N-Type Bismuth Telluride Nanocomposite Materials Optimization for Thermoelectric Generators in Wearable Applications
Abstract
:1. Introduction
2. Experimental Section
3. Results and Discussion
3.1. Effect of Dopant Addition
3.2. Optimizing Te Vacancy
3.3. Effect of Glass Inclusion and Soaking Time
3.4. Effect of Microwave Processing with Glass Inclusion
3.5. Effect of Initial SPS Temperature
3.6. Effect of Annealing
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Goldsmid, H.J. Introduction to Thermoelectricity; Springer: Heidelberg, Germany, 2009; Volume 121. [Google Scholar]
- Vining, C.B. Thermoelectric Properties of Silicides. In CRC Handbook of Thermoelectrics; CRC Press: Boca Raton, FL, USA, 1995; pp. 277–286. [Google Scholar]
- Heremans, J.P.; Dresselhaus, M.S.; Bell, L.E.; Morelli, D.T. When thermoelectrics reached the nanoscale. Nat. Nanotechnol. 2013, 8, 471–473. [Google Scholar] [CrossRef]
- Ioffe, A.F.; Stil’Bans, L.S.; Iordanishvili, E.K.; Stavitskaya, T.S.; Gelbtuch, A.; Vineyard, G. Semiconductor thermoelements and thermoelectric cooling. Phys. Today 1959, 12, 42. [Google Scholar] [CrossRef]
- Schmidt, G.R.; Sutliff, T.J.; Dudzinksi, L.A. Radioisotope Power: A Key Technology for Deep Space Exploration. In Radioisotopes—Applications in Physical Science; Singh, N., Ed.; InTech: London, UK, 2011; p. 419. [Google Scholar]
- Snyder, G.J.; Toberer, E.S. Complex thermoelectric materials. Nat. Mater. 2008, 7, 105–114. [Google Scholar] [CrossRef]
- Bux, S.K.; Fleurial, J.P.; Kaner, R.B. Nanostructured materials for thermoelectric applications. Chem. Commun. 2010, 46, 8311–8324. [Google Scholar] [CrossRef] [PubMed]
