Sulfonic Acid Derivative-Modified SBA-15, PHTS and MCM-41 Mesoporous Silicas as Carriers for a New Antiplatelet Drug: Ticagrelor Adsorption and Release Studies
Abstract
:1. Introduction
2. Materials and Methods
2.1. Reagents and Materials
2.2. Preparation of Mesoporous Adsorbents
2.2.1. SBA-15 Synthesis
2.2.2. PHTS Synthesis
2.3. Functionalization of Siliceous Matrices
2.4. Ticagrelor Adsorption Experiments
2.5. Modeling of Asorption Eperimental Data
2.6. Preparation of Dosage Forms
2.7. Ticagrelor Release Studies
2.8. Ticagrelor Release Modeling
2.9. Instrumentation
3. Results and Discussion
3.1. Characterization of Mesoporous Adsorbents
3.2. Adsorption Studies
3.3. Estimation of Isotherm Parameters Using Nonlinear Fitting Analysis
3.4. Release Studies
3.5. Ticagrelor Release Modeling
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
List of Abbreviations and Symbols
| a | scale parameter of Weibull equation |
| aRP | constant of Redlich-Peterson isotherm (dm3β/mgβ) |
| ARE | average relative error (%) |
| b | shape parameter of Weibull equation |
| bT | Temkin constant related to the adsorption heat (J g/mol mg) |
| BET | Brunauer-Emmett-Teller isotherm |
| BJH | Barrett-Joyner-Halenda isotherm |
| β | exponential constant of Redlich-Peterson isotherm |
| C0 | initial adsorbate concentration (mg/dm3) |
| Ce | equilibrium adsorbate concentration (mg/dm3) |
| Cs | solubility (mg/dm3) |
| DSC | differential scanning calorimetry |
| Eads | efficiency of adsorption (%) |
| EDA | adsorption energy calculated from Dubinin-Astakhov model (J/mol) |
| EDR | adsorption energy calculated from Dubinin-Radushkevich model (J/mol) |
| F | fraction of drug release |
| Fcalc. | fraction of drug release (calculated) |
| Fexp. | fraction of drug release (experimental data) |
| KDA | constant of Dubinin-Astakhov isotherm related to the adsorption energy (molnDA/JnDA) |
| KDR | constant of Dubinin-Radushkevich isotherm related to the adsorption energy (mol2/J2) |
| KF | Freundlich constant (mg1-1/ndm3/n/g) |
| KJ | Jovanovich constant (dm3/mg) |
| KL | Langmuir constant (dm3/mg) |
| KRP | constant of Redlich-Peterson isotherm (dm3/g) |
| KT | Temkin binding constant (dm3/mg) |
| m | mass of adsorbent (g) |
| MTGR | molar weight of ticagrelor molecule (g/mol) |
| MPTMS | (3-mercaptopropyl)trimethoxysilane |
| nTGR | number of ticagrelor molecules (mol) |
| nDA | heterogeneity factor of Dubinin-Astakhov isotherm |
| nF | exponential constant of Freundlich equation |
| n-(CH2)3-SO3H | number of propyl-sulfonic acid groups (mol) |
| p/p0 | relative pressure |
| Qads(max) | maximum adsorption capacity calculated from given isotherm model (mg/g) |
| QDA(max) | maximum adsorption capacity calculated from Dubinin-Astakhov isotherm (mg/g) |
| QDR(max) | maximum adsorption capacity calculated from Dubinin-Radushkevich equation (mg/g) |
| Qe | amount of adsorbate in equilibrium solid state (mg/g) |
| Qe,calc. | amount of adsorbate in equilibrium solid state (calculated) (mg/g) |
| Qe,exp. | amount of adsorbate in equilibrium solid state (experimental data) (mg/g) |
| QJ(max) | maximum adsorption capacity calculated from Jovanovich equation (mg/g) |
| QL(max) | maximum adsorption capacity calculated from Langmuir equation (mg/g) |
| Qs(max) | surface area-normalized adsorption capacity (mg/m2) |
| Q-(CH2)3-SO3H | content of propyl-sulfonic acid groups (mol/g) |
| R | gas constant (8.314 J/mol K) |
| SBET | specific surface area calculated from BET model (m2/g) |
| STP | standard temperature and pressure |
| T | absolute temperature (Kelvin) |
| t | time (h) |
| TEM | transmission electron microscopy |
| TEOS | tetraethyl orthosilicate |
| TGR | ticagrelor |
References
