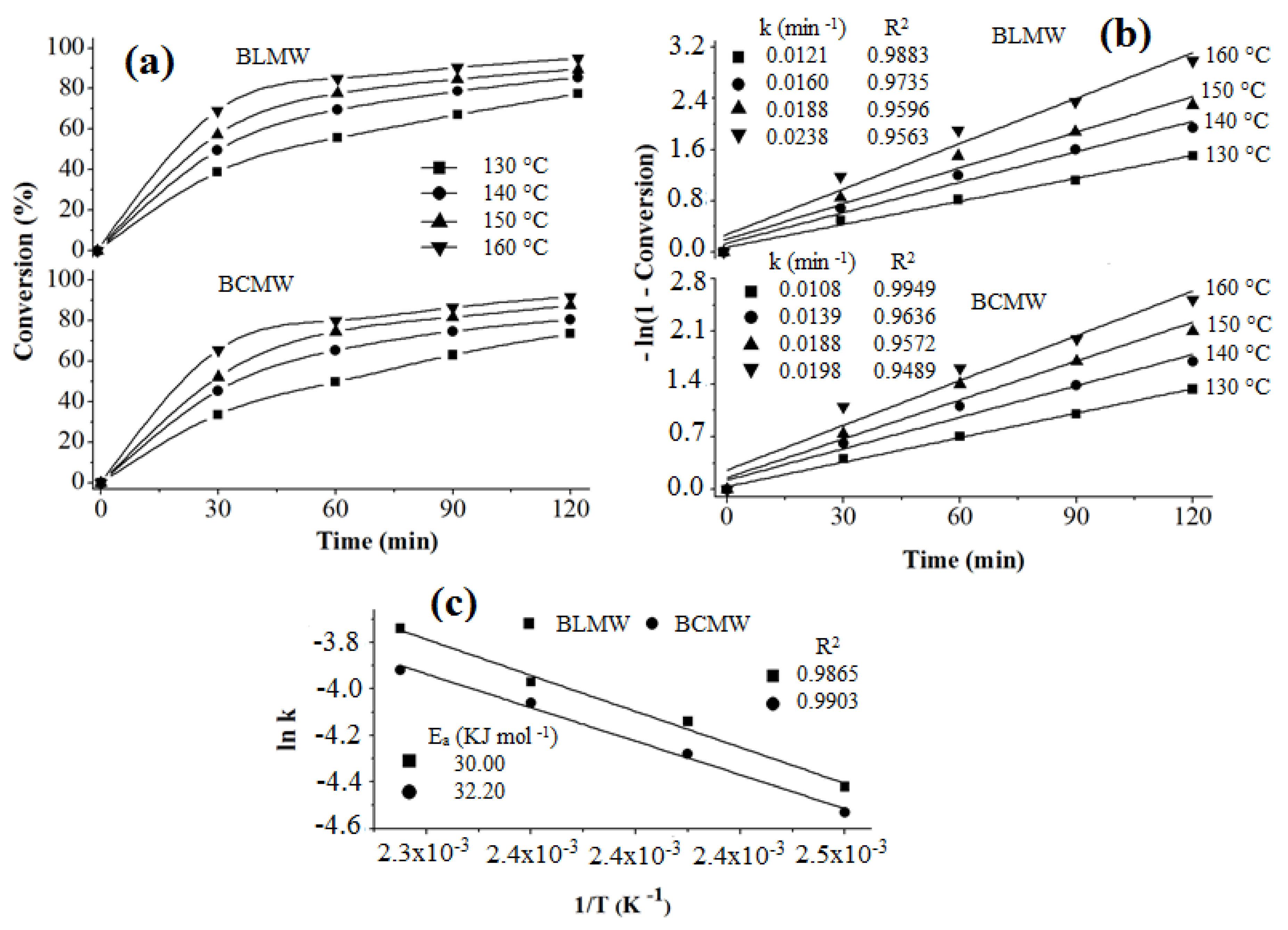
3.9.5. Reaction Kinetics
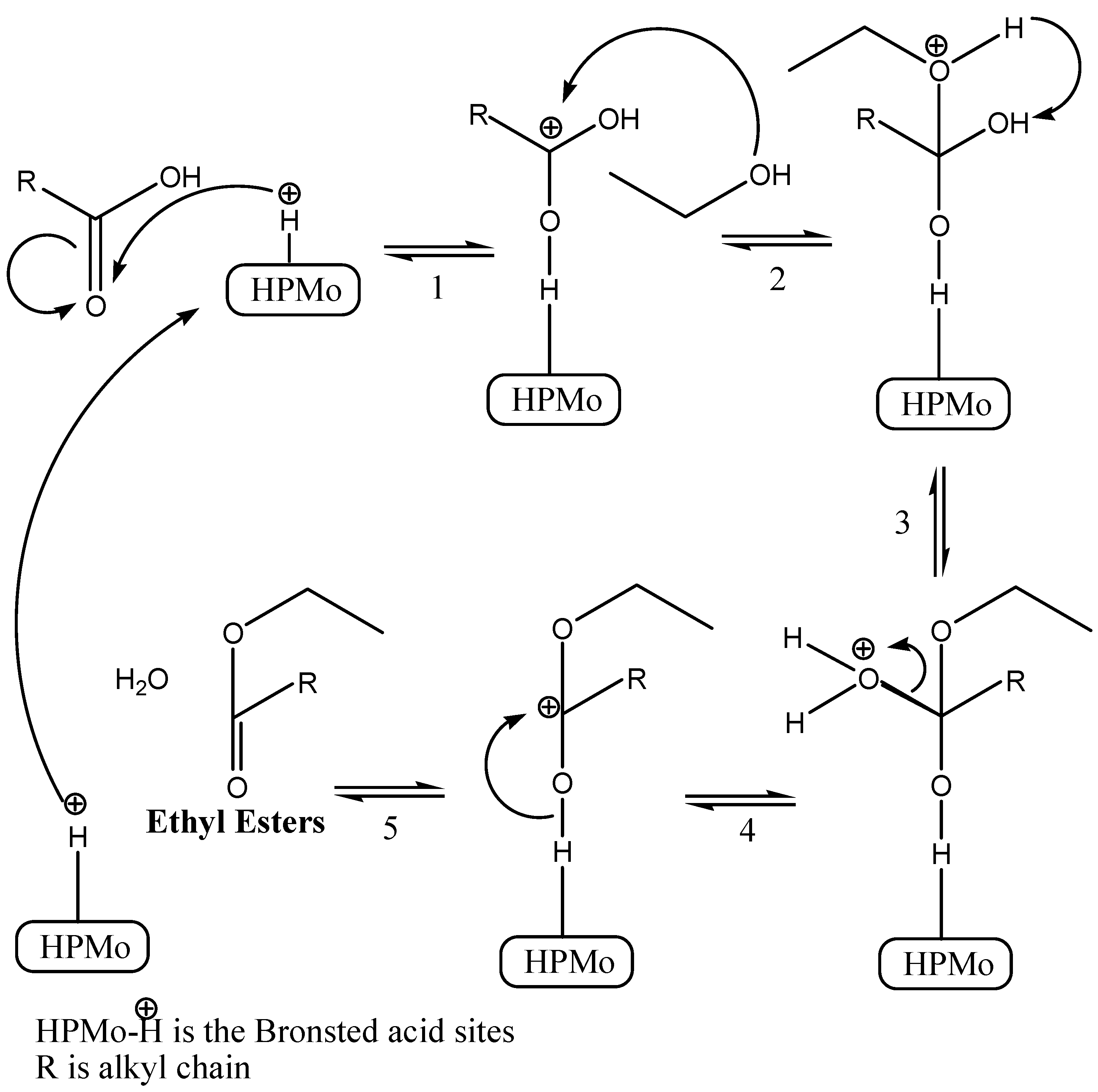
3.9.6. Proposed Mechanism for the Esterification of DDPO
3.9.7. Reuse of Catalysts
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Jeenpadiphat, S.; Tungasmita, D.N. Esterification of oleic acid and high acid content palm oil over an acid-activated bentonite catalyst. Appl. Clay Sci. 2014, 87, 272–277. [Google Scholar] [CrossRef]
- Paladino, O.; Neviani, M. A closed loop biowaste to biofuel integrated process fed with waste frying oil, organic waste and algal biomass: Feasibility at pilot scale. Renew. Energy 2018, 124, 61–74. [Google Scholar] [CrossRef]
- Demirbas, M.F.; Balat, M.; Balat, H. Biowastes-to-biofuels. Energy Convers. Manag. 2011, 52, 1815–1828. [Google Scholar] [CrossRef]
- Alamgholiloo, H.; Zhang, S.; Ahadi, A.; Rostamnia, S.; Banaei, R.; Li, Z.; Liu, X.; Shokouhimehr, M. Synthesis of bimetallic 4-PySI-Pd@Cu ( BDC) via open metal site Cu-MOF: Effect of metal and support of Pd@Cu-MOFs in H2 generation from formic acid. Mol. Catal. 2019, 467, 30–37. [Google Scholar] [CrossRef]
- Luna, F.M.T.; Cecilia, J.A.; Saboya, R.M.A.; Barrera, D.; Sapag, K.; Rodríguez-Castellón, E.; Cavalcante, C.L., Jr. Natural and Modified Montmorillonite Clays as Catalysts for Synthesis of Biolubricants. Materials (Basel) 2018, 11, 1764. [Google Scholar] [CrossRef] [PubMed]
- Carmo, A.C.; de Souza, L.K.C.; da Costa, C.E.F.; Longo, E.; Zamian, J.R.; da Rocha Filho, G.N. Production of biodiesel by esterification of palmitic acid over mesoporous aluminosilicate Al-MCM-41. Fuel 2009, 88, 461–468. [Google Scholar] [CrossRef]
- Nascimento, L.A.S.; Tito, L.M.Z.; Angélica, R.S.; Costa, C.E.F.; Zamian, J.R.; Rocha Filho, G.N. Esterification of oleic acid over solid acid catalysts prepared from Amazon flint kaolin. Appl. Catal. B Environ. 2011, 101, 495–503. [Google Scholar] [CrossRef] [Green Version]
- Oliveira, A.D.N.; da Silva Costa, L.R.; Pires, L.H.D.O.; Nascimento, L.A.S.; Angélica, R.S.; Da Costa, C.E.F.; Zamian, J.R.; Da Rocha Filho, G.N. Microwave-assisted preparation of a new esterification catalyst from wasted flint kaolin. Fuel 2013, 103, 626–631. [Google Scholar] [CrossRef] [Green Version]
- Pires, L.H.O.; de Oliveira, A.N.; Junior, O.V.M.; Angélica, R.S.; da Costa, C.E.F.; Zamian, J.R.; do Nascimento, L.A.S.; Filho, G.N.R. Esterification of a waste produced from the palm oil industry over 12-tungstophosforic acid supported on kaolin waste and mesoporous materials. Appl. Catal. B Environ. 2014, 160–161, 122–128. [Google Scholar] [CrossRef]