- Kishi, M.; Nemoto, H.; Hamao, T.; Yamamoto, M.; Sudou, S.; Mandai, M.; Yamamoto, S. Micro thermoelectric modules and their application to wristwatches as an energy source. In Proceedings of the 18th International Conference on Thermoelectrics, Baltimore, MD, USA, 29 August–2 September 1999; pp. 301–307. [Google Scholar]
- Misra, V.; Bozkurt, A.; Calhoun, B.; Jackson, T.; Jur, J.S.; Lach, J.; Lee, B.; Muth, J.; Oralkan, Ö.; Öztürk, M.; Trolier-McKinstry, S.; et al. Flexible technologies for self-powered wearable health and environmental sensing. Proc. IEEE 2015, 103, 661–685. [Google Scholar] [CrossRef]
- Minnich, A.J.L.; Dresselhaus, M.S.; Ren, Z.F.; Chen, G. Bulk nanostructured thermoelectric materials: Current research and future prospects. Energy Environ. Sci. 2009, 2, 466–479. [Google Scholar] [CrossRef]
- Dresselhaus, M.S.; Chen, G.; Tang, M.Y.; Yang, R.G.; Lee, H.; Wang, D.Z.; Ren, Z.F.; Fleurial, J.; Gogna, P. New Directions for Low-Dimensional Thermoelectric Materials. Adv. Mater. 2007, 19, 1043–1053. [Google Scholar] [CrossRef]
- Zebarjadi, M.; Esfarjani, K.; Dresselhaus, M.S.; Ren, Z.F.; Chen, G. Perspectives on thermoelectrics: From fundamentals to device applications. Energy Environ. Sci. 2012, 5, 5147–5162. [Google Scholar] [CrossRef]
- Vineis, C.J.; Shakouri, A.; Majumdar, A.; Kanatzidis, M.G. Nanostructured thermoelectrics: Big efficiency gains from small features. Adv. Mater. 2010, 22, 3970–3980. [Google Scholar] [CrossRef] [PubMed]
- Biswas, K.; He, J.; Blum, I.D.; Wu, C.I.; Hogan, T.P.; Seidman, D.N.; Dravid, V.P.; Kanatzidis, M.G. High-performance bulk thermoelectrics with all-scale hierarchical architectures. Nature 2012, 489, 414–418. [Google Scholar] [CrossRef]
