Utilization of Waste Polysilicon Sludge in Concrete
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Mix Proportions and Methodology
3. Results and Discussion
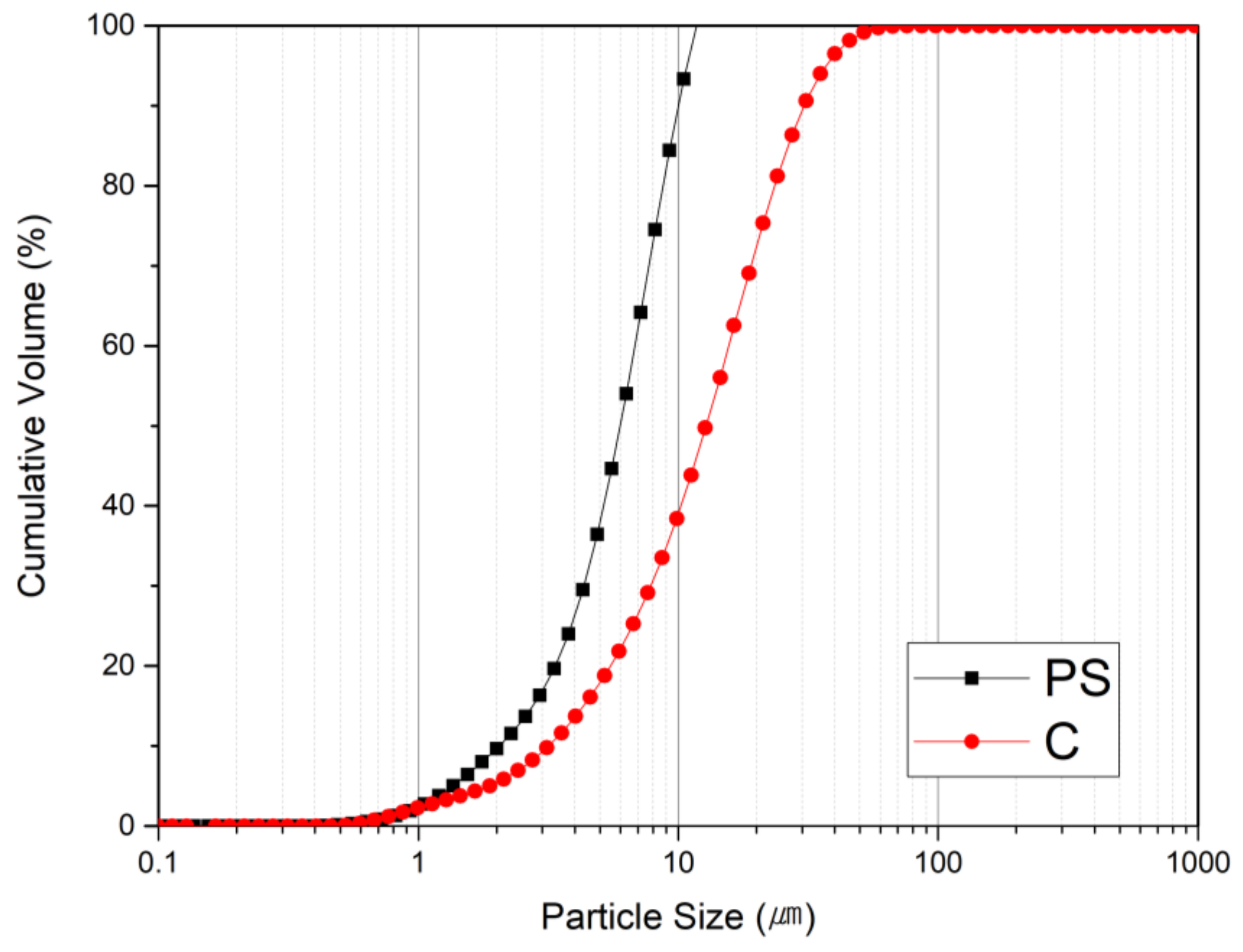
3.1. Properties of Polysilicon Sludge
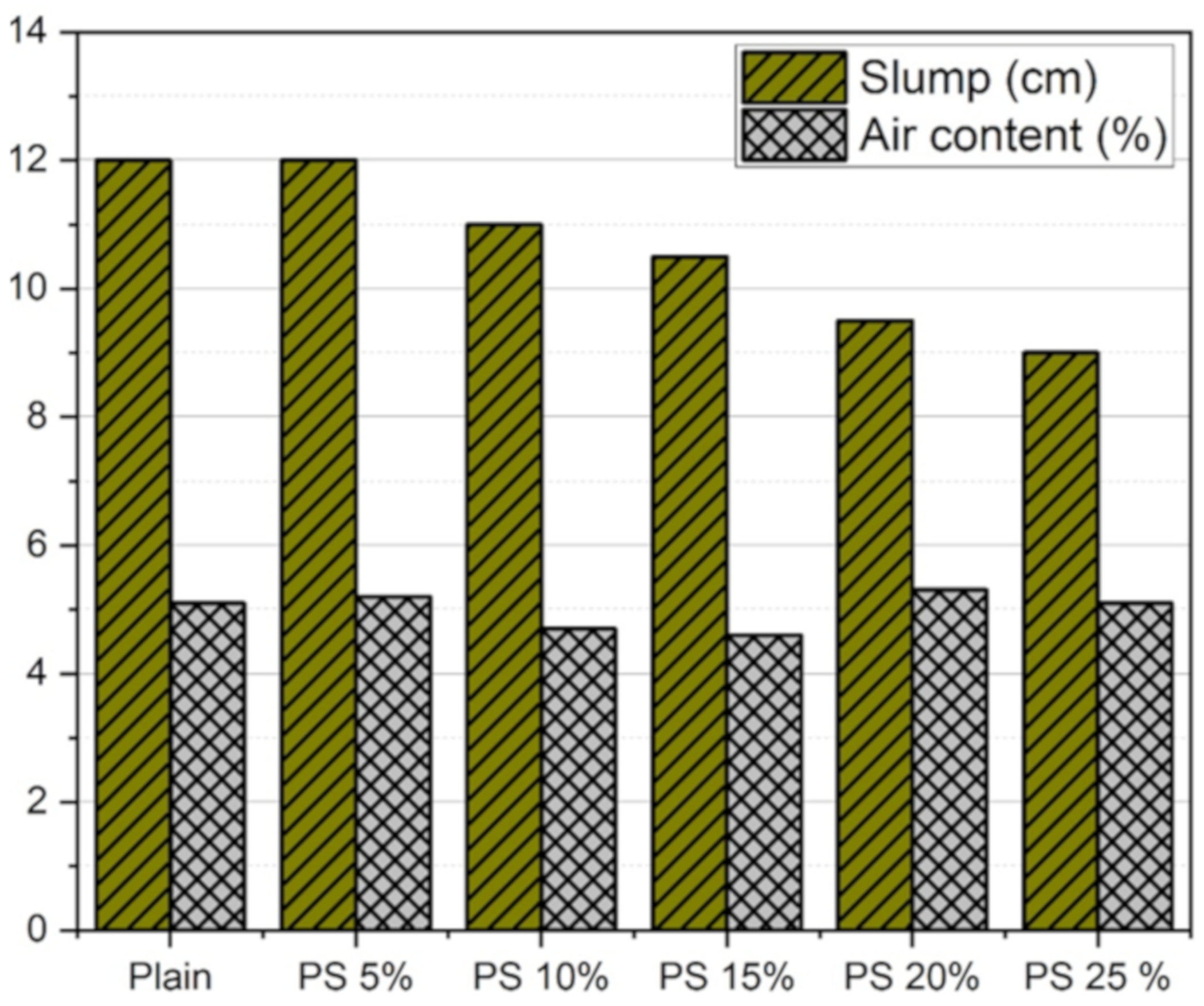
3.2. Fresh Concrete Properties
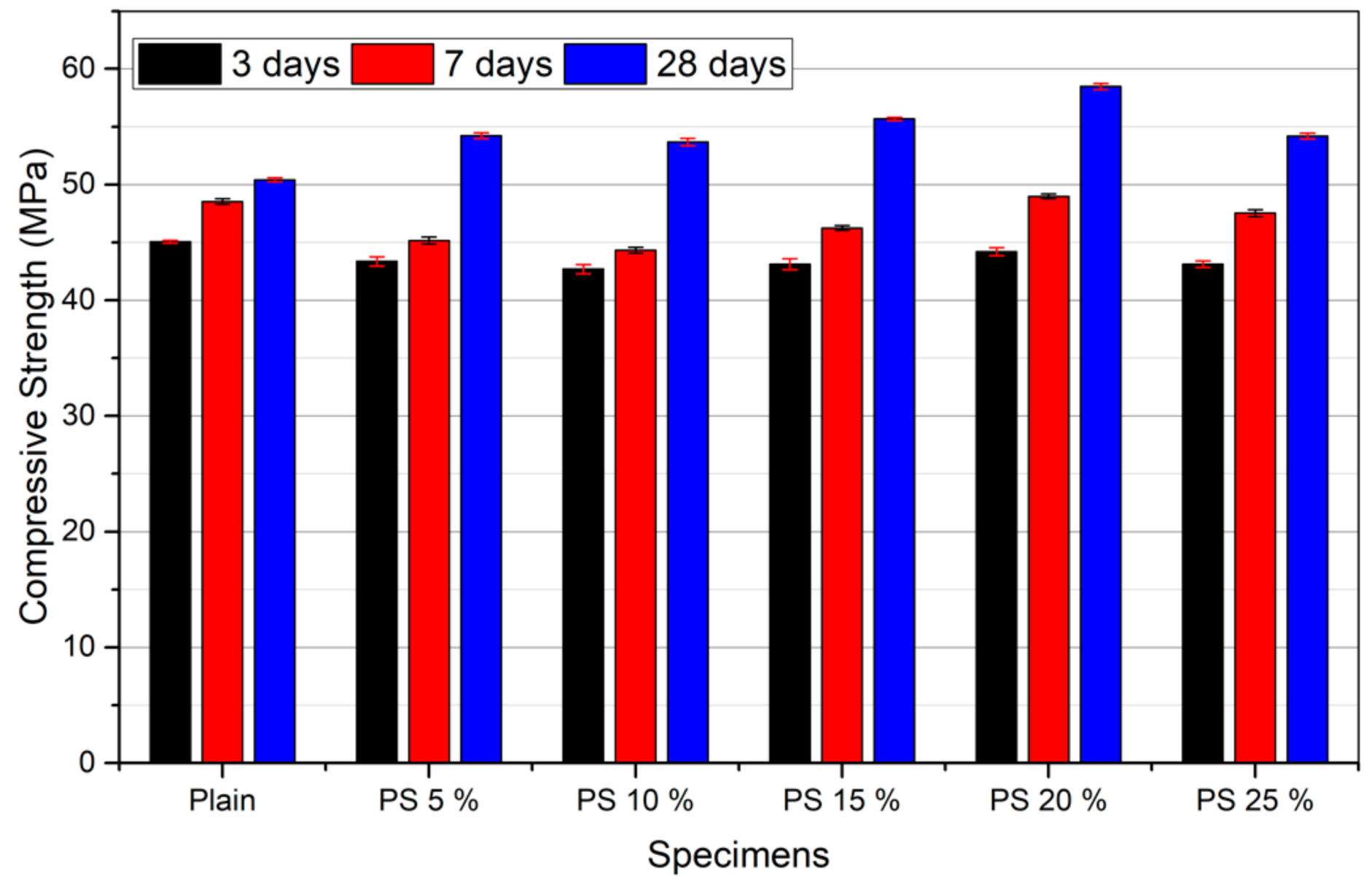
3.3. Compressive Strength
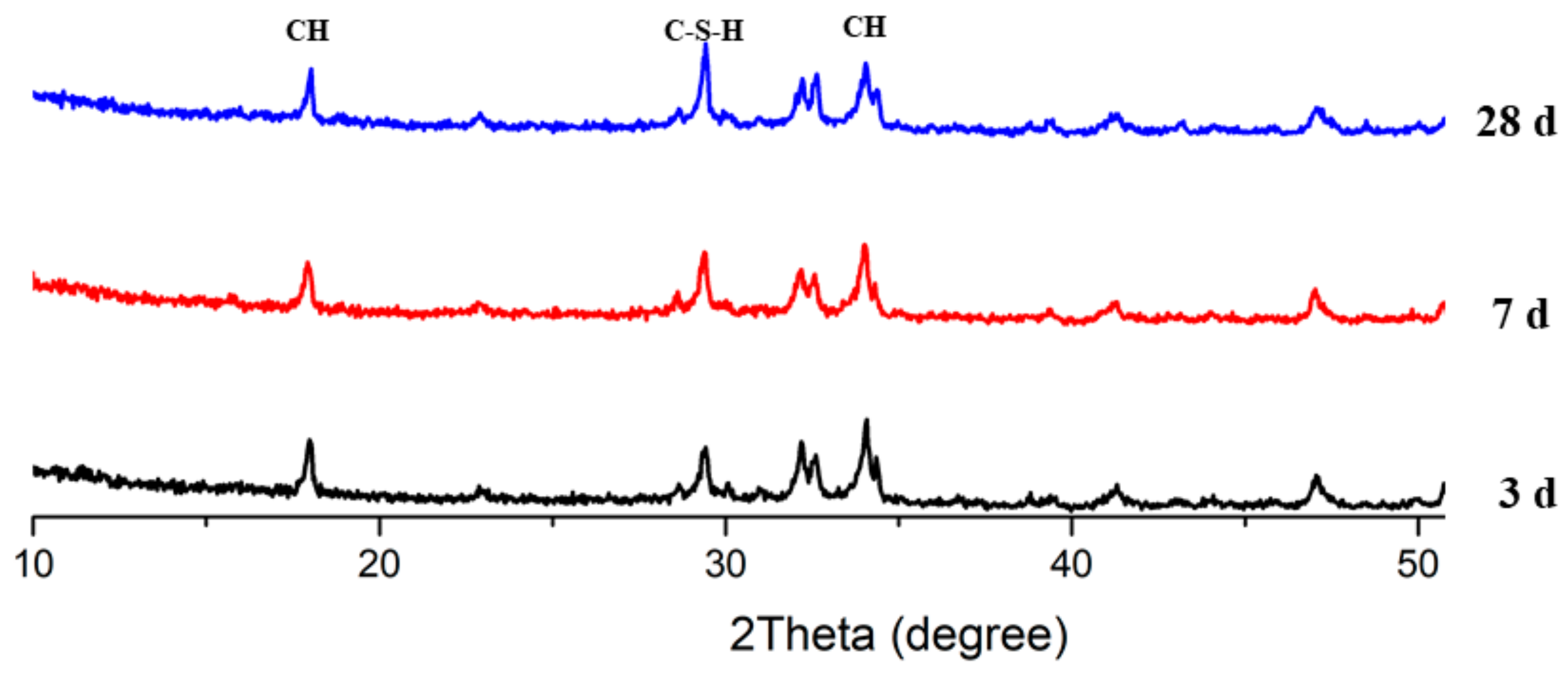
2C2S + 4H2O (H) → C-S-H + Ca(OH)2 (CH),
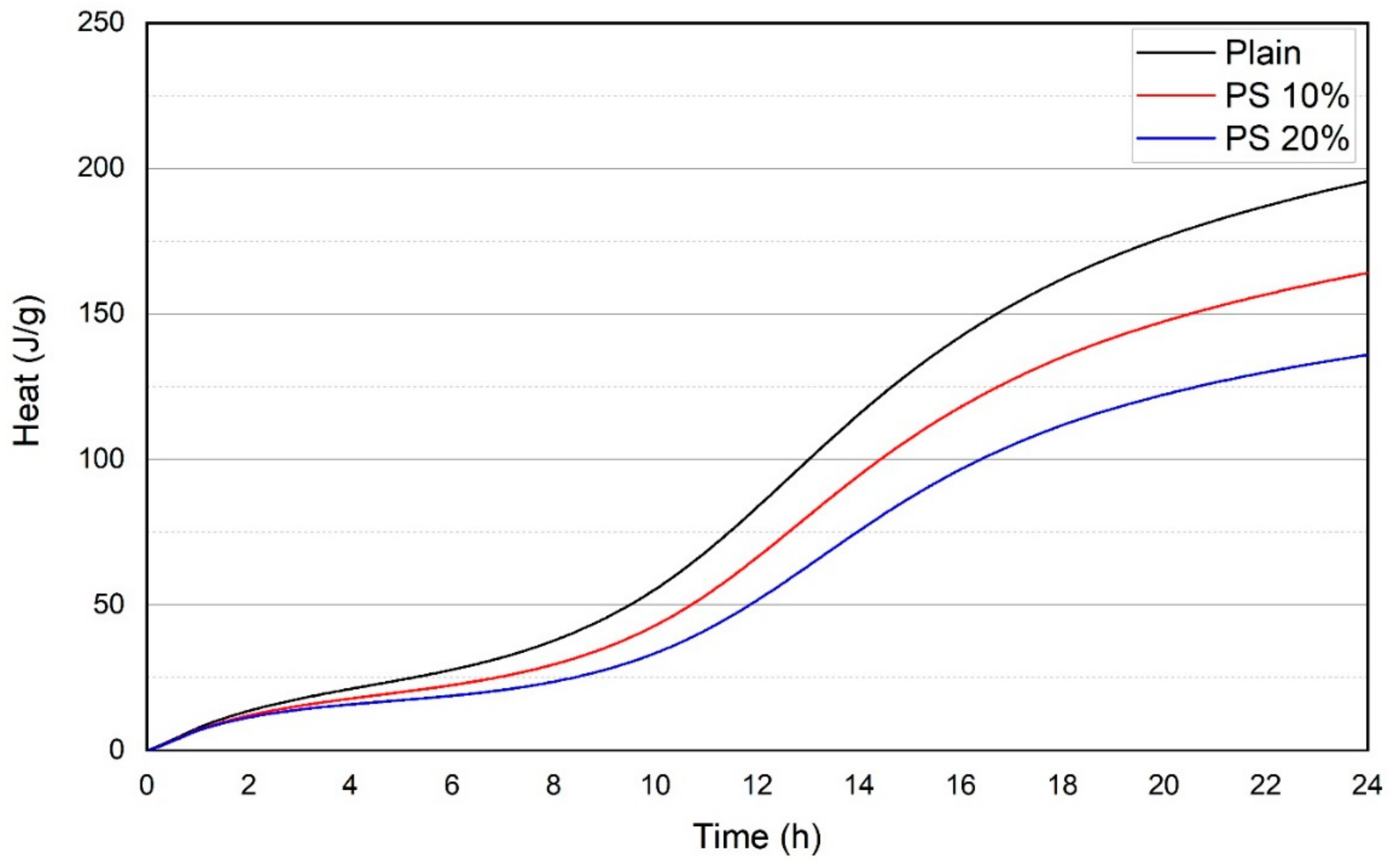
3.4. Heat Release Profiles
3.5. Chloride Penetration
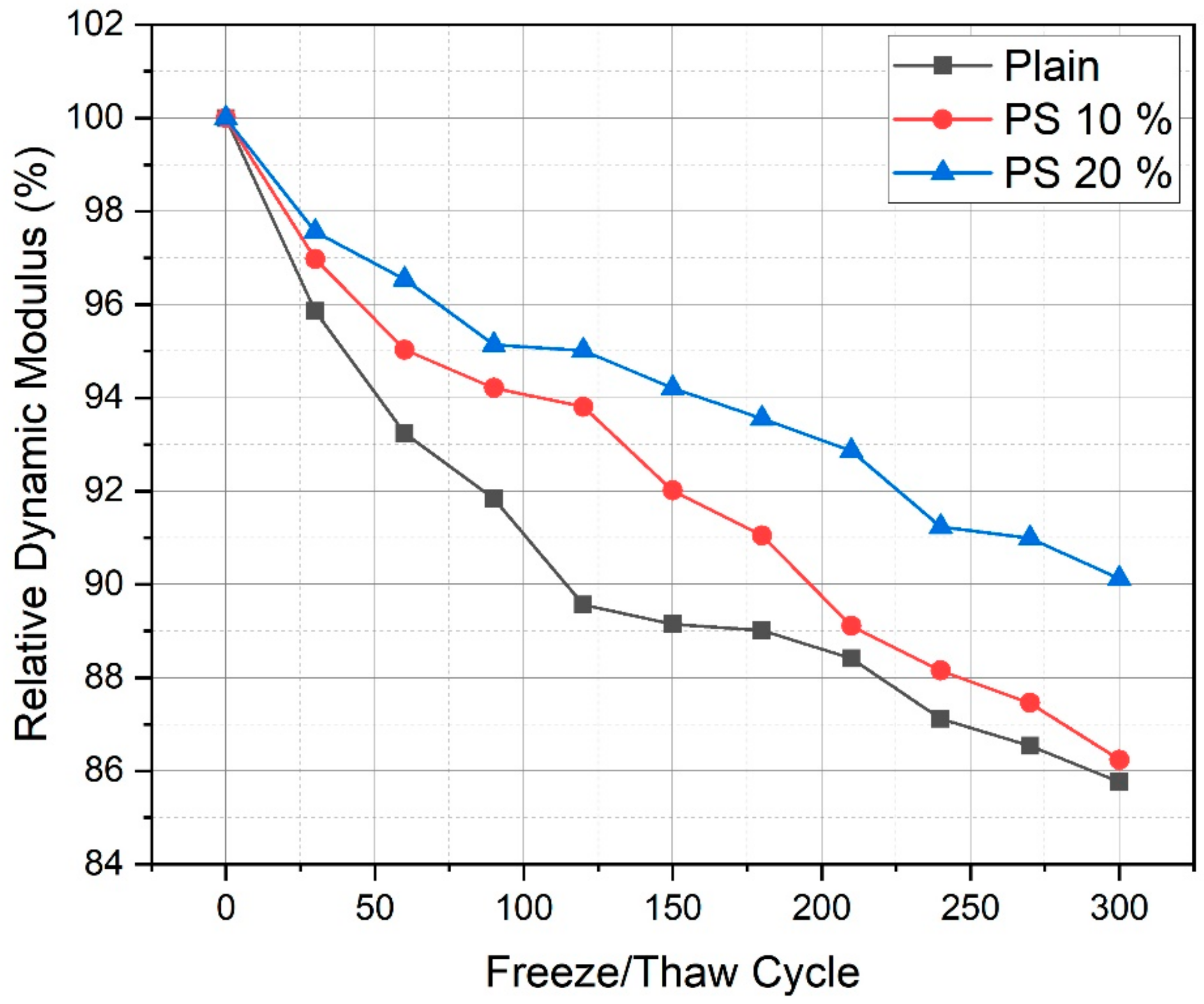
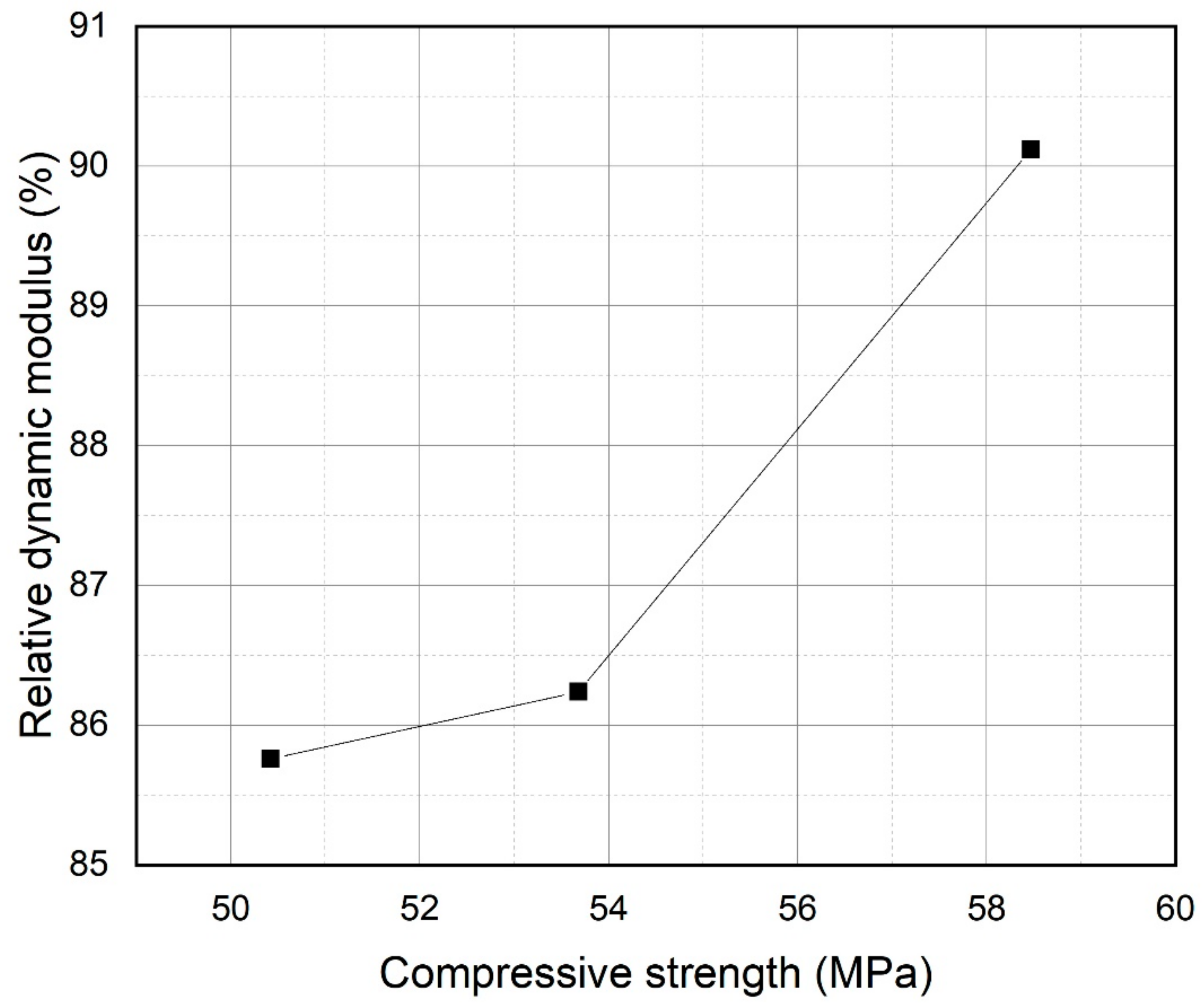
3.6. Freeze/Thaw Resistance
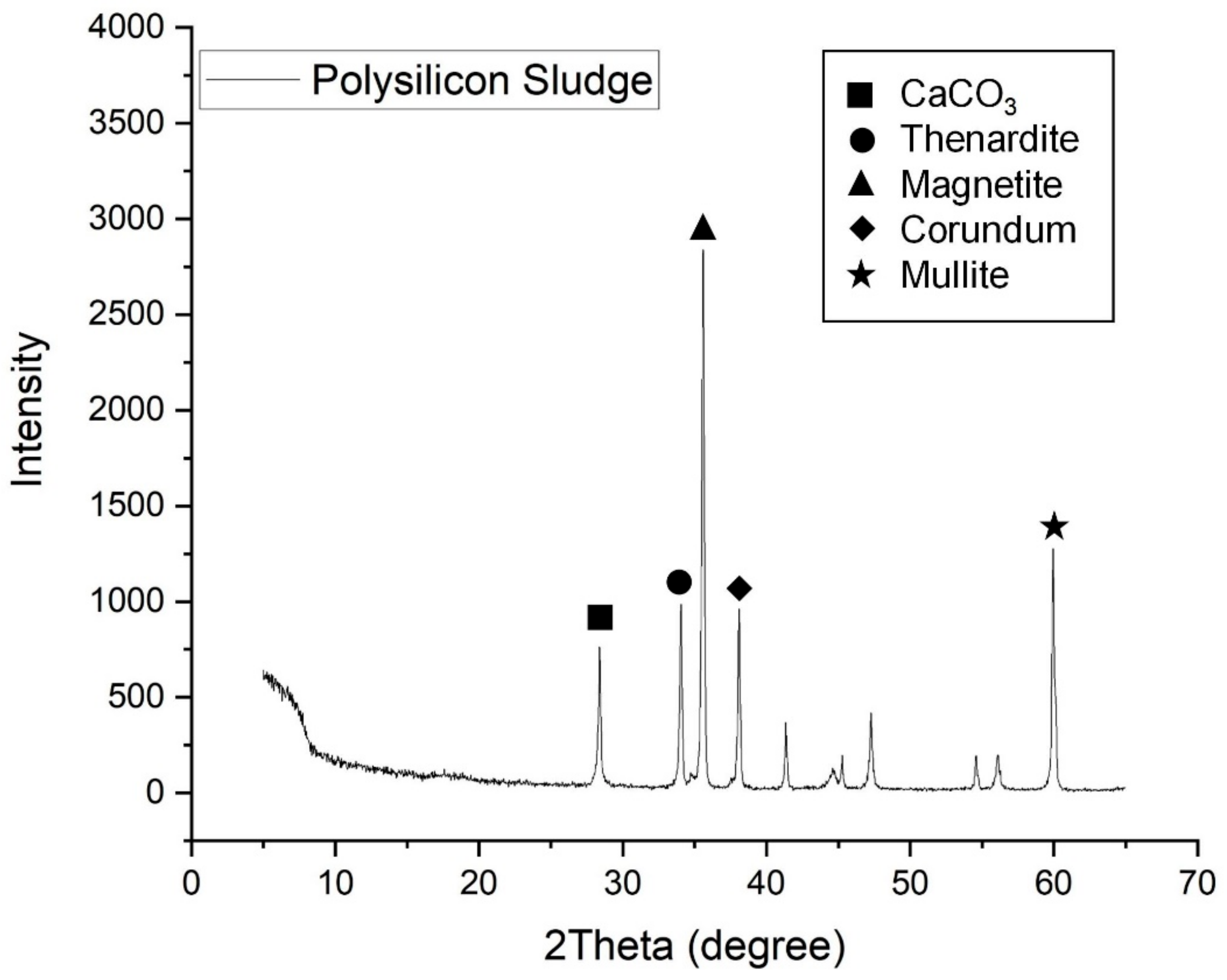
3.7. X-Ray Diffraction Analysis
3.8. Fourier-Transform Infrared Spectroscopy
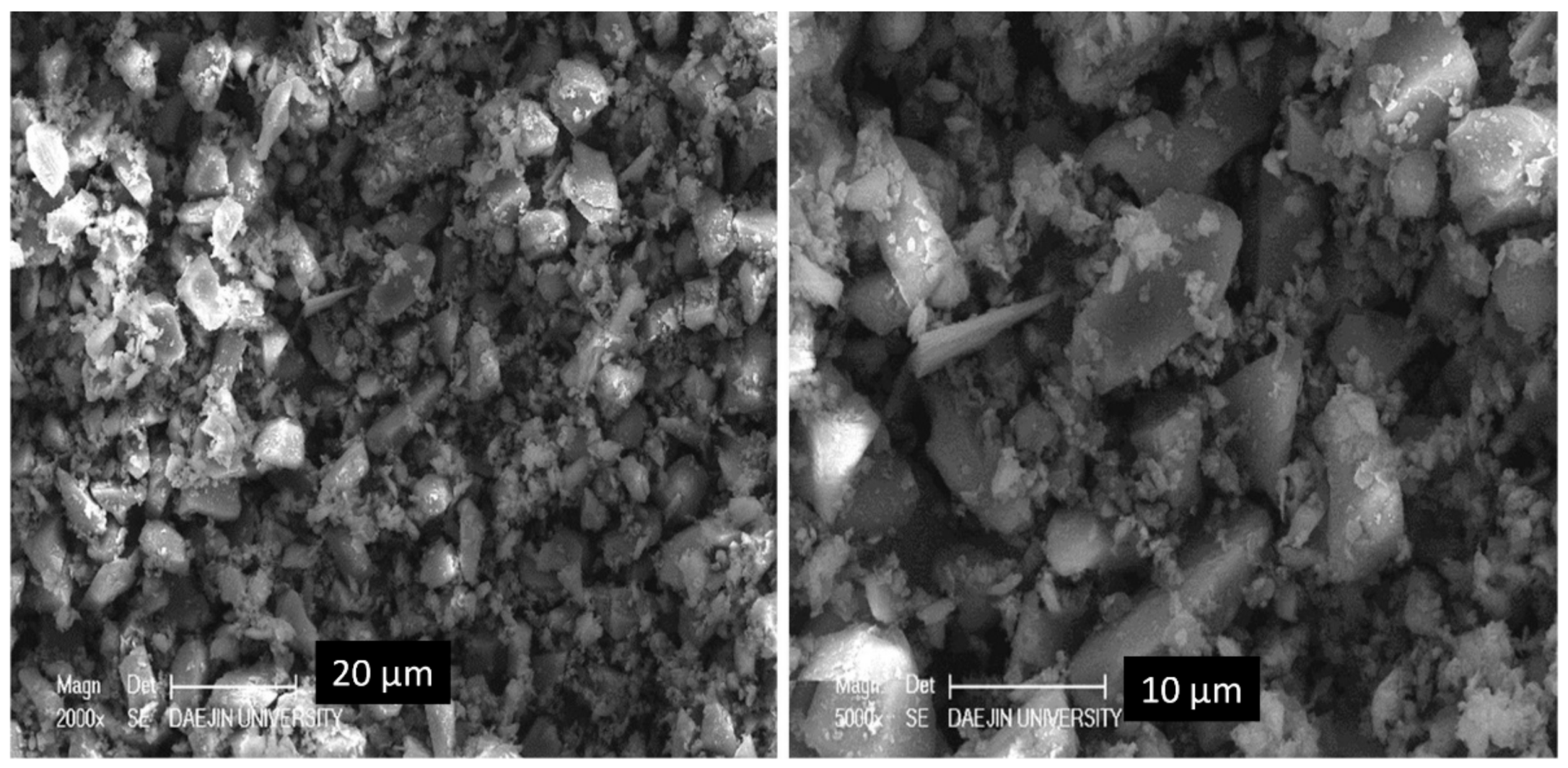
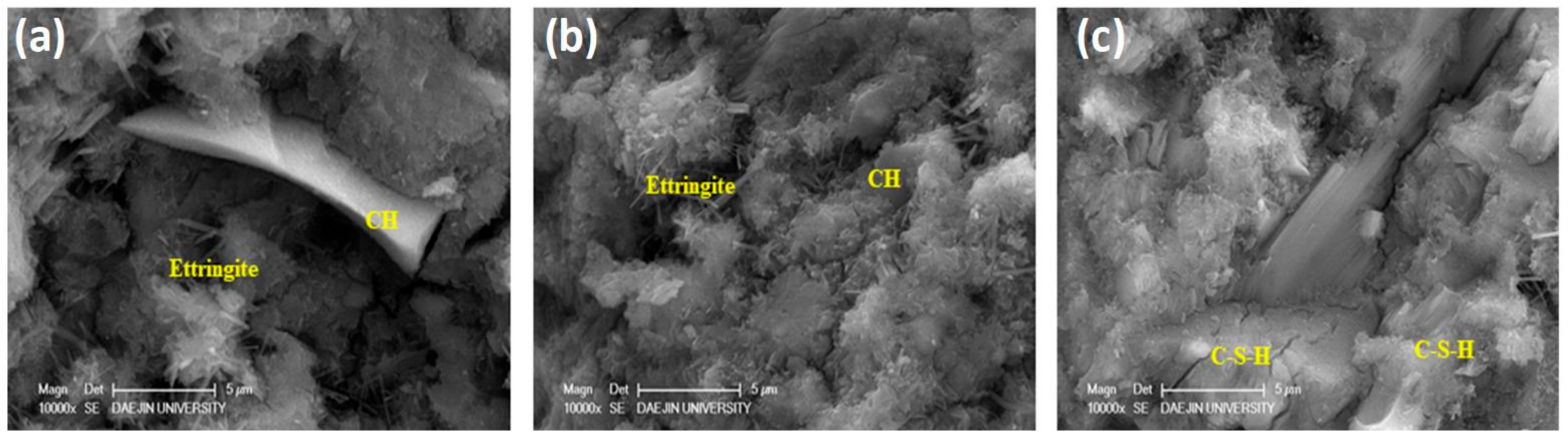
3.9. Scanning Electron Microscopy
4. Conclusions
- Polysilicon sludge particles are finer than cement particles and contain a large amount of SiO2 in amorphous form.
- The incorporation of PS particles as a cement replacement material reduced the early heat of hydration, suggesting a slower reaction rate of PS particles.
- Compressive strength at 28 days, chloride penetration resistance, and freeze/thaw resistance increased with incorporation of PS particles up to a 20% replacement level.
- Polysilicon sludge particles consumed calcium hydroxide and produced additional calcium silica hydrate resulting in an improved microstructure of the concrete specimens.
Author Contributions
Funding
Conflicts of Interest
References
- Shen, W.; Cao, L.; Li, Q.; Zhang, W.; Wang, G.; Li, C. Quantifying CO2 emissions from China’s cement industry. Renew. Sustain. Energy Rev. 2015, 50, 1004–1012. [Google Scholar] [CrossRef]
- Kajaste, R.; Hurme, M. Cement industry greenhouse gas emissions–management options and abatement cost. J. Clean. Prod. 2016, 112, 4041–4052. [Google Scholar] [CrossRef]