- Kim, A.; Rafiaei, S.M.; Abolhosseini, S.; Shokouhimehr, M. Palladium Nanocatalysts Confined in Mesoporous Silica for Heterogeneous Reduction of Nitroaromatics. Energy Environ. Focus 2015, 4, 18–23. [Google Scholar] [CrossRef]
- Atchadapiban, K.; Praserthdam, P.; Tungasmita, D.N.; Tangku, C.; Anutrasakda, W. Effect of Surface Modifications of SBA-15 with Aminosilanes and 12-Tungstophosphoric Acid on Catalytic Properties in Environmentally Friendly Esterification of Glycerol with Oleic Acid to Produce Monoolein. Catalysts 2018, 8, 360. [Google Scholar] [CrossRef]
- Feng, N.; Zheng, A.; Huang, S.J.; Zhang, H.; Yu, N.; Yang, C.Y.; Liu, S.B.; Deng, F. Combined solid-state NMR and theoretical calculation studies of Brønsted acid properties in anhydrous 12-molybdophosphoric acid. J. Phys. Chem. C 2010, 114, 15464–15472. [Google Scholar] [CrossRef]
- Pacula, A.; Pamin, K.; Krysciak-Czerwenka, J.; Olejniczak, Z.; Gil, B.; Bielanska, E.; Dula, R.; Serwicka, E.M.; Drelinkiewicz, A. Physicochemical and catalytic properties of hybrid catalysts derived from 12-molybdophosphoric acid and montmorillonites. Appl. Catal. A Gen. 2015, 498, 192–204. [Google Scholar] [CrossRef]
- Conceição, L.R.V.; Carneiro, L.M.; Giordani, D.S.; de Castro, H.F. Synthesis of biodiesel from macaw palm oil using mesoporous solid catalyst comprising 12-molybdophosphoric acid and niobia. Renew. Energy 2017, 113, 119–128. [Google Scholar] [CrossRef]
- Wimonrat, T. Supported cesiun polyoxotungstates as catalysts for the esterification of palm fatty acid distillate. Mendeleev Commun. 2013, 23, 46–48. [Google Scholar] [CrossRef]
- Liu, R.; Xia, X.; Niu, X.; Zhang, G.; Lu, Y.; Jiang, R.; He, S. 12-Phosphotungstic acid immobilized on activated-bentonite as an efficient heterogeneous catalyst for the hydroxyalkylation of phenol. Appl. Clay Sci. 2015, 105–106, 71–77. [Google Scholar] [CrossRef]
- Timofeeva, M.N. Acid catalysis by heteropoly acids. Appl. Catal. A Gen. 2003, 256, 19–35. [Google Scholar] [CrossRef]
- Patel, A.; Brahmkhatri, V. Kinetic study of oleic acid esterification over 12-tungstophosphoric acid catalyst anchored to different mesoporous silica supports. Fuel Process. Technol. 2013, 113, 141–149. [Google Scholar] [CrossRef]
- Méndez, F.J.; Llanos, A.; Echeverría, M.; Jáuregui, R.; Villasana, Y.; Díaz, Y.; Liendo-Polanco, G.; Ramos-García, M.A.; Zoltan, T.; Brito, J.L. Mesoporous catalysts based on Keggin-type heteropolyacids supported on MCM-41 and their application in thiophene hydrodesulfurization. Fuel 2013, 110, 249–258. [Google Scholar] [CrossRef]
- Lacerda Júnior, O.S.; Cavalcanti, R.M.; De Matos, T.M.; Angélica, R.S.; Da Rocha Filho, G.N.; Barros, I.D.C.L. Esterification of oleic acid using 12-tungstophosphoric supported in flint kaolin of the Amazonia. Fuel 2013, 108, 604–611. [Google Scholar] [CrossRef] [Green Version]