- Dobesh, P.P.; Oestreich, J.H. Ticagrelor: Pharmacokinetics, pharmacodynamics, clinical efficacy, and safety. Pharmacotherapy 2014, 34, 1077–1090. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Deeks, E.D. Ticagrelor. A review of its use in the management of acute coronary syndromes. Drugs 2011, 71, 909–933. [Google Scholar] [CrossRef] [PubMed]
- Nylander, S.; Femia, A.E.; Scavone, M.; Bertsson, P.; Asztély, A.-K.; Nelander, K.; Löfgren, L.; Nilsson, R.G.; Cattaneo, M. Ticagrelor inhibits human platelet aggregation via adenosine in addition to P2Y12 antagonism. J. Thromb. Haemost. 2013, 11, 1867–1876. [Google Scholar] [PubMed]
- Cattaneo, M.; Schulz, R.; Nylander, S. Adenosine-mediated effects of ticagrelor. J. Am. Coll. Cardiol. 2014, 63, 2503–2508. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wittfeldt, A.; Emanuelsson, H.; Brandrup-Wognsen, G.; Van Giezen, J.J.J.; Jonasson, J.; Nylander, S.; Gan, L.-M. Ticagrelor enhances adenosine-induced coronary vasodilatory responses in humans. J. Am. Coll. Cardiol. 2013, 61, 723–727. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nylander, S.; Schulz, R. Effects of P2Y12 receptor antagonists beyond platelet inhibition—Comparison of ticagrelor with thienopyridines. Brit. J. Pharmacol. 2016, 173, 1163–1178. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Storey, R.F.; Angiolillo, D.J.; Bonaca, M.P.; Thomas, M.R.; Judge, H.M.; Rollini, F.; Franchi, F.; Ahsan, A.J.; Bhatt, D.L.; Kuder, J.F.; et al. Platelet inhibition with ticagrelor 60 mg versus 90 mg twice daily in the PEGASUS-TIMI 54 trial. J. Am. Coll. Cardiol. 2016, 67, 1145–1154. [Google Scholar] [CrossRef] [Green Version]
- Gurbel, P.A.; Bliden, K.P.; Butler, K.; Antonino, M.J.; Wei, C.; Teng, R.; Rasmussen, L.; Storey, R.F.; Nielsen, T.; Eikelboom, J.W.; et al. Response to ticagrelor in clopidogrel nonresponders and responders and effect of switching therapies. The RESPOND study. Circulation 2010, 121, 1188–1199. [Google Scholar] [CrossRef] [Green Version]
- Son, G.-H.; Na, Y.-G.; Huh, H.W.; Wang, M.; Kim, M.-K.; Han, M.-G.; Byeon, J.-J.; Lee, H.-K.; Cho, C.-W. Systemic design and evaluation of ticagrelor-loaded nanostructured lipid carriers for enhancing bioavailability and anitplatelet activity. Pharmaceutics 2019, 11, 222. [Google Scholar] [CrossRef] [Green Version]
- Goto, S.; Huang, C.-H.; Park, S.-J.; Emanuelsson, H.; Kimura, T. Ticagrelor vs. clopidogrel in Japanese, Korean and Taiwanese patients with acute coronary syndrome. Circ. J. 2015, 79, 2452–2460. [Google Scholar] [CrossRef] [Green Version]
- Storey, R.F.; Bliden, K.P.; Patil, S.B.; Karunakaran, A.; Ecob, R.; Butler, K.; Teng, R.; Wei, C.; Tantry, U.S.; Gurbel, P.A. Incidence of dyspnea and assessment of cardiac and pulmonary function in patients with stable coronary artery disease receiving ticagrelor, clopidogrel, or placebo in the ONSET/OFFSET study. J. Am. Coll. Cardiol. 2010, 56, 185–193. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Parodi, G.; Storey, R.F. Dyspnoea management in acute coronary syndrome patients treated with ticagrelor. Eur. Heart J. Acute Cardiovasc. Care 2014, 4, 555–560. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Parodi, G.; Xanthopoulou, I.; Bellandi, B.; Gkizas, V.; Valenti, R.; Karanikas, S.; Migliorini, A.; Angelidis, C.; Abbate, R.; Patsilinakos, S.; et al. Ticagrelor crushed tablets administration in STEMI patients. J. Am. Coll. Cardiol. 2015, 65, 511–512. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vallet-Regí, M.; Rámilla, A.; del Real, R.P.; Pérez-Pariente, J. A new property of MCM-41: Drug delivery system. Chem. Mater. 2001, 13, 308–311. [Google Scholar] [CrossRef]
