Atomic Layer Deposition of GdCoO3 and Gd0.9Ca0.1CoO3
Abstract
:1. Introduction
2. Experimental
2.1. ALD and Precursors
2.2. Substrates and Annealing
2.3. Characterization
3. Results and Discussion
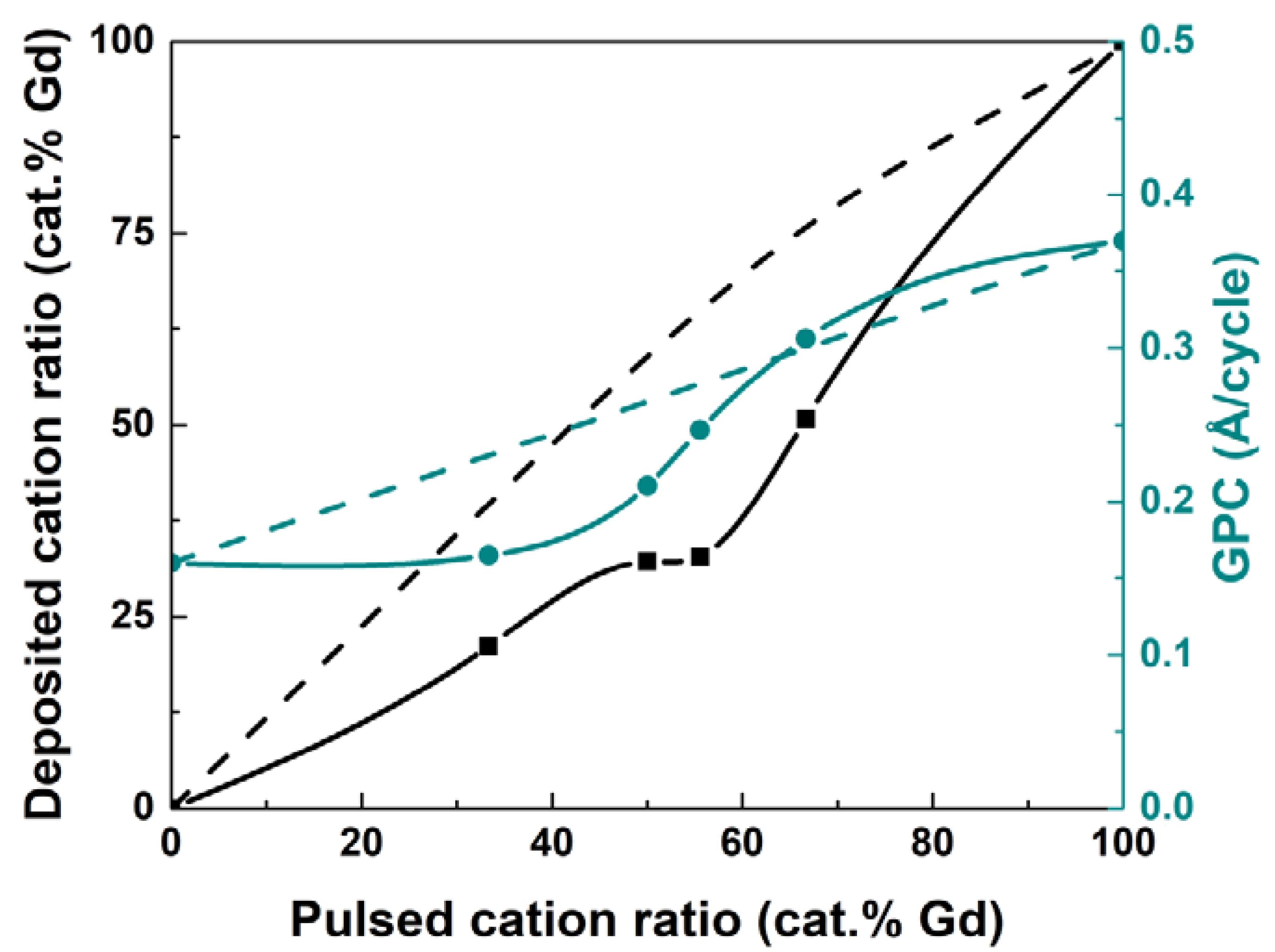
3.1. Deposition of GdCoO3
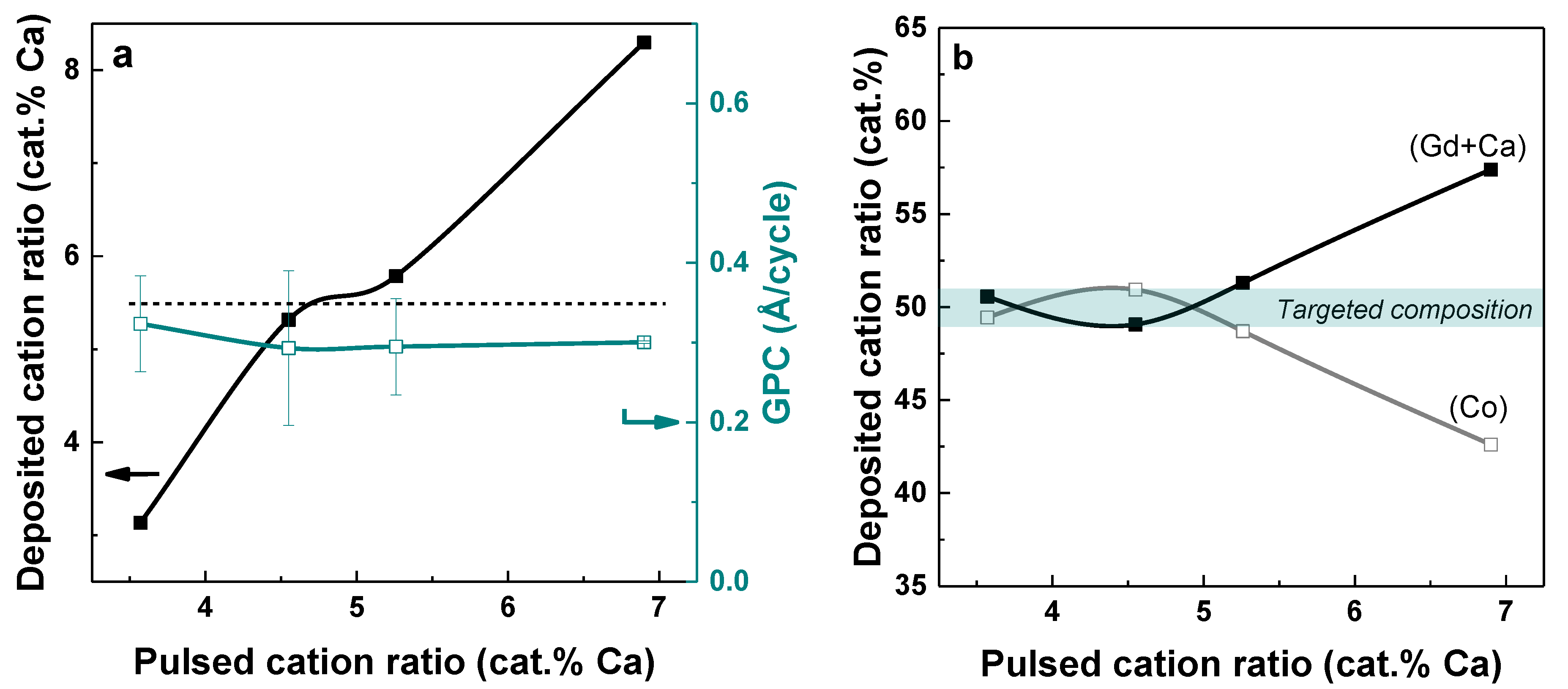
3.2. Deposition of Gd1−xCaxCoO3
3.3. Characterization of GdCoO3 and Gd0.9Ca0.1CoO3 Thin Films
3.3.1. X-Ray Diffraction (XRD)
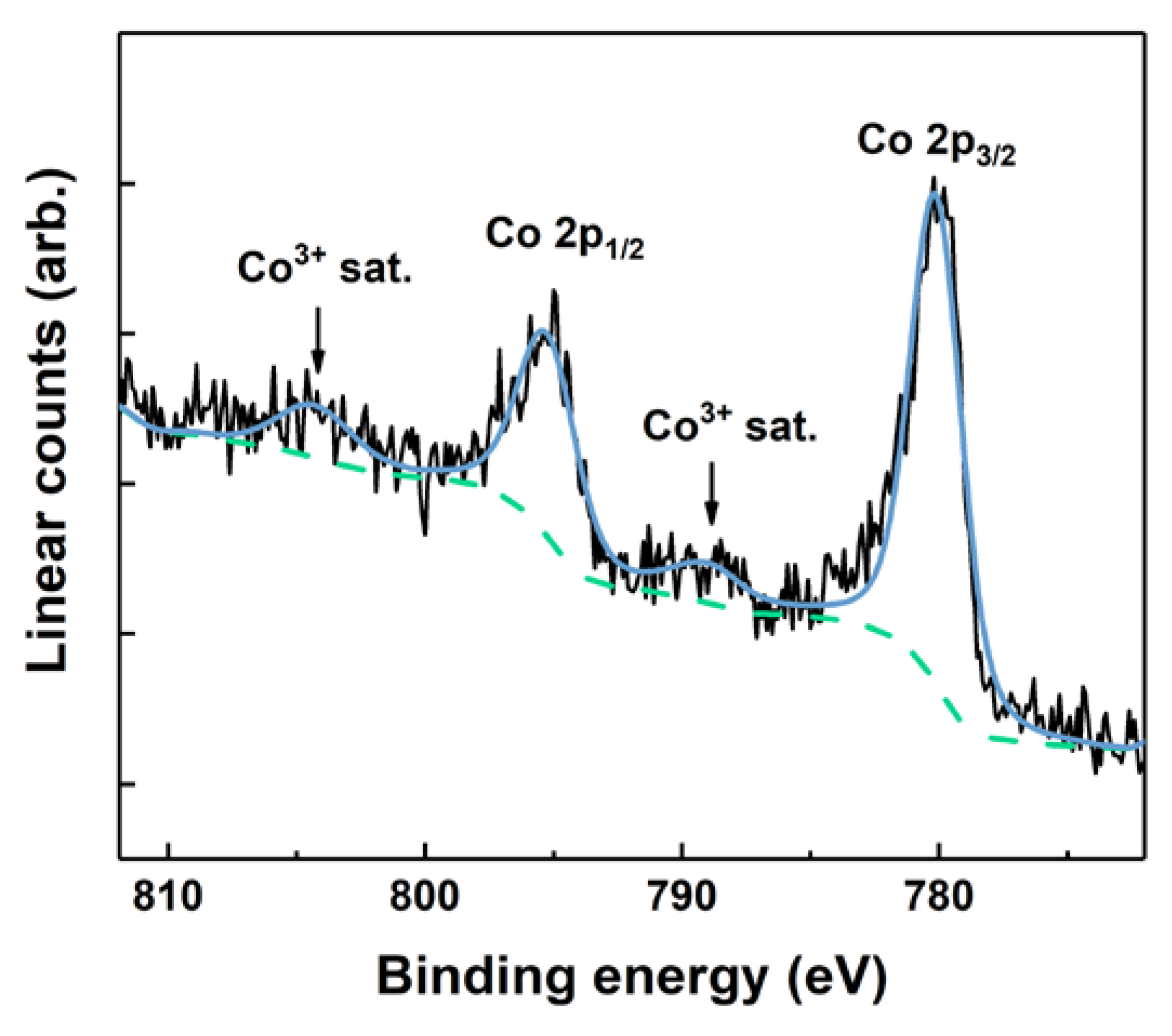
3.3.2. XPS
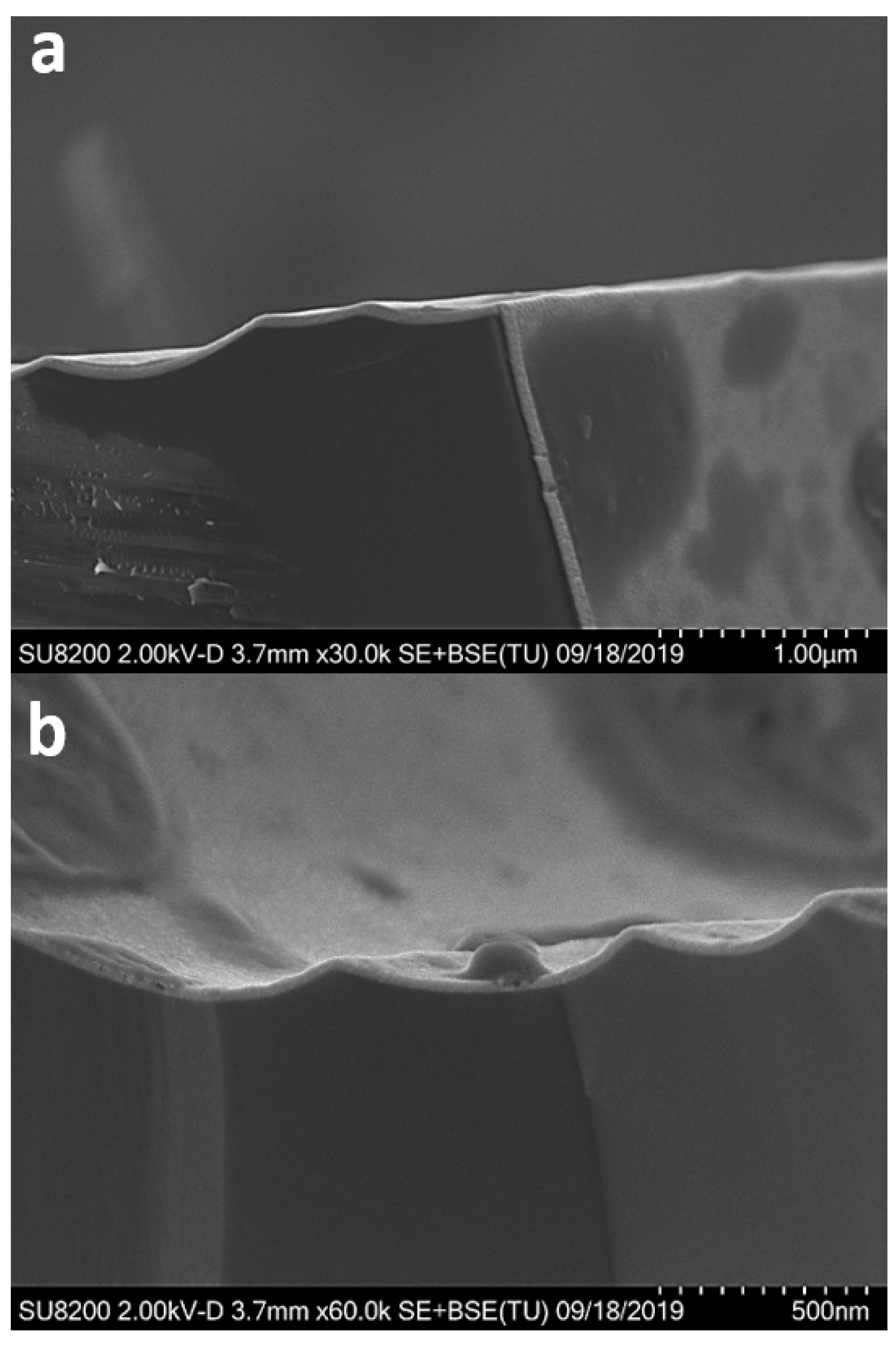
3.3.3. Deposition on a High-Aspect-Ratio Substrate
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Royer, S.; Duprez, D.; Can, F.; Courtois, X.; Batiot-Dupeyrat, C.; Laassiri, S.; Alamdari, H. Perovskites as Substitutes of Noble Metals for Heterogeneous Catalysis: Dream or Reality. Chem. Rev. 2014, 114, 10292–10368. [Google Scholar] [CrossRef] [PubMed]
- Pena, M.A.; Fierro, J.L.G. Chemical Structures and Performance of Perovskite Oxides. Chem. Rev. 2011, 101, 1981–2018. [Google Scholar] [CrossRef] [PubMed]