- Sootsman, J.R.; Chung, D.Y.; Kanatzidis, M.G. New and old concepts in thermoelectric materials. Angew. Chem. Int. Ed. 2009, 48, 8616–8639. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Wang, Z.M. Nanoscale Thermoelectrics; Springer International Publishing: Berlin/Heidelberg, Germany, 2014. [Google Scholar]
- LaLonde, A.D.; Pei, Y.; Wang, H.; Snyder, G.J. Lead telluride alloy thermoelectrics. Mater. Today 2011, 14, 526–532. [Google Scholar] [CrossRef]
- Kim, S.I.; Lee, K.H.; Mun, H.A.; Kim, H.S.; Hwang, S.W.; Roh, J.W.; Yang, D.J.; Shin, W.H.; Li, X.S.; Lee, Y.H.; et al. Dense dislocation arrays embedded in grain boundaries for high-performance bulk thermoelectrics. Science 2015, 348, 109–114. [Google Scholar] [CrossRef] [Green Version]
- Zhao, L.D.; Lo, S.H.; Zhang, Y.; Sun, H.; Tan, G.; Uher, C.; Wolverton, C.; Dravid, V.P.; Kanatzidis, M.G. Ultralow thermal conductivity and high thermoelectric figure of merit in SnSe crystals. Nature 2014, 508, 373–377. [Google Scholar] [CrossRef]
- Nozariasbmarz, A.; Zamanipour, Z.; Norouzzadeh, P.; Krasinski, J.S.; Vashaee, D. Enhanced Thermoelectric Performance in Metal/Semiconductor Nanocomposite of Iron Silicide/Silicon Germanium. RSC Adv. 2016, 6, 49643–49650. [Google Scholar] [CrossRef]
- Nozariasbmarz, A.; Roy, P.; Zamanipour, Z.; Dycus, J.H.; Cabral, M.J.; LeBeau, J.M.; Krasinski, J.S.; Vashaee, D. Comparison of Thermoelectric Properties of Nanostructured Mg2Si, FeSi2, SiGe, and Nanocomposites of SiGe-Mg2Si, SiGe-FeSi2. APL Mater. 2016, 4, 104814. [Google Scholar] [CrossRef]
- Bell, L.E. Cooling, Heating, Generating Power, and Recovering Waste Heat with Thermoelectric Systems. Science 2008, 321, 1457–1461. [Google Scholar] [CrossRef] [Green Version]