- Huang, L.; Krigsvoll, G.; Johansen, F.; Liu, Y.; Zhang, X. Carbon emission of global construction sector. Renew. Sustain. Energy Rev. 2018, 81, 1906–1916. [Google Scholar] [CrossRef] [Green Version]
- Li, B.; Ling, T.-C.; Yu, J.-G.; Wu, J.; Chen, W. Cement pastes modified with recycled glass and supplementary cementitious materials: Properties at the ambient and high temperatures. J. Clean. Prod. 2019, 241, 118155. [Google Scholar] [CrossRef]
- Papadakis, V.; Tsimas, S. Supplementary cementing materials in concrete: Part I: efficiency and design. Cem. Concr. Res. 2002, 32, 1525–1532. [Google Scholar] [CrossRef]
- Poon, C.S.; Lam, L.; Wong, Y.L. Effects of fly ash and silica fume on interfacial porosity of concrete. J. Mater. Civil Eng. 1999, 11, 197–205. [Google Scholar] [CrossRef]
- Mardani-Aghabaglou, A.; Sezer, G.İ.; Ramyar, K. Comparison of fly ash, silica fume and metakaolin from mechanical properties and durability performance of mortar mixtures view point. Constr. Build. Mater. 2014, 70, 17–25. [Google Scholar] [CrossRef]
- Gupta, L.K.; Vyas, A.K. Impact on mechanical properties of cement sand mortar containing waste granite powder. Constr. Build. Mater. 2018, 191, 155–164. [Google Scholar] [CrossRef]
- Usman, M.; Khan, A.Y.; Farooq, S.H.; Hanif, A.; Tang, S.; Khushnood, R.A.; Rizwan, S.A. Eco-friendly self-compacting cement pastes incorporating wood waste as cement replacement: A feasibility study. J. Clean. Prod. 2018, 190, 679–688. [Google Scholar] [CrossRef]
- Aliabdo, A.A.; Elmoaty, A.E.M.A.; Aboshama, A.Y. Utilization of waste glass powder in the production of cement and concrete. Constr. Build. Mater. 2016, 124, 866–877. [Google Scholar] [CrossRef]
- Pahlevani, F.; Sahajwalla, V. From waste glass to building materials–An innovative sustainable solution for waste glass. J. Clean. Prod. 2018, 191, 192–206. [Google Scholar]