- Meynen, V.; Cool, P.; Vansant, E.F. Verified syntheses of mesoporous materials. Microporous Mesoporous Mater. 2009, 125, 170–223. [Google Scholar] [CrossRef]
- Díaz, A.; López, T.; Manjarrez, J.; Basaldella, E.; Martínez-Blanes, J.M.; Odriozola, J.A. Growth of hydroxyapatite in a biocompatible mesoporous ordered silica. Acta Biomater. 2006, 2, 173–179. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Zhu, I.; Zhao, T.; Lan, J.; Yan, W.; Zhang, H. Synthesis and characterization of sulfonic acid-functionalized SBA-15 for adsorption of biomolecules. Microporous Mesoporous Mater. 2011, 142, 614–620. [Google Scholar] [CrossRef]
- Moritz, M.; Łaniecki, M. SBA-15 mesoporous material modified with APTES as the carrier for 2-(3-benzoylphenyl)propionic acid. Appl. Surf. Sci. 2012, 258, 7523–7529. [Google Scholar] [CrossRef]
- Hu, Y.; Wang, J.; Zhi, Z.; Jiang, T.; Wang, S. Facile synthesis of 3D cubic mesoporous silica microspheres with a controllable pore size and their application for improved delivery of a water-insoluble drug. J. Colloid Interface Sci. 2011, 363, 410–417. [Google Scholar] [CrossRef]
- Marques, I.J.; Vaz, P.D.; Fernandes, A.C.; Nunes, C.D. Advantageous delivery of nifedipine from inorganic materials showing increased solubility and biobompatibility. Microporous Mesoporous Mater. 2014, 183, 192–200. [Google Scholar] [CrossRef]
- Zhao, Q.; Wang, T.; Wang, J.; Zheng, L.; Jiang, T.; Cheng, G.; Wang, S. Template-directed hydrothermal synthesis of hydroxyapatite as a drug delivery system for the poorly water-soluble drug carvedilol. Appl. Surf. Sci. 2011, 257, 10126–10133. [Google Scholar] [CrossRef]
- Basaldella, E.I.; Legnoverde, M.S. Functionalized silica matrices for controlled delivery of cephalexin. J. Sol-Gel Sci. Technol. 2010, 56, 191–196. [Google Scholar] [CrossRef]
- Doadrio, A.L.; Doadrio, J.C.; Sànchez-Montero, J.M.; Salinas, A.J.; Vallet-Regí, M. A rational explanation of the vankomycin release from SBA-15 and its derivative by molecular modelling. Microporous Mesoporous Mater. 2010, 132, 559–566. [Google Scholar] [CrossRef]
- Moritz, M. Solvent opitimization for niacinamide adsorption on organo-functionalized SBA-15 mesoporous silica. Appl. Surf. Sci. 2013, 283, 537–545. [Google Scholar] [CrossRef]
- Wu, Z.; Jiang, Y.; Kim, T.; Lee, K. Effects of surface coating on the controlled release of vitamin B1 from mesoporous silica tablets. J. Control. Release 2007, 119, 215–221. [Google Scholar] [CrossRef] [PubMed]
- Xu, Z.; Ji, Y.; Guan, M.; Huang, H.; Zhao, C.; Zhang, H. Preparation and characterization of L-leucine-modified amphiprotic bifunctional mesoporous SBA-15 molecular sieve as a drug carrier for ribavirin. Appl. Surf. Sci. 2010, 256, 3160–3165. [Google Scholar] [CrossRef]
- Tang, Q.; Xu, Y.; Wu, D.; Sun, Y. pH-Controlled drug release from mesoporous silica tablets coated with hydroxypropyl methylcellulose phthalate. Mater. Res. Bull. 2006, 44, 606–612. [Google Scholar]
- Colila, M.; Izquierdo-Barba, I.; Vallet-Regí, M. Phosphorus-containing SBA-15 materials as bisphosphonate carriers for osteoporosis treatment. Microporous Mesoporous Mater. 2010, 135, 51–59. [Google Scholar] [CrossRef]
- Moritz, M.; Geszke-Moritz, M. The effect of SBA-15 surface modification on the process of 18β-glycyrrhetinic acid adsorption: Modeling of experimental adsorption isotherm data. Materials 2019, 12, 3671. [Google Scholar] [CrossRef] [Green Version]
- Baeza, A.; Guisasola, E.; Ruiz-Hernández, E.; Vallet-Regí, M. Magnetically triggered multidrug release by hybrid mesoporous silica nanoparticles. Chem. Mater. 2012, 24, 517–524. [Google Scholar] [CrossRef]