- Oliveira, C.F.; Dezaneti, L.M.; Garcia, F.A.C.; de Macedo, J.L.; Dias, J.A.; Dias, S.C.L.; Alvim, K.S.P. Esterification of oleic acid with ethanol by 12-tungstophosphoric acid supported on zirconia. Appl. Catal. A Gen. 2010, 372, 153–161. [Google Scholar] [CrossRef]
- Damyanova, S.; Gomez, L.M.; Bañares, M.A.; Fierro, J.L.G. Thermal behavior of 12-molybdophosphoric acid supported on zirconium-loaded silica. Chem. Mater. 2000, 12, 501–510. [Google Scholar] [CrossRef]
- Kuzminska, M.; Kovalchuk, T.V.; Backov, R.; Gaigneaux, E.M. Immobilizing heteropolyacids on zirconia-modified silica As catalysts for oleochemistry transesterification and esterification reactions. J. Catal. 2014, 320, 1–8. [Google Scholar] [CrossRef]
- Dharne, S.; Bokade, V.V. Esterification of levulinic acid to n-butyl levulinate over heteropolyacid supported on acid-treated clay. J. Nat. Gas Chem. 2011, 20, 18–24. [Google Scholar] [CrossRef]
- Pacuła, A.; Pamin, K.; Zieba, A.; Kryściak-Czerwenka, J.; Olejniczak, Z.; Serwicka, E.M.; Drelinkiewicz, A. Physicochemical and catalytic properties of montmorillonites modified with 12-tungstophosphoric acid. Appl. Clay Sci. 2014, 95, 220–231. [Google Scholar] [CrossRef]
- Wang, X.Y.; Dong, Q.; Meng, Q.L.; Yang, J.Y.; Feng, W.; Han, X.K. Visible-light photochromic nanocomposite thin films based on polyvinylpyrrolidone and polyoxometalates supported on clay minerals. Appl. Surf. Sci. 2014, 316, 637–642. [Google Scholar] [CrossRef]
- Zatta, L.; Nepel, A.; Barison, A.; Wypych, F. Modified montmorillonite as a heterogeneous catalyst in ( m ) ethyl esterification reaction of lauric acid. Quim. Nov. 2012, 35, 1711–1718. [Google Scholar] [CrossRef]
- Zatta, L.; Ramos, L.P.; Wypych, F. Acid-activated montmorillonites as heterogeneous catalysts for the esterification of lauric acid acid with methanol. Appl. Clay Sci. 2013, 80–81, 236–244. [Google Scholar] [CrossRef]
- Pacuła, A.; Bielańska, E.; Gaweł, A.; Bahranowski, K.; Serwicka, E.M. Textural effects in powdered montmorillonite induced by freeze-drying and ultrasound pretreatment. Appl. Clay Sci. 2006, 32, 64–72. [Google Scholar] [CrossRef]
- Motokura, K.; Nakagiri, N.; Mizugaki, T.; Ebitani, K.; Kaneda, K. Nucleophilic substitution reactions of alcohols with use of montmorillonite catalysts as solid Brønsted acids. J. Org. Chem. 2007, 72, 6006–6015. [Google Scholar] [CrossRef]
- Rezende, M.J.C.; Pinto, A.C. Esteri fi cation of fatty acids using acid-activated Brazilian smectite natural clay as a catalyst. Renew. Energy 2016, 92, 171–177. [Google Scholar] [CrossRef]