- Quercia, G.; Van der Putten, J.; Hüsken, G.; Brouwers, H. Photovoltaic’s silica-rich waste sludge as supplementary cementitious material (SCM). Cem. Concr. Res. 2013, 54, 161–179. [Google Scholar] [CrossRef] [Green Version]
- Kalaw, M.; Culaba, A.; Hinode, H.; Kurniawan, W.; Gallardo, S.; Promentilla, M. Optimizing and characterizing geopolymers from ternary blend of Philippine coal fly ash, coal bottom ash and rice hull ash. Materials 2016, 9, 580. [Google Scholar] [CrossRef] [PubMed]
- Du, H.; Tan, K.H. Properties of high volume glass powder concrete. Cem. Concr. Res. 2017, 75, 22–29. [Google Scholar] [CrossRef]
- Lin, Y.-C.; Wang, T.-Y.; Lan, C.-W.; Tai, C.Y. Recovery of silicon powder from kerf loss slurry by centrifugation. Powder Technol. 2010, 200, 216–223. [Google Scholar] [CrossRef]
- Billiet, R.L.; Nguyen, H.T. Photovoltaic cells from silicon kerf. U.S. Patent 20,030,041,895A1, 6 March 2003. [Google Scholar]
- Green, M.A. Silicon solar cells: evolution, high-efficiency design and efficiency enhancements. Semicond. Sci. Technol. 1993, 8, 1. [Google Scholar] [CrossRef]
- Wang, T.; Lin, Y.; Tai, C.; Sivakumar, R.; Rai, D.; Lan, C. A novel approach for recycling of kerf loss silicon from cutting slurry waste for solar cell applications. J. Crystal Growth 2008, 310, 3403–3406. [Google Scholar] [CrossRef]
- Meng, K.; Guo, H.; Wang, Z.; Li, X.; Su, M.; Huang, B.; Hu, Q.; Peng, W. Self-assembly of porous-graphite/silicon/carbon composites for lithium-ion batteries. Powder Technol. 2014, 254, 403–406. [Google Scholar] [CrossRef]
- Ding, H.; Li, J.; Gao, Y.; Zhao, D.; Shi, D.; Mao, G.; Liu, S.; Tan, X. Preparation of silica nanoparticles from waste silicon sludge. Powder Technol. 2015, 284, 231–236. [Google Scholar] [CrossRef]