- Coll, C.; Mondragón, I.; Martínez-Máñez, R.; Sancenón, F.; Marcos, M.D.; Soto, J.; Amarós, P.; Pérez-Payá, E. Enzyme-mediated controlled release systems by anchoring peptide sequences on mesoporous silica supports. Angew. Chem. Int. Ed. 2011, 50, 2138–2140. [Google Scholar] [CrossRef] [PubMed]
- Yang, S.; Li, N.; Chen, D.; Qi, X.; Xu, Y.; Li, H.; Lu, J. Visible-light degradable polymer coated hollow mesoporous silica nanoparticles for controlled drug release and cell imaging. J. Mater. Chem. B 2013, 1, 4628–4636. [Google Scholar] [CrossRef] [PubMed]
- Van der Voort, P.; Ravikovitch, P.I.; de Jong, K.P.; Neimark, A.V.; Janssen, A.H.; Benjelloun, M.; van Bavel, E.; Cool, P.; Weckhuysen, B.M.; Vansant, E.F. Plugged hexagonal templated silica: A unique micro- and mesoporous composite material with internal silica nanocapsules. Chem. Commun. 2002, 9, 1010–1011. [Google Scholar] [CrossRef]
- Li, Q.; Wu, Z.; Feng, D.; Tu, B.; Zhao, D. Hydrothermal stability of mesostructured cellular silica foams. J. Phys. Chem. C 2010, 114, 5012–5019. [Google Scholar] [CrossRef]
- Bavel, E.V.; Cool, P.; Aerts, K.; Vansant, E.V. Plugged hexagonal templated silica (PHTS): An in-depth study of the structural characteristics. J. Phys. Chem. B 2004, 108, 5263–5268. [Google Scholar] [CrossRef]
- Moritz, M.; Geszke-Moritz, M. Mesoporous materials as multifunctional tools in biosciences: Principles and applications. Mater. Sci. Eng. C 2015, 49, 114–151. [Google Scholar] [CrossRef]
- Le, T.-T.; Elyafi, A.K.E.; Mohammed, A.R.; Al-Khattawi, A. Delivery of poorly soluble drugs via mesoporous silica: Impact of drug overloading on release and thermal profiles. Pharmaceutics 2019, 11, 269. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhao, D.; Huo, Q.; Feng, J.; Chmelka, B.F.; Stucky, G.D. Nonionic triblock and star diblock copolymer and oligomeric surfactant syntheses of highly ordered, hydrothermally stable, mesoporous silica structures. J. Am. Chem. Soc. 1998, 120, 6024–6036. [Google Scholar] [CrossRef]
- Foo, K.Y.; Hameed, B.H. Insights into the modeling of adsorption isotherm systems. Chem. Eng. J. 2010, 156, 2–10. [Google Scholar] [CrossRef]
- Markovski, J.S.; Marković, D.D.; Ɖokić, V.R.; Mitrić, M.; Ristić, M.Ɖ.; Onjia, A.E.; Marinković, A.D. Arsenate adsorption on waste eggshell modified by goethite, α-MnO2 and goethite/α-MnO2. Chem. Eng. J. 2014, 237, 430–442. [Google Scholar] [CrossRef]
- Inglezakis, V.J. Solubility-normalized Dubinin-Astakhov adsorption isotherm for ion-exchange systems. Microporous Mesoporous Mater. 2007, 103, 72–81. [Google Scholar] [CrossRef]
- Hizal, J.; Demirçivi, P.; Karadirek, Ş.; Apak, R. Investigation of individual and competitive adsorption of Cu(II), Cd(II), and Pb(II) on montmorillonite in terms of surface complexation and kinetic properties of Cu(II) adsorption. Desalin. Water Treat. 2015, 47, 22441–22453. [Google Scholar] [CrossRef]
- Kundu, S.; Gupta, A.K. Arsenic adsorption onto iron oxide-coated cement (IOCC): Regression analysis of equilibrium data with several isotherm models and their optimization. Chem. Eng. J. 2006, 122, 93–106. [Google Scholar] [CrossRef]
- Garbacz, G.; Golke, B.; Wedemeyer, R.-S.; Axell, M.; Söderlind, E.; Abrahamsson, B.; Weitschies, W. Comparison of dissolution profiles obtained from nifedipine extended release once a day products using different dissolution test apparatuses. Eur. J. Pharm. Sci. 2009, 38, 147–155. [Google Scholar] [CrossRef]
- Phillips, D.J.; Pygall, S.R.; Cooper, B.B.; Mann, J.C. Overcoming sink limitations in dissolution testing: A review of traditional methods and the potential utility of biphasic systems. J. Pharm. Pharmacol. 2012, 64, 1549–1559. [Google Scholar] [CrossRef]
- European Pharmacopoeia 6.0. Available online: https://www.worldcat.org/title/european-pharmacopoeia/oclc/170932841 (accessed on 29 June 2020).