- Kim, C.H.; Qi, G.; Dahlberg, K.; Li, W. Strontium-doped perovskites rival platinum catalysts for treating NOx in simulated diesel exhaust. Science 2010, 327, 1624–1627. [Google Scholar] [CrossRef] [PubMed]
- Fleming, P.; Farrell, R.A.; Holmes, J.D.; Morris, M.A. The Rapid Formation of La(OH)3 from La2O3 Powders on Exposureto Water Vapor. J. Am. Ceram. Soc. 2010, 93, 1187–1194. [Google Scholar] [CrossRef]
- Waller, D.; Grønvold, M.S.; Sahli, N. Ammonia Oxidation Catalyst for the Production of Nitric Acid Based on Yttrium-Gadolinium Ortho Cobaltates; World Intellectual Property Organization: New York, NY, USA, 2017. [Google Scholar]
- Duparc, M.; et al. 2019; Unpublished manuscript.
- Nagao, M.; Hamano, H.; Hirata, K. Hydration Process of Rare-Earth Sesquioxides Having Different Crystal Structures. Langmuir 2003, 19, 9201–9209. [Google Scholar] [CrossRef]
- Krishnamurthy, N.; Gupta, C.K. Extractive Metallurgy of Rare Earths; CRC Press: Boca Raton, FL, USA, 2004. [Google Scholar]
- Marichy, C.; Bechalany, M.; Pinna, N. Atomic Layer Deposition of Nanostructured Materials for Energy and Environmental Applications. Adv. Mater. 2012, 24, 1017–1032. [Google Scholar] [CrossRef]