- Yamamoto, Y.; Yamada, T.; Satou, T.; Ishizuka, M.; Kinoshita, T.; Yasunaga, N. Development of Polishing Technique for Silicon Wafer; Advanced Materials and Optoelectronics, 3rd Technical report; Sumitomo Osaka Cement Co. Ltd.: Tokyo, Japan, 2002. [Google Scholar]
- Drouiche, N.; Cuellar, P.; Kerkar, F.; Medjahed, S.; Boutouchent-Guerfi, N.; Hamou, M.O. Recovery of solar grade silicon from kerf loss slurry waste. Renew. Sustain. Energy Rev. 2014, 32, 936–943. [Google Scholar] [CrossRef]
- Sinke, W.C.; Ballif, C.; Bett, A.; Dimmler, B.; Dimova-Malinovska, D.; Fath, P.; Mason, N.; Ferrazza, F.; Gabler, H.; Hall, M. A Strategic Research Agenda for Photovoltaic Solar Energy Technology; OPOCE: Brussels, Belgium, 2007. [Google Scholar]
- Lee, T.-C.; Liu, F.-J. Recovery of hazardous semiconductor-industry sludge as a useful resource. J. Hazardous Mater. 2009, 165, 359–365. [Google Scholar] [CrossRef] [PubMed]
- Lee, T.-C. Recycling of municipal incinerator fly-ash slag and semiconductor waste sludge as admixtures in cement mortar. Constr. Build. Mater. 2009, 23, 3305–3311. [Google Scholar] [CrossRef]
- Lee, T.-C.; Lin, K.-L.; Su, X.-W.; Lin, K.-K. Recycling CMP sludge as a resource in concrete. Constr. Build. Mater. 2012, 30, 243–251. [Google Scholar] [CrossRef]
- Chang, C.-J.; Tseng, L.; Lin, T.-S.; Wang, W.-J.; Lee, T.-C. Recycling of modified MSWI ash-mix slag and CMP sludge as a cement substitute and its optimal composition. Indian J. Eng. Mater. Sci. 2012, 19, 31–40. [Google Scholar]
- Yang, Z.; Zhang, Y.; Liu, L.; Seetharaman, S.; Wang, X.; Zhang, Z. Integrated utilization of sewage sludge and coal gangue for cement clinker products: promoting tricalcium silicate formation and trace elements immobilization. Materials 2016, 9, 275. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Du, L.; Folliard, K.J. Mechanisms of air entrainment in concrete. Cem. Concr. Res. 2005, 35, 1463–1471. [Google Scholar] [CrossRef]