- Tang, J.; Srinivasan, S.; Yuan, W.; Ming, R.; Liu, Y.; Dai, Z.; Noble, C.O.; Hayes, M.E.; Zheng, N.; Jiang, W.; et al. Development of a flow-through USP 4 apparatus drug release assay for the evaluation of amphotericin B liposome. Eur. J. Pharm. Biopharm. 2019, 134, 107–116. [Google Scholar] [CrossRef]
- Costa, P.; Lobo, J.M.S. Modeling and comparison of dissolution profiles. Eur. J. Pharm. Sci. 2001, 13, 123–133. [Google Scholar] [CrossRef]
- Zhang, Y.; Huo, M.; Zhou, J.; Zou, A.; Li, W.; Yao, C.; Xie, S. DDSolver: An add-in program for modeling and comparison of drug dissolution profiles. AAPS J. 2010, 12, 263–271. [Google Scholar] [CrossRef] [Green Version]
- Geszke-Moritz, M.; Moritz, M. APTES-modified mesoporous silicas as the carriers for poorly water-soluble drug. Modeling of diflunisal adsortpion and release. Appl. Surf. Sci. 2016, 368, 348–359. [Google Scholar] [CrossRef]
- Sing, K.S.W.; Everett, D.H.; Haul, R.A.W.; Moscou, L.; Pierotti, R.A.; Rouquérol, J.; Siemieniewska, T. Reporting physisorption data for gas/solid systems with special references to the determination of surface area and porosity. Pure Appl. Chem. 1985, 57, 603–619. [Google Scholar] [CrossRef]