- Suarez, F.; Nozariasbmarz, A.; Vashaee, D.; Öztürk, M.C. Designing thermoelectric generators for self-powered wearable electronics. Energy Environ. Sci. 2016, 9, 2099–2113. [Google Scholar] [CrossRef]
- Goldsmid, H.J. Recent Studies of Bismuth Telluride and Its Alloys. J. Appl. Phys. 1961, 32, 2198. [Google Scholar] [CrossRef]
- Wang, S.; Tan, G.; Xie, W.; Zheng, G.; Li, H.; Yang, J.; Tang, X. Enhanced thermoelectric properties of Bi2(Te1−xSex)3-based compounds as n-type legs for low-temperature power generation. J. Mater. Chem. 2012, 22, 20943–20951. [Google Scholar] [CrossRef]
- Yan, X.; Poudel, B.; Ma, Y.; Liu, W.S.; Joshi, G.; Wang, H.; Lan, Y.C.; Wang, D.Z.; Chen, G.; Ren, Z.F. Experimental studies on anisotropic thermoelectric properties and structures of n-type Bi2Te2.7Se0.3. Nano Lett. 2010, 10, 3373. [Google Scholar] [CrossRef]
- Liu, W.S.; Zhang, Q.; Lan, Y.; Chen, S.; Yan, X.; Zhang, Q.; Wang, H.; Wang, D.; Chen, G.; Ren, Z. Thermoelectric Property Studies on Cu-Doped n-type CuxBi2Te2.7Se0.3 Nanocomposites. Adv. Energy Mater. 2011, 1, 577–587. [Google Scholar] [CrossRef]
- Hong, M.; Chasapis, T.C.; Chen, Z.G.; Yang, L.; Kanatzidis, M.G.; Snyder, G.J.; Zou, J. n-Type Bi2Te3−xSex Nanoplates with Enhanced Thermoelectric Efficiency Driven by Wide Frequency Phonon Scatterings and Synergistic Carrier Scatterings. ACS Nano 2016, 10, 4719–4727. [Google Scholar] [CrossRef]
- Song, S.; Wang, J.; Xu, B.; Lei, X.; Jiang, H.; Jin, Y.; Zhang, Q.; Ren, Z. Thermoelectric properties of n-type Bi2Te2.7Se0.3 with addition of nano-ZnO:Al particles. Mater. Res. Express 2014, 1, 035901. [Google Scholar] [CrossRef]
- Hu, L.P.; Liu, X.H.; Xie, H.H.; Shen, J.J.; Zhu, T.J.; Zhao, X.B. Improving thermoelectric properties of n-type bismuth–telluride-based alloys by deformation-induced lattice defects and texture enhancement. Acta Mater. 2012, 60, 4431–4437. [Google Scholar] [CrossRef]