- Moon, H.; Ramanathan, S.; Suraneni, P.; Shon, C.-S.; Lee, C.-J.; Chung, C.-W. Revisiting the effect of slag in reducing heat of hydration in concrete in comparison to other supplementary cementitious materials. Materials 2018, 11, 1847. [Google Scholar] [CrossRef] [Green Version]
- Jeon, I.K.; Ryou, J.S.; Jakhrani, S.H.; Kim, H.G. Effects of Light-Burnt Dolomite Incorporation on the Setting, Strength, and Drying Shrinkage of One-Part Alkali-Activated Slag Cement. Materials 2019, 12, 2874. [Google Scholar] [CrossRef] [Green Version]
- 492, NT Build. Concrete, Mortar and Cement-Based Repair Materials: Chloride Migration Coefficient from Non-Steady-State Migration Experiments; Nordtest: Espoo, Finland, 1999. [Google Scholar]
- ASTM, C., 666/C666M-03. Standard Test Method for Resistance of Concrete to Rapid Freezing and Thawing; ASTM International: West Conshohocken, PA, USA, 2008. [Google Scholar]
- Walker, H.N.; Lane, D.S.; Stutzman, P.E. Petrographic Methods of Examining Hardened Concrete: A Petrographic Manual; Virginia Transportation Research Council: Charlottesville, Virginia, July 2006. [Google Scholar]
- Neville, A.M. Properties of Concrete; Longman London: London, UK, 1995; Volume 4. [Google Scholar]
- Mehta, P.K. Pozzolanic and cementitious byproducts as mineral admixtures for concrete-a critical review. Spec. Publication 1983, 79, 1–46. [Google Scholar]
- Memon, A.; Radin, S.; Zain, M.F.M.; Trottier, J.-F. Effects of mineral and chemical admixtures on high-strength concrete in seawater. Cem. Concr. Res. 2002, 32, 373–377. [Google Scholar] [CrossRef]
- Deboucha, W.; Oudjit, M.N.; Bouzid, A.; Belagraa, L. Effect of incorporating blast furnace slag and natural pozzolana on compressive strength and capillary water absorption of concrete. Procedia Eng. 2015, 108, 254–261. [Google Scholar] [CrossRef]