- O’Neill, B. Catalyst Design with Atomic Layer Deposition. ACS Catal. 2015, 5, 1804–1825. [Google Scholar] [CrossRef] [Green Version]
- Camacho-Bunquin, J.; Shou, H.; Aich, P.; Beaulieu, D.R.; Klotzsch, H.; Bachman, S.; Marshall, C.L.; Hock, A.; Stair, P. Catalyst synthesis and evaluation using an integrated atomic layer deposition synthesis–catalysis testing tool. Rev. Sci. Instrum. 2015, 86, 84–103. [Google Scholar] [CrossRef] [Green Version]
- Suntola, T. Atomic layer epitaxy. Mater. Sci. Rep. 1989, 4, 261–312. [Google Scholar] [CrossRef]
- Leskelä, M.; Ritala, M. Atomic layer deposition (ALD): From precursors to thin film structures. Thin Solid Films 2002, 409, 138–146. [Google Scholar] [CrossRef]
- George, S.M. Atomic layer deposition: An overview. Chem. Rev. 2009, 110, 111–131. [Google Scholar] [CrossRef] [PubMed]
- Onn, T.M.; Dai, S.; Chen, J.; Pan, X.; Graham, G.W.; Gorte, R.J. High-Surface Area Ceria-Zirconia Films Prepared by Atomic Layer Deposition. Catal. Lett. 2017, 147, 1464–1470. [Google Scholar] [CrossRef]