- Chaari, A.; Neji, S.B.; Frikha, M.H. Fatty Acid Esterification with Polyols over Acidic Montmorillonite. J. Oleo Sci. 2017, 66, 455–461. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, S.Z.; Wu, P.X. Characterization of sodium dodecyl sulfate modified iron pillared montmorillonite and its application for the removal of aqueous Cu(II) and Co(II). J. Hazard. Mater. 2010, 173, 62–70. [Google Scholar] [CrossRef] [PubMed]
- Moraes, D.S.; Angélica, R.S.; Costa, C.E.F.; Rocha Filho, G.N.; Zamian, J.R. Bentonite functionalized with propyl sulfonic acid groups used as catalyst in esterification reactions. Appl. Clay Sci. 2011, 51, 209–213. [Google Scholar] [CrossRef] [Green Version]
- Venkatathri, N. Characterization and catalytic properties of a naturally occurring clay, Bentonite. Bull. Catal. Soc. India 2006, 5, 61–72. [Google Scholar]
- Wu, H.; Hu, L. Microfabric change of electro-osmotic stabilized bentonite. Appl. Clay Sci. 2014, 101, 503–509. [Google Scholar] [CrossRef] [Green Version]
- Zivica, V.; Palou, M.T. Physico-chemical characterization of thermally treated bentonite. Compos. Part B Eng. 2015, 68, 436–445. [Google Scholar] [CrossRef]
- Nascimento, L.A.S.; Angélica, R.S.; Costa, C.E.F.; Zamian, J.R.; Rocha Filho, G.N. Comparative study between catalysts for esterification prepared from kaolins. Appl. Clay Sci. 2011, 51, 267–273. [Google Scholar] [CrossRef] [Green Version]
- Nascimento, L.A.S.; Angélica, R.S.; Costa, C.E.F.; Zamian, J.R.; Rocha Filho, G.N. Conversion of waste produced by the deodorization of palm oil as feedstock for the production of biodiesel using a catalyst prepared from waste material. Bioresour. Technol. 2011, 102, 8314–8317. [Google Scholar] [CrossRef] [Green Version]
- Zatta, L.; da Costa Gardolinski, J.E.F.; Wypych, F. Raw halloysite as reusable heterogeneous catalyst for esterification of lauric acid. Appl. Clay Sci. 2011, 51, 165–169. [Google Scholar] [CrossRef] [Green Version]
- Bhorodwaj, S.K.; Dutta, D.K. Activated clay supported heteropoly acid catalysts for esterification of acetic acid with butanol. Appl. Clay Sci. 2011, 53, 347–352. [Google Scholar] [CrossRef]
- Khayoon, M.S.; Hameed, B.H. Single-step esterification of crude karanj (Pongamia pinnata) oil to fatty acid methyl esters over mesostructured SBA-16 supported 12-molybdophosphoric acid catalyst. Fuel Process. Technol. 2013, 114, 12–20. [Google Scholar] [CrossRef]
- Carvalho, A.K.F.; Conceição, L.R.V.; Silva, J.P.V.; Perez, V.H.; De Castro, H.F. Biodiesel production from Mucor circinelloides using ethanol and heteropolyacid in one and two-step transesterification. Fuel 2017, 202, 503–511. [Google Scholar] [CrossRef]