- Cerny, R.; Rovnaníková, P. Transport. Processes in Concrete; CRC Press: Boca Raton, FL, USA, 2002. [Google Scholar]
- ASTM Standards. Standard Practice for Measuring Hydration Kinetics of Hydraulic Cementitious Mixtures Using Isothermal Calorimetry; ASTM, C. 1679-08; Annual Book of ASTM Standards: West Conshohocken, PA, USA, 2008. [Google Scholar]
- Long, W.-J.; Tan, X.-W.; Xiao, B.-X.; Han, N.-X.; Xing, F. Effective use of ground waste expanded perlite as green supplementary cementitious material in eco-friendly alkali activated slag composites. J. Clean. Prod. 2019, 213, 406–414. [Google Scholar] [CrossRef]
- Qudoos, A.; Kim, H.G.; Ryou, J.-S. Effect of mechanical processing on the pozzolanic efficiency and the microstructure development of wheat straw ash blended cement composites. Constr. Build. Mater. 2018, 193, 481–490. [Google Scholar] [CrossRef]
- Leng, F.; Feng, N.; Lu, X. An experimental study on the properties of resistance to diffusion of chloride ions of fly ash and blast furnace slag concrete. Cem. Concr. Res. 2000, 30, 989–992. [Google Scholar] [CrossRef]
- Kong, Y.; Wang, P.; Liu, S.; Zhao, G.; Peng, Y. SEM analysis of the interfacial transition zone between cement-glass powder paste and aggregate of mortar under microwave curing. Materials 2016, 9, 733. [Google Scholar] [CrossRef] [Green Version]
- Fraj, A.B.; Bonnet, S.; Leklou, N.; Khelidj, A. Investigating the early-age diffusion of chloride ions in hardening slag-blended mortars on the light of their hydration progress. Constr. Build. Mater. 2019, 225, 485–495. [Google Scholar] [CrossRef] [Green Version]
- Mehta, P.K.; Monteiro, P.J. Concrete Microstructure, Properties and Materials; McGraw-Hill Education: New York, NY, USA, 2017. [Google Scholar]
- Coussy, O.; Monteiro, P.J. Poroelastic model for concrete exposed to freezing temperatures. Cem. Concr. Res. 2008, 38, 40–48. [Google Scholar] [CrossRef]