- Hu, L.; Zhu, T.; Liu, X.; Zhao, X. Point Defect Engineering of High-Performance Bismuth-Telluride-Based Thermoelectric Materials. Adv. Funct. Mater. 2014, 24, 5211–5218. [Google Scholar] [CrossRef]
- Cronin, N.J. Microwave and Optical Waveguides; IOP Publishing: Philadelphia, PA, USA, 1995. [Google Scholar]
- Nozariasbmarz, A. In-Situ Sintering Decrystallization of Thermoelectric Materials Using Microwave Radiation. Ph.D. Thesis, North Carolina State University, Raleigh, NC, USA, 2017. [Google Scholar]
- Golio, M. The RF and Microwave Handbook; CRC Press, Taylor and Francis Group: Boca Raton, FL, USA, 2000. [Google Scholar]
- Oghbaei, M.; Mirzaee, O. Microwave versus conventional sintering: A review of fundamentals, advantages and applications. J. Alloy. Compd. 2010, 494, 175–189. [Google Scholar] [CrossRef]
- Thostenson, E.T.; Chou, T.W. Microwave Processing: Fundamentals and Applications. Compos. Part A 1999, 30, 1055–1071. [Google Scholar] [CrossRef]
- Miller, G.R.; Li, C.Y. Evidence for the existence of antistructure defects in bismuth telluride by density measurements. J. Phys. Chem. Solids 1965, 26, 173–177. [Google Scholar] [CrossRef]
- Stterthwaite, C.B.; Ure, R.W., Jr. Electrical and Thermal Properties of Bi2Te3. Phys. Rev. 1957, 108, 1164. [Google Scholar] [CrossRef]
- Mishra, S.K.; Satpathy, S.; Jepsen, O. Electronic structure and thermoelectric properties of bismuth telluride and bismuth selenide. J. Phys. Cond. Matter 1997, 9, 461. [Google Scholar] [CrossRef]