- Moraes, D.S.; Miranda, L.C.R.; Angélica, R.S.; Rocha Filho, G.N.; Zamian, J.R. Functionalization of bentonite and vermiculite after the creation of structural defects through an acid leaching process. J. Braz. Chem. Soc. 2018, 29, 320–327. [Google Scholar] [CrossRef]
- Kuroki, V.; Bosco, G.E.; Fadini, P.S.; Mozeto, A.A.; Cestari, A.R.; Carvalho, W.A. Use of a La(III)-modified bentonite for effective phosphate removal from aqueous media. J. Hazard. Mater. 2014, 274, 124–131. [Google Scholar] [CrossRef] [PubMed]
- Monteiro, E.P.; Santana, G.P. Lead removal from battery solutions using kaolinite modified with manganese oxide. Quim. Nova 2012, 35, 1151–1156. [Google Scholar] [CrossRef]
- Bhorodwaj, S.K.; Dutta, D.K. Heteropoly acid supported modified Montmorillonite clay: An effective catalyst for the esterification of acetic acid with sec-butanol. Appl. Catal. A Gen. 2010, 378, 221–226. [Google Scholar] [CrossRef]
- Aranda, D.A.G.; Santos, R.T.P.; Tapanes, N.C.O.; Ramos, A.L.D.; Antunes, O.A.C. Acid-Catalyzed Homogeneous Esterification Reaction for Biodiesel Production from Palm Fatty Acids. Catal. Letters 2008, 122, 20–25. [Google Scholar] [CrossRef]
- Lima, E.T.L.; Queiroz, L.S.; de Oliveira Pires, L.H.; Angélica, R.S.; Costa, C.E.F.; Zamian, J.R.; Rocha Filho, G.N.; Luque, R.; Nascimento, L.A.S. Valorization of Mining Waste in the Synthesis of Organofunctionalized Aluminosilicates for the Esteri fi cation of Waste from Palm Oil Deodorization. ACS Sustain. Chem. Eng 2019, 7, 7543–7551. [Google Scholar] [CrossRef]
- Moraes, D.S.; Angélica, R.S.; Costa, C.E.F.; Rocha Filho, G.N.; Zamian, J.R. Mineralogy and chemistry of a new bentonite occurrence in the eastern Amazon region, northern Brazil. Appl. Clay Sci. 2010, 48, 475–480. [Google Scholar] [CrossRef]
- Pezzotta, C.; Fleury, G.; Soetens, M.; Van der Perre, S.; Denayer, J.F.M.; Riant, O.; Gaigneaux, E.M. Improving the selectivity to 4-tert-butylresorcinol by adjusting the surface chemistry of heteropolyacid-based alkylation catalysts. J. Catal. 2018, 359, 198–211. [Google Scholar] [CrossRef]
- Reddy, C.R.; Bhat, Y.S.; Nagendrappa, G.; Prakash, B.S.J. Brønsted and Lewis acidity of modified montmorillonite clay catalysts determined by FT-IR spectroscopy. Catal. Today 2009, 141, 157–160. [Google Scholar] [CrossRef] [Green Version]
- Reddy, C.R.; Nagendrappa, G.; Prakash, B.S.J. Surface acidity study of Mn+-montmorillonite clay catalysts by FT-IR spectroscopy: Correlation with esterification activity. Catal. Commun. 2007, 8, 241–246. [Google Scholar] [CrossRef]
- Vazquez, P.G.; Blanco, M.N.; Caceres, C.V. Catalysts based on supported 12-molybdophosphoric acid. Catal. Letters 1999, 60, 205–215. [Google Scholar] [CrossRef]