- Onn, T.M.; Monai, M.; Dai, S.; Arroyo-Ramirez, L.; Zhang, S.; Pan, X.; Graham, G.W.; Fornasiero, P.; Gorte, R.J. High-surface-area, iron-oxide films prepared by atomic layer deposition on γ-Al2O3. Appl. Catal. A Gen. 2017, 534, 70–77. [Google Scholar] [CrossRef] [Green Version]
- Miikkulainen, V.; Leskelä, M.; Ritala, M.; Puurunen, R.L. Crystallinity of inorganic films grown by atomic layer deposition: Overview and general trends. J. Appl. Phys. 2013, 113, 2. [Google Scholar] [CrossRef]
- Sønsteby, H.H.; Fjellvåg, H.; Nilsen, O. Functional Perovskites by Atomic Layer Deposition—An Overview. Adv. Mater. Interfaces 2017, 4, 1600903. [Google Scholar] [CrossRef]
- Johnson, R.W.; Hultqvist, A.; Bent, S.F. A brief review of atomic layer deposition: From fundamentals to applications. Mater. Today 2014, 17, 236–246. [Google Scholar] [CrossRef]
- Ahvenniemi, E.; Matvejeff, M.; Karppinen, M. Atomic layer deposition of quaternary oxide (La,Sr)CoO3−δ thin films. Dalton Trans. 2015, 44, 8001–8006. [Google Scholar] [CrossRef]
- Seim, H.; Nieminen, M.; Niinistö, L.; Fjellvåg, H.; Johansson, L.S. Growth of LaCoO3 thin films from β-diketonate precursors. Appl. Surf. Sci. 1997, 112, 243–250. [Google Scholar] [CrossRef]