- Horak, J.; Cermak, K.; Koudelka, L. Energy formation of antisite defects in doped Sb2Te3 and Bi2Te3 crystals. J. Phys. Chem. Solids 1986, 47, 805. [Google Scholar] [CrossRef]
- Pecheur, P.; Toussaint, G. Tight-binding studies of crystal stability and defects in Bi2Te3. J. Phys. Chem. Solids 1994, 35, 327. [Google Scholar] [CrossRef]
- Nozariasbmarz, A.; Hosseini, M.; Vashaee, D. Solid-solution Material Decomposition Induced by Electromagnetic Field: A Non-Equilibrium Thermodynamic Process. 2019; submitted. [Google Scholar]
- Nozariasbmarz, A.; Dsouza, K.; Vashaee, D. Field Induced Decrystallization of Silicon: Evidence of a Microwave Non-Thermal Effect. Appl. Phys. Lett. 2018, 112, 093103. [Google Scholar] [CrossRef]
- Vashaee, D.; Nozariasbmarz, A.; Tayebi, L.; Krasinski, J.S. Microwave processing of thermoelectric materials and use of glass inclusions for improving the mechanical and thermoelectric properties. US Patent App. 15/778, 704, 2018. International Application No.: PCT/US2016/064292. [Google Scholar]
- Yamashita, O.; Sadatomi, N. Dependence of Seebeck Coefficient on Carrier Concentration in Heavily B- and P Doped Si1−xGex (x ≤ 0.05) System. Jpn. J. Appl. Phys. 1999, 38, 6394–6400. [Google Scholar] [CrossRef]

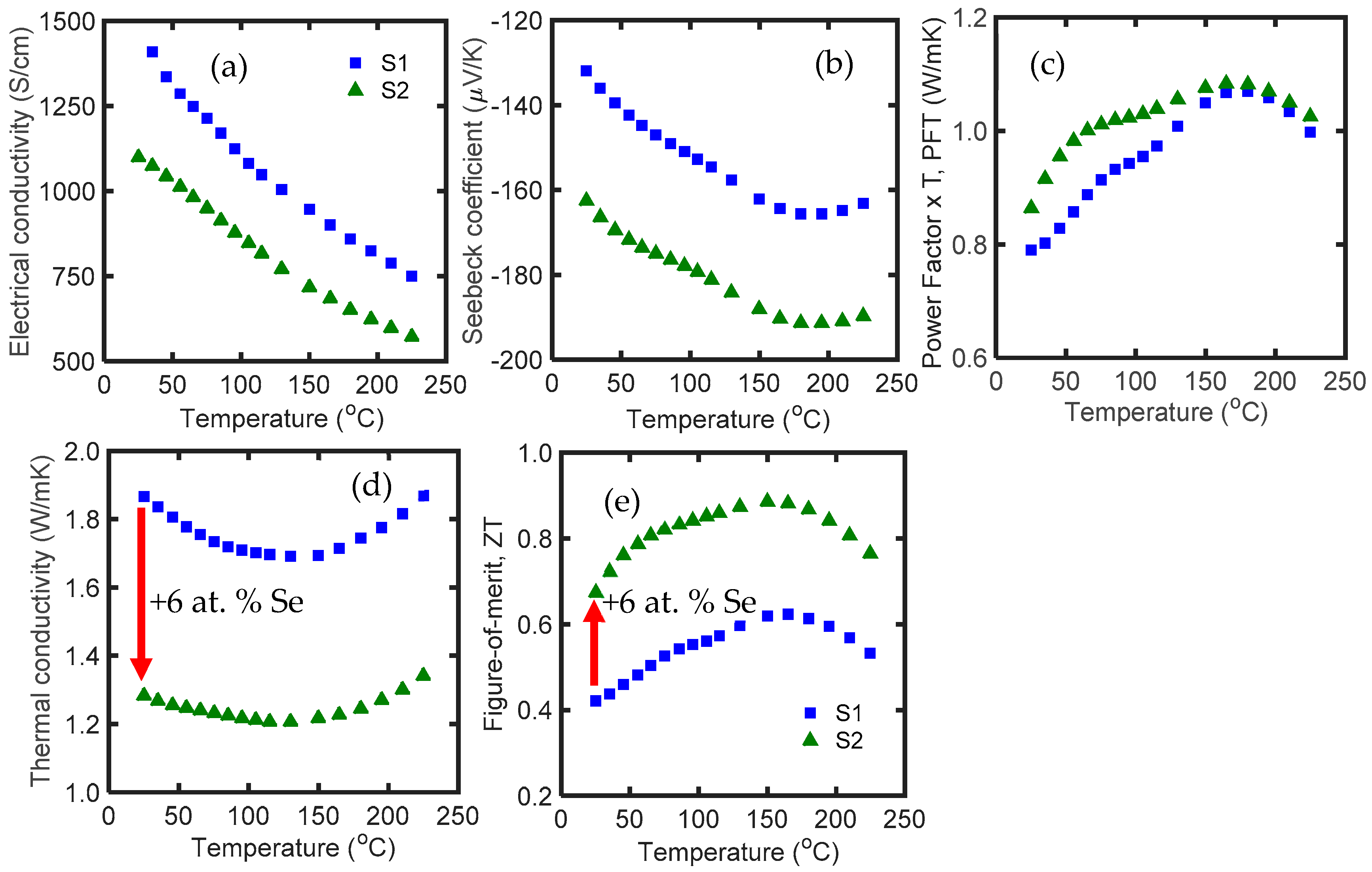
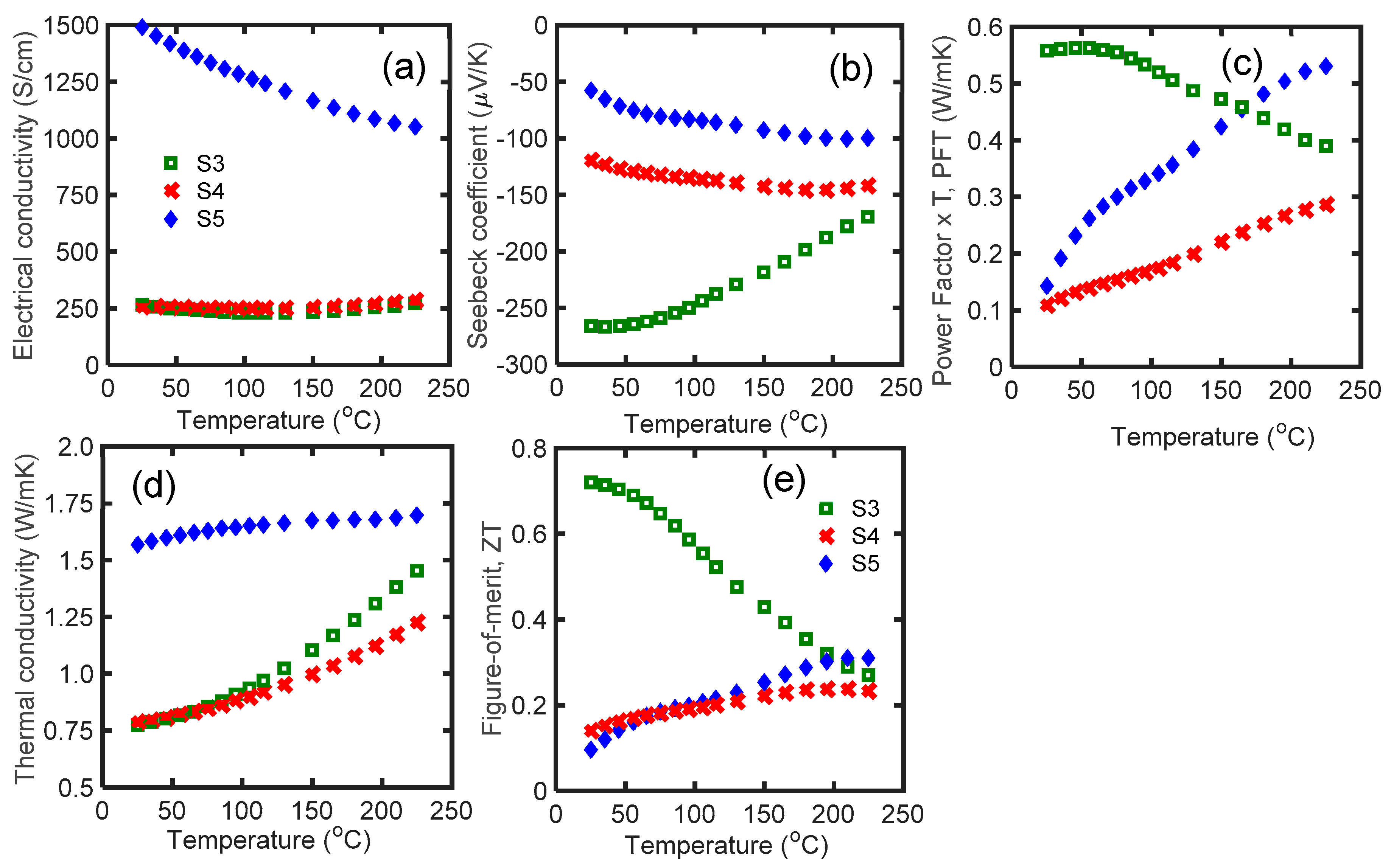



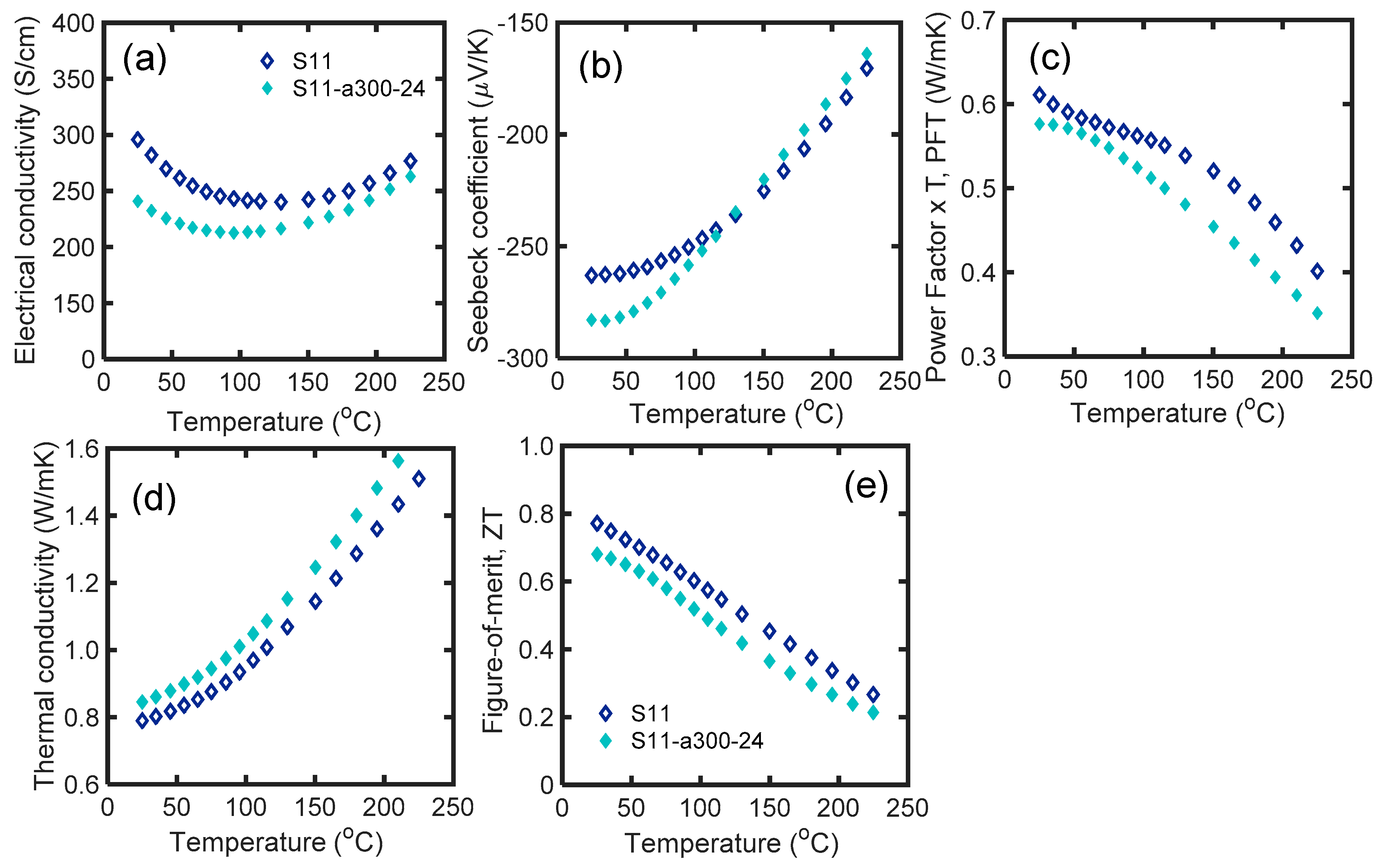
| Name | Material | 1st SPS T (°C)/Soak Time (min) * | MW Process | 2nd SPS T (°C)/Soak Time (min) | Room T S (μV/K) | Room T κ (W/mK) | Room T zT | Max zT |
|---|---|---|---|---|---|---|---|---|
| S1 | Bi2Te3 | 540/1 | Yes | 450/1 | −132 | 1.95 | 0.37 | 0.57 |
| S2 | Bi2Te3-0.3%Se ** | 540/1 | Yes | 450/1 | −163 | 1.28 | 0.70 | 0.89 |
| S3 | Bi2Te2.7Se0.3 | 540/2 | No | - | −266 | 0.78 | 0.72 | 0.72 |