- Conceição, L.R.V.; Carneiro, L.M.; Rivaldi, J.D.; de Castro, H.F. Solid acid as catalyst for biodiesel production via simultaneous esterification and transesterification of macaw palm oil. Ind. Crop. Prod. 2016, 89, 416–424. [Google Scholar] [CrossRef]
- Wang, Y.; Fang, Z.; Zhang, F. Esterification of oleic acid to biodiesel catalyzed by a highly acidic carbonaceous catalyst. Catal. Today 2019, 319, 172–181. [Google Scholar] [CrossRef]
- Tamborini, L.H.; Militello, M.P.; Balach, J.; Moyano, J.M.; Barbero, C.A.; Acevedo, D.F. Application of sulfonated nanoporous carbons as acid catalysts for Fischer esterification reactions. Arab. J. Chem. 2015. [Google Scholar] [CrossRef] [Green Version]
- Barbosa, C.S.; Santana, S.A.A.; Bezerra, C.W.B.; dos Santos Silva, H.A. Removal of phenolic compounds from aqueous solutions using activated carbon prepared from water hyacinth (Eichhornia crassipes): Kinetic and thermodynamic equilibrium studies. Quim. Nova 2014, 37, 447–453. [Google Scholar] [CrossRef]
- Liu, D.; Quek, X.Y.; Hu, S.; Li, L.; Lim, H.M.; Yang, Y. Mesostructured TUD-1 supported molybdophosphoric acid (HPMo/TUD-1) catalysts for n-heptane hydroisomerization. Catal. Today 2009, 147, 51–57. [Google Scholar] [CrossRef]
- Menezes, R.R.; Souto, P.M.; Santana, L.N.L.; Neves, G.A.; Kiminami, R.H.G.A.; Ferreira, H.C. Bentonite clay from Cubati, Paraíba, Brazil: Physical and mineralogical characterization. Cerâmcia 2009, 55, 163–169. [Google Scholar] [CrossRef]
- Foletto, E.L.; Volzone, C.; Morgado, A.F.; Porto, L.M. Influence of the type and concentration of the used acid on the activation of bentonite clays. Cerâmica 2001, 47, 208–211. [Google Scholar] [CrossRef]
- Gardolinski, J.E.; Martins Filho, H.P.; Wypych, F. Themal behavior of hydrated kaolinite. Quim. Nova 2003, 26, 30–35. [Google Scholar] [CrossRef]
- Carneiro, B.S.; Angélica, R.S.; Scheller, T.; Castro, E.A.S.d.; Neves, R.d.F. Mineralogical and geochemical characterization of the hard kaolin from the Capim region, Pará, northern Brazil. Cerâmica 2003, 49, 237–244. [Google Scholar] [CrossRef]
- Ezquerro, C.S.; Ric, G.I.; Miñana, C.C.; Bermejo, J.S. Characterization of montmorillonites modified with organic divalent phosphonium cations. Appl. Clay Sci. 2015, 111, 1–9. [Google Scholar] [CrossRef]
- Xiong, J.; Zhu, W.; Ding, W.; Yang, L.; Zhang, M.; Jiang, W.; Zhao, Z.; Li, H. Hydrophobic mesoporous silica-supported heteropolyacid induced by ionic liquid as a high efficiency catalyst for the oxidative desulfurization of fuel. RSC Adv. 2015, 5, 16847–16855. [Google Scholar] [CrossRef]