- Valenza, J.J.; Scherer, G.W. Mechanism for salt scaling. J. Am. Ceram. Soc. 2006, 89, 1161–1179. [Google Scholar] [CrossRef]
- Valenza II, J.J.; Scherer, G.W. A review of salt scaling: II. Mechanisms. Cem. Concr. Res. 2007, 37, 1022–1034. [Google Scholar] [CrossRef]
- Ramachandran, V.S.; Beaudoin, J.J. Handbook of Analytical Techniques in Concrete Science and Technology: Principles, Techniques and Applications; Elsevier: Amsterdam, The Netherlands, 2000. [Google Scholar]
- Fernández-Carrasco, L.; Torrens-Martín, D.; Morales, L.; Martínez-Ramírez, S. Infrared spectroscopy in the analysis of building and construction materials. Infrared Spectrosc.—Mater. Sci. Eng. Technol. INTECH 2012, 369–382. [Google Scholar] [CrossRef] [Green Version]
- Mollah, M.; Yu, W.; Schennach, R.; Cocke, D.L. A Fourier transform infrared spectroscopic investigation of the early hydration of Portland cement and the influence of sodium lignosulfonate. Cem. Concr. Res. 2000, 30, 267–273. [Google Scholar] [CrossRef]
- Varas, M.; de Buergo, M.A.; Fort, R. Natural cement as the precursor of Portland cement: Methodology for its identification. Cem. Concr. Res. 2005, 35, 2055–2065. [Google Scholar] [CrossRef]
- Cordeiro, G.; Toledo Filho, R.; Tavares, L.; Fairbairn, E. Pozzolanic activity and filler effect of sugar cane bagasse ash in Portland cement and lime mortars. Cem. Concr. Res. 2008, 30, 410–418. [Google Scholar] [CrossRef]






| Description | OPC | PS |
|---|---|---|
| SiO2 (%) | 20.8 | 96.44 |
| TiO2 (%) | - | 0.01 |
| Al2O3 (%) | 6.3 | 0.03 |
| Fe2O3 (%) | 3.2 | 3.276 |
| CaO (%) | 62.0 | 0.06 |
| Cl (%) | - | 0.03 |
| CuO (%) | - | 0.07 |
| ZnO (%) | - | 0.03 |
| MgO (%) | 2.9 | - |
| MnO (%) | - | 0.007 |
| SO3 (%) | 2.1 | 0.03 |
| Ignition loss (%) | 1.5 | - |
| Specific gravity (g/cm3) | 3.15 | 1.95 |
| Surface area (cm2/g) | 3410 | 7122 |