| S4 | Bi2.04Te2.66Se0.3 | 540/2 | No | - | −120 | 0.79 | 0.15 | 0.24 |
| S5 | Bi2.15Te2.55Se0.3 | 540/2 | No | - | −58 | 1.57 | 0.11 | 0.33 |
| S6 | Bi2Te2.7Se0.3-2.5%Glass | 540/1 | No | - | −256 | 0.78 | 0.72 | 0.72 |
| S7 | Bi2Te2.7Se0.3-2.5%Glass | 540/2 | No | - | −268 | 0.70 | 0.57 | 0.57 |
| S8 | Bi2Te2.7Se0.3-2.5%Glass | 540/3 | No | - | −283 | 0.73 | 0.39 | 0.39 |
| S9 | Bi2Te2.7Se0.3-2.5%Glass | 540/2 | Yes | 450/1 | −284 | 0.77 | 0.59 | 0.59 |
| S10 | Bi2Te2.7Se0.3-2.5%Glass | 540/3 | Yes | 450/1 | −297 | 0.65 | 0.63 | 0.63 |
| S11 | Bi2Te2.7Se0.3-2.5%Glass | 310/1 | Yes | 450/1 | −188 | 0.78 | 0.67 | 0.87 |
| S12 | Bi2Te2.7Se0.3-2.5%Glass | 270/1 | Yes | 450/1 | −204 | 0.91 | 0.63 | 0.70 |
| S13 | Bi2Te3-0.3%Se | 310/1 | Yes | 450/1 | −121 | 1.34 | 0.46 | 0.77 |
| S14 | Bi2Te2.7Se0.3 | 540/2 | Yes | 450/1 | −263 | 0.79 | 0.76 | 0.76 |
| S15-a300-24 | Bi2Te2.7Se0.3 (anneal at 300°C/24h) | 540/2 | Yes | 450/1 | −283 | 0.85 | 0.68 | 0.68 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nozariasbmarz, A.; Krasinski, J.S.; Vashaee, D. N-Type Bismuth Telluride Nanocomposite Materials Optimization for Thermoelectric Generators in Wearable Applications. Materials 2019, 12, 1529. https://doi.org/10.3390/ma12091529
Nozariasbmarz A, Krasinski JS, Vashaee D. N-Type Bismuth Telluride Nanocomposite Materials Optimization for Thermoelectric Generators in Wearable Applications. Materials. 2019; 12(9):1529. https://doi.org/10.3390/ma12091529
Chicago/Turabian StyleNozariasbmarz, Amin, Jerzy S. Krasinski, and Daryoosh Vashaee. 2019. "N-Type Bismuth Telluride Nanocomposite Materials Optimization for Thermoelectric Generators in Wearable Applications" Materials 12, no. 9: 1529. https://doi.org/10.3390/ma12091529