- Chabukswar, D.D.; Heer, P.K.K.S.; Gaikar, V.G. Esterification of Palm Fatty Acid Distillate Using Heterogeneous Sulfonated Microcrystalline Cellulose Catalyst and Its Comparison with H2SO4 Catalyzed Reaction. Eng. Chem. Res 2013, 52, 7316–7326. [Google Scholar] [CrossRef]
- Pantoja, S.; Mescouto, V.; Costa, C.; Zamian, J.; Rocha Filho, G.; Nascimento, L. High-Quality Biodiesel Production from Buriti (Mauritia flexuosa) Oil Soapstock. Molecules 2019, 24, 94. [Google Scholar] [CrossRef]
- Wan, Z.; Lim, J.K.; Hameed, B.H. Chromium-tungsten heterogeneous catalyst for esterification of palm fatty acid distillate to fatty acid methyl ester. J. Taiwan Inst. Chem. Eng. 2015, 54, 64–70. [Google Scholar] [CrossRef]
- Wan, Z.; Hameed, B.H. Chromium-tungsten-titanium mixed oxides solid catalyst for fatty acid methyl ester synthesis from palm fatty acid distillate. Energy Convers. Manag. 2014, 88, 669–676. [Google Scholar] [CrossRef]
- Brahmkhatri, V.; Patel, A. 12-Tungstophosphoric acid anchored to SBA-15: An efficient, environmentally benign reusable catalysts for biodiesel production by esterification of free fatty acids. Appl. Catal. A Gen. 2011, 403, 161–172. [Google Scholar] [CrossRef]







| Sample | BC | BL | BCM | BLM | BCMW | BLMW |
|---|---|---|---|---|---|---|
| O | 42.11 | 47.73 | 35.94 | 39.89 | 37.74 | 30.88 |
| Si | 36.55 | 28.39 | 27.29 | 22.92 | 28.44 | 28.79 |
| Al | 12.49 | 10.50 | 9.51 | 7.28 | 10.22 | 10.10 |
| Fe | 5.06 | 9.26 | 3.76 | 8.21 | 4.72 | 7.83 |
| Mg | 2.36 | 1.75 | 1.58 | 1.36 | 0.62 | 1.53 |
| K | 0.21 | 0.82 | 0.21 | 0.25 | 0.45 | 1.02 |
| Ca | 0.23 | 0.64 | 0.39 | 0.28 | 0.43 | 1.25 |
| Na | 0.79 | 0.42 | 0.90 | 0.91 | 0.98 | 0.62 |
| Ti | 0.20 | 0.88 | 0.20 | 0.50 | 0.85 | 0.36 |
| P | - | - | 0.08 | 0.07 | 0.054 | 0.065 |
| Mo | - | - | 18.15 | 17.72 | 15.01 | 16.97 |
| Total | 100 | 100 | 100 | 100 | 100 | 100 |
| Catalyst | a SSA (m2 g−1) | b Sμ (m2 g−1) | c Vp (cm 3 g−1) | d Dp (nm) | mmol of H+ g−1 |
|---|---|---|---|---|---|
| HPMo | - | - | - | - | 20.7 |
| BC | 24 | 22 | 0.02 | 7.8 | 4.2 |
| BL | 43 | 22 | 0.04 | 7.1 | 5.0 |
| BCM | <10 | <10 | - | - | 10.7 |
| BCMW | 17 | 17 | 0.01 | 12.5 | 9.1 |
| BLM | <10 | <10 | - | - | 13.3 |
| BLMW | 23 | 23 | 0.02 | 18.1 | 12.5 |
 | ||||
|---|---|---|---|---|
| Catalyst | Conv. (%) a | Conv. (%) b | TOF (min−1) c | TOF (min−1) d |
| HPMo | 88.3 | 94.4 | 76 | 82 |
| BC | 25.3 | 27.5 | ||
| BCM | 95.1 | 97.1 | 82 | 84 |
| BCMW | 90.7 | 93.3 | 105 | 108 |
| BL | 28.4 | 30.7 | ||
| BLM | 94.4 | 95.3 | 82 | 83 |
| BLMW | 93.3 | 94.5 | 93 | 110 |
| Catalyst | Temperature (°C) | Time (h) | FFA | Alcohol | Mole Ratio | Conversion (%) | Reference |
|---|---|---|---|---|---|---|---|
| Al-MCM-41 Si/Al = 8 | 130 | 2 | palmitic | Methanol | 1:60 | 79 | [6] |