- Kruk, M.; Jaroniec, M.; Ko, C.H.; Ryoo, R. Characterization of the porous structure of SBA-15. Chem. Mater. 2000, 12, 1961–1968. [Google Scholar] [CrossRef]
- Selvam, P.; Bhatia, S.K.; Sonwane, C.G. Recent advances in processing and characterization of periodic mesoporous MCM-41 silicate molecular sieves. Ind. Eng. Chem. Res. 2001, 40, 3237–3261. [Google Scholar] [CrossRef]
- Moritz, M.; Geszke-Moritz, M. Mesoporous silica materials with different structures as the carriers for antimicrobial agent. Modeling of chlorhexidine adsorption and release. Appl. Surf. Sci. 2015, 256, 415–426. [Google Scholar] [CrossRef]
- Geszke-Moritz, M.; Moritz, M. Modeling of boldine alkaloid adsorption onto pure and propyl-sulfonic acid-modified mesoporous silicas. A comparative study. Mater. Sci. Eng. C 2016, 69, 815–830. [Google Scholar] [CrossRef]
- Giles, C.H.; MacEwan, T.H.; Nakhwa, S.N.; Smith, D. Studies in adsorption. Part XI. A system of classification of solution adsorption isotherms, and its use in diagnosis of adsorption mechanisms and in measurement of specific surface areas of solids. J. Chem. Soc. 1960, 111, 1973–3993. [Google Scholar] [CrossRef]