| Description | Fine Aggregate | Coarse Aggregate |
|---|---|---|
| Gmax (mm) | 4.75 | 25 |
| Gmin (mm) | 0.075 | 4.75 |
| Density (g/cm3) | 2.62 | 2.66 |
| Absorption rate (%) | 1.05 | 0.72 |
| Fineness modulus (FM) | 2.70 | 6.91 |
| Abrasion rate (AR) (%) | - | 25.1 |
| Unit volume mass (g/cm3) | - | 1.564 |
| - | Specific Gravity | pH (25 °C) | Cl− Content (%) | Alkali Content (%) | Color/Type | Usage |
|---|---|---|---|---|---|---|
| SP | 1.06 ± 0.05 | 6.5 ± 1.0 | < 0.01 | < 0.02 | Brown/liquid | C × 0.5% |
| AE | 1.04 ± 0.01 | - | - | - | Translucent/liquid | C × 0.2% |
| Types | W/B (%) | S/A (%) | Unit Weight (kg/m3) | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Water | OPC | Sand | Gravel | PS | AE | SP | |||
| Plain | 40.0 | 48 | 175 | 337 | 859 | 919 | - | 0.68 (C × 0.2%) | 1.69 (C × 0.5%) |
| PS 5% | 320 | 17 | |||||||
| PS 10% | 303 | 34 | |||||||
| PS 15% | 286 | 51 | |||||||
| PS 20% | 270 | 67 | |||||||
| PS 25% | 253 | 84 | |||||||
| Concrete Mix | Pozzolanic Activity Index (PAI) | ||
|---|---|---|---|
| 3 days | 7 days | 28 days | |
| Plain | 100 | 100 | 100 |
| PS 5% | 96.2 | 93.0 | 107.6 |
| PS 10% | 94.7 | 91.3 | 106.5 |
| PS 15% | 95.7 | 95.3 | 110.4 |
| PS 20% | 98.1 | 100.9 | 116.0 |
| PS 25% | 95.7 | 97.9 | 107.5 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Qudoos, A.; Jeon, I.K.; Kim, S.S.; Lee, J.B.; Kim, H.G. Utilization of Waste Polysilicon Sludge in Concrete. Materials 2020, 13, 251. https://doi.org/10.3390/ma13010251
Qudoos A, Jeon IK, Kim SS, Lee JB, Kim HG. Utilization of Waste Polysilicon Sludge in Concrete. Materials. 2020; 13(1):251. https://doi.org/10.3390/ma13010251
Chicago/Turabian StyleQudoos, Abdul, In Kyu Jeon, Seong Soo Kim, Jeong Bae Lee, and Hong Gi Kim. 2020. "Utilization of Waste Polysilicon Sludge in Concrete" Materials 13, no. 1: 251. https://doi.org/10.3390/ma13010251