| MF9S4 | 160 | 4 | Oleic | Methanol | 1:60 | 98.9 | [7] |
| MF9S4 | 160 | 4 | DDPO | Methanol | 1:60 | 92.8 | [39] |
| 20%H3PW/ZrO2 | 100 | 4 | Oleic | Ethanol | 1:6 | 88 | [21] |
| MF8S4M4W15 | 115 | 2/3 | Oleic | Methanol | 1:60 | 96.5 | [8] |
| 25%HPW/MK700 | 200 | 2 | DDPO | Ethanol | 1:10 | 83 | [9] |
| MP-S-16 (15) | 140 | 5 | CKO | Methanol | 1:8 | 82 | [41] |
| HPMo/Nb2O5 | 210 | 4 | macaw | Ethanol | 1:90 | 99.7 | [14] |
| AM41-2H-O | 130 | 2 | DDPO | Methanol | 1:30 | 98 | [49] |
| H2SO4 | 130 | 1 | DDPO | Methanol | 1:3 | 90 | [48] |
| H2SO4 | 60 | 3 | PFAD | Methanol | 1:3 | 62 | [66] |
| H2SO4 | 50 | 14 | Oil Soapstock | Methanol | 1:18 | 99.9 | [67] |
| CrWO2 | 170 | 3 | PFAD | Methanol | 1:2 | 86 | [68] |
| CrWTiO2 | 170 | 3 | PFAD | Methanol | 1:2 | 83 | [69] |
| BLMW | 160 | 2 | DDPO | Ethanol | 1:30 | 93.3 | Present work |
| BCMW | 160 | 2 | DDPO | Ethanol | 1:30 | 90.7 | Present work |
| Cycle | Mo (%)a | HPMo (mg)b | TOF (min−1) | Acidity (mmol H+g−1)c | Conversion (%) |
|---|---|---|---|---|---|
| Blank | <20 | ||||
| BCMW | 15.01 | 31.5 | 105 | 9.2 | 90.7 |
| BCMWR 1 | 13.88 | 30.2 | 104 | 8.6 | 86.3 |
| BCMWR 2 | 12.25 | 28.2 | 101 | 8.1 | 79.0 |
| BCMWR 3 | 12.11 | 27.5 | 98 | 7.5 | 73.1 |
| BLMW | 16.97 | 36.9 | 93 | 12.5 | 93.3 |
| BLMWR 1 | 16.90 | 36.2 | 90 | 11.9 | 89.1 |
| BLMWR 2 | 16.48 | 35.3 | 86 | 11.1 | 83.5 |
| BLMWR 3 | 15.32 | 34.0 | 84 | 10.1 | 78.3 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
de Oliveira, A.d.N.; Barbosa de Lima, M.A.; de Oliveira Pires, L.H.; Rosas da Silva, M.; Souza da Luz, P.T.; Angélica, R.S.; da Rocha Filho, G.N.; F. da Costa, C.E.; Luque, R.; Santos do Nascimento, L.A. Bentonites Modified with Phosphomolybdic Heteropolyacid (HPMo) for Biowaste to Biofuel Production. Materials 2019, 12, 1431. https://doi.org/10.3390/ma12091431
de Oliveira AdN, Barbosa de Lima MA, de Oliveira Pires LH, Rosas da Silva M, Souza da Luz PT, Angélica RS, da Rocha Filho GN, F. da Costa CE, Luque R, Santos do Nascimento LA. Bentonites Modified with Phosphomolybdic Heteropolyacid (HPMo) for Biowaste to Biofuel Production. Materials. 2019; 12(9):1431. https://doi.org/10.3390/ma12091431
Chicago/Turabian Stylede Oliveira, Alex de Nazaré, Marco Aurélio Barbosa de Lima, Luíza Helena de Oliveira Pires, Moisés Rosas da Silva, Patrícia Teresa Souza da Luz, Rômulo S. Angélica, Geraldo N. da Rocha Filho, Carlos Emmerson F. da Costa, Rafael Luque, and Luís Adriano Santos do Nascimento. 2019. "Bentonites Modified with Phosphomolybdic Heteropolyacid (HPMo) for Biowaste to Biofuel Production" Materials 12, no. 9: 1431. https://doi.org/10.3390/ma12091431