- Iriel, A.; Bruneel, S.P.; Schenone, N.; Cirelli, A.F. The removal of fluoride from aqueous solution by a lateritic soil adsorption: Kinetic and equilibrium studies. Ecotoxicol. Environ. Saf. 2018, 149, 166–172. [Google Scholar] [CrossRef] [PubMed]
- Cestari, A.R.; Vieira, E.F.S.; Vieira, G.S.; Almeida, L.E. Aggregation and adsorption of reactive dyes in the presence of an anionic surfactant on mesoporous aminopropyl silica. J. Colloid Interface Sci. 2007, 309, 402–411. [Google Scholar] [CrossRef]
- Ho, Y.-S.; Ofomaja, A.E. Biosorption thermodynamics of cadmium on coconut copra meal as biosorbent. Biochem. Eng. J. 2006, 30, 117–123. [Google Scholar] [CrossRef]
- Remko, M.; Remková, A.; Broer, R. A comparative study of molecular structure, pKa, lipophilicity, solubility, absorption and polar surface area of some antiplatelet drugs. Int. J. Mol. Sci. 2016, 17, 388. [Google Scholar] [CrossRef] [Green Version]
- Geszke-Moritz, M.; Moritz, M. Use of mesoporous silica modified with a sulfonic acid-derivative as adsorbent for boldine. Przem. Chem. 2017, 96, 2001–2004. [Google Scholar]