- Sønsteby, H.H.; Bratvold, J.E.; Weibye, K.; Fjellvåg, H.; Nilsen, O. Phase Control in Thin Films of Layered Cuprates. Chem. Mater. 2018, 30, 1095–1101. [Google Scholar] [CrossRef]
- Klepper, K.B.; Nilsen, O.; Fjellvåg, H. Growth of thin films of Co3O4 by atomic layer deposition. Thin Solid Fims 2007, 515, 7772–7781. [Google Scholar] [CrossRef]
- Niinistö, J.; Petrova, N.; Putkonen, M.; Niinistö, L.; Arstila, K.; Sajavaara, T. Gadolinium oxide thin films by atomic layer deposition. J. Cryst. Growth 2005, 285, 191–200. [Google Scholar] [CrossRef]
- Sønsteby, H.H.; Østreng, E.; Fjellvåg, H.; Nilsen, O. Deposition and x-ray characterization of epitaxial thin films of LaAlO3. Thin Solid Films 2014, 550, 90–94. [Google Scholar] [CrossRef]
- Leskelä, M.; Ritala, M.; Nilsen, O. Novel materials by atomic layer deposition and molecular layer deposition. MRS Bull. 2011, 36, 877–884. [Google Scholar] [CrossRef]
- Bretos, I.; Ricote, J.; Jiménez, R.; Mendiola, J.; Jiménez Riobóo, R.J.; Calzada, M.L. Crystallisation of Pb1−xCaxTiO3 ferroelectric thin films as a function of the Ca2+ content. J. Eur. Ceram. Soc. 2005, 25, 2325–2329. [Google Scholar] [CrossRef]
- Orlov, Y.S.; Solovyov, L.A.; Dudnikov, V.A.; Fedorov, A.S.; Kuzubov, A.A.; Kazak, N.V.; Voronov, V.N.; Vereshchagin, S.N.; Shishkina, N.N.; Perov, N.S.; et al. Structural properties and high-temperature spin and electronic transitions in GdCoO3: Experiment and theory. Phys. Rev. B 2013, 81, 235105. [Google Scholar] [CrossRef]

© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Duparc, M.; Sønsteby, H.H.; Nilsen, O.; Sjåstad, A.O.; Fjellvåg, H. Atomic Layer Deposition of GdCoO3 and Gd0.9Ca0.1CoO3. Materials 2020, 13, 24. https://doi.org/10.3390/ma13010024
Duparc M, Sønsteby HH, Nilsen O, Sjåstad AO, Fjellvåg H. Atomic Layer Deposition of GdCoO3 and Gd0.9Ca0.1CoO3. Materials. 2020; 13(1):24. https://doi.org/10.3390/ma13010024
Chicago/Turabian StyleDuparc, Marion, Henrik Hovde Sønsteby, Ola Nilsen, Anja Olafsen Sjåstad, and Helmer Fjellvåg. 2020. "Atomic Layer Deposition of GdCoO3 and Gd0.9Ca0.1CoO3" Materials 13, no. 1: 24. https://doi.org/10.3390/ma13010024