- Charnay, C.; Bégu, S.; Tourné-Péteilh, C.; Nicole, L.; Lerner, D.A.; Devoisselle, J.M. Inclusion of ibuprofen in mesoporous templated silica: Drug loading and release property. Eur. J. Pharm. Biopharm. 2004, 57, 533–540. [Google Scholar] [CrossRef] [PubMed]
- Zhu, W.; Wan, L.; Zhang, C.; Gao, Y.; Zheng, X.; Jiang, T.; Wang, S. Exloitation of 3D face-centered cubic mesoporous silica as a carrier for poorly water soluble drug: Influence of pore size on release rate. Mater. Sci. Eng. C 2014, 34, 78–85. [Google Scholar] [CrossRef] [PubMed]
- Wu, C.; Sun, X.; Zhao, Z.; Zhao, Y.; Hao, Y.; Liu, Y.; Gao, Y. Synthesis of novel core-shell structured dual mesoporous silica nanospheres and their application for enhancing the dissolution rate of poorly water-soluble drugs. Mater. Sci. Eng. C 2014, 44, 262–267. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Jiang, T.; Zhang, Q.; Wang, S. Inclusion of telmisartan in mesocellular foam nanoparticles: Drug loading and release property. Eur. J. Pharm. Biopharm. 2010, 76, 17–23. [Google Scholar] [CrossRef] [PubMed]

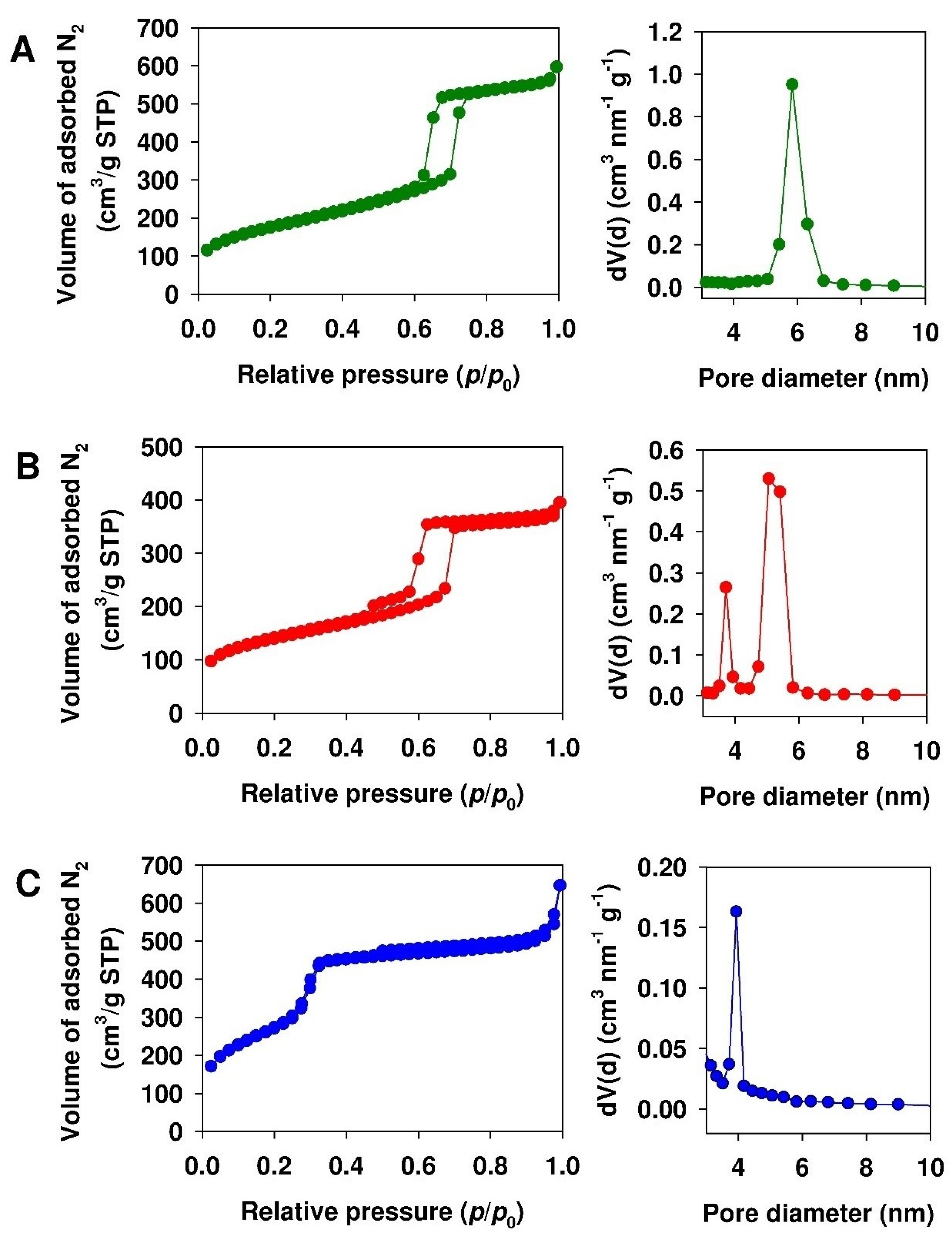
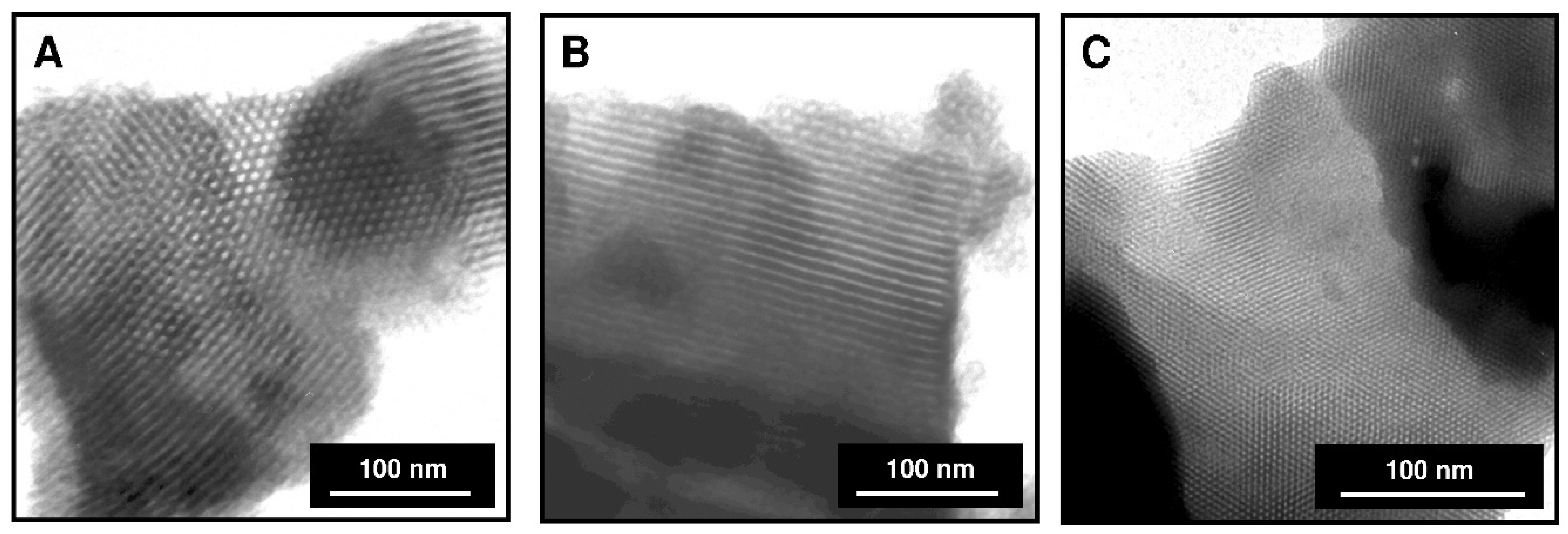
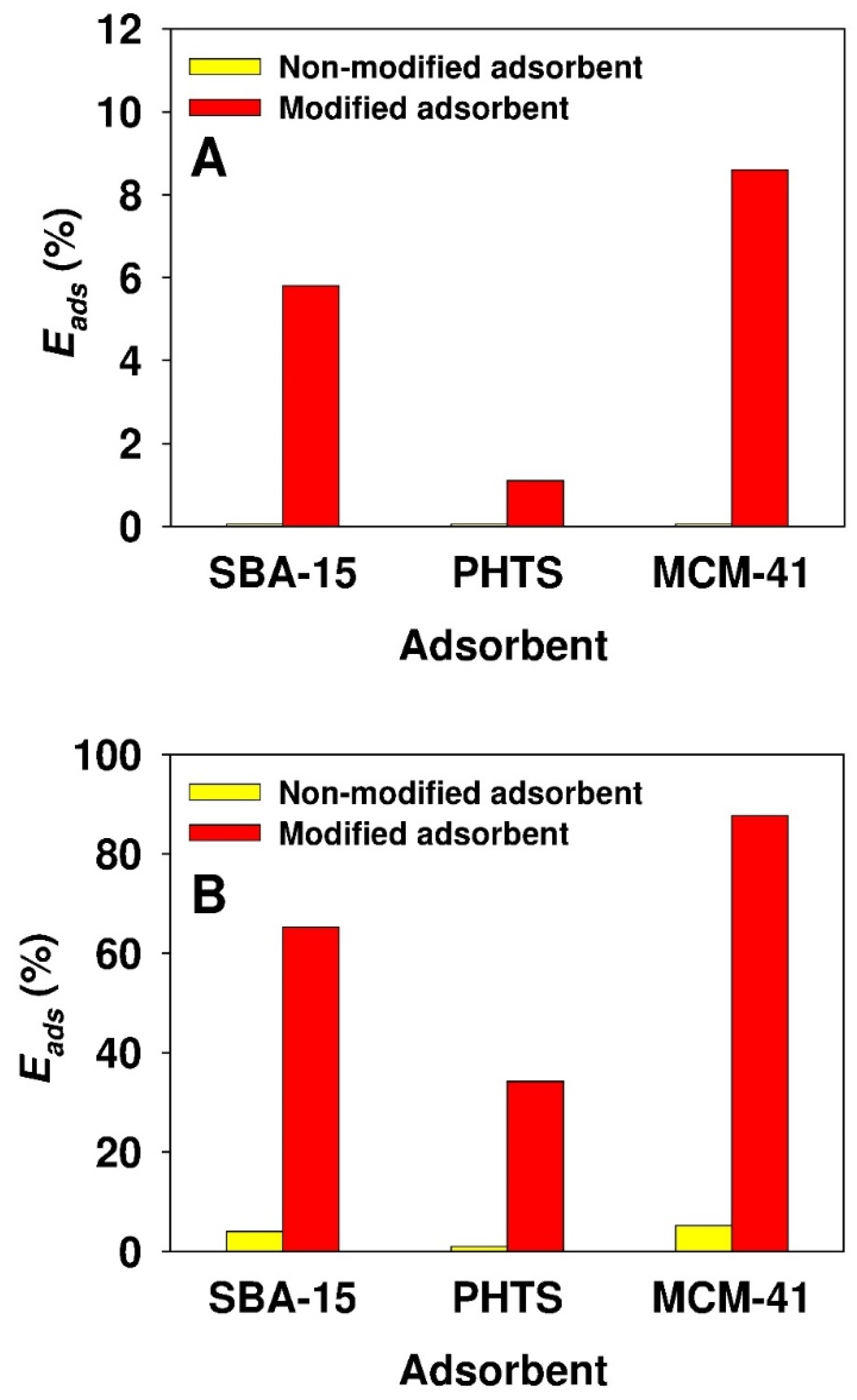
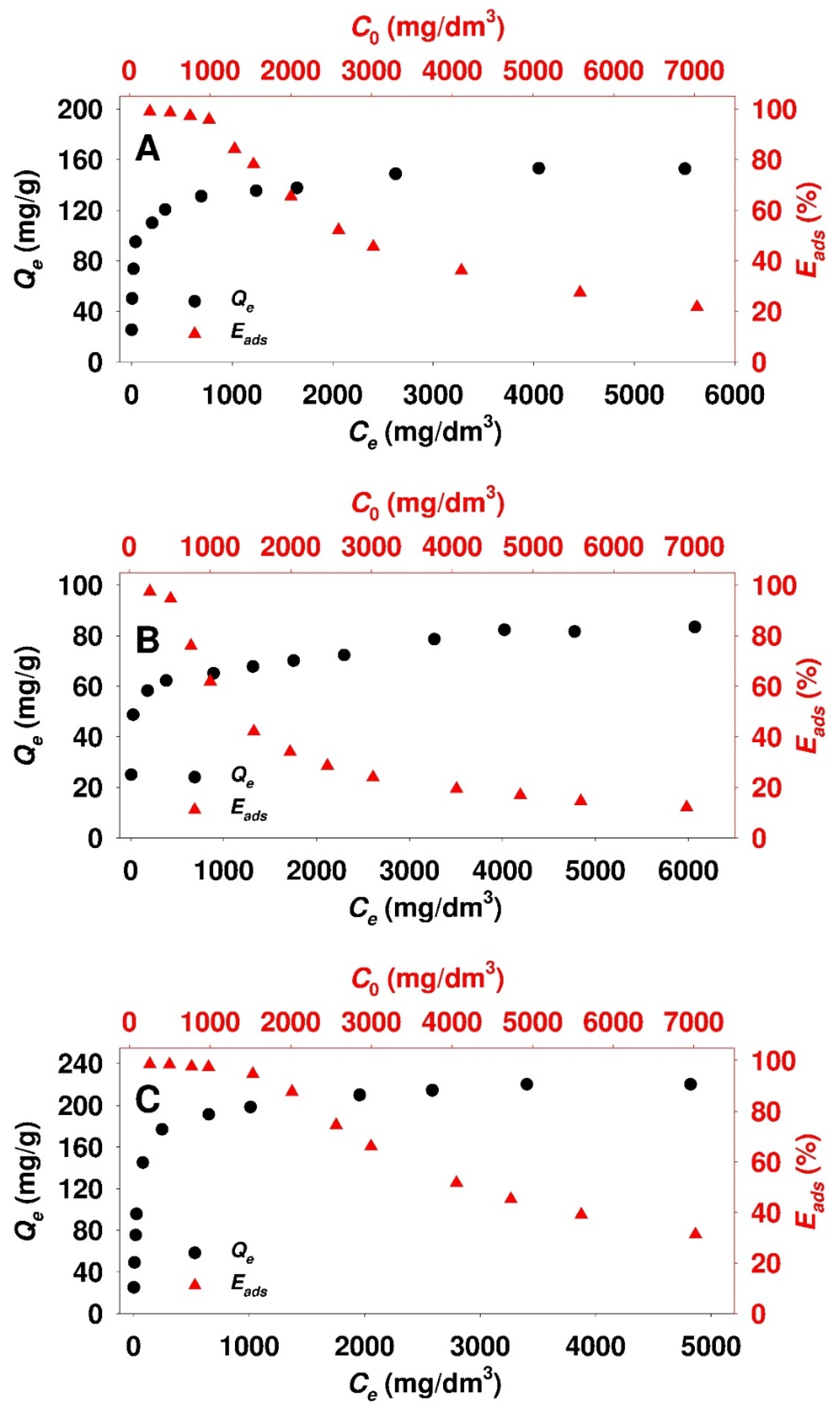



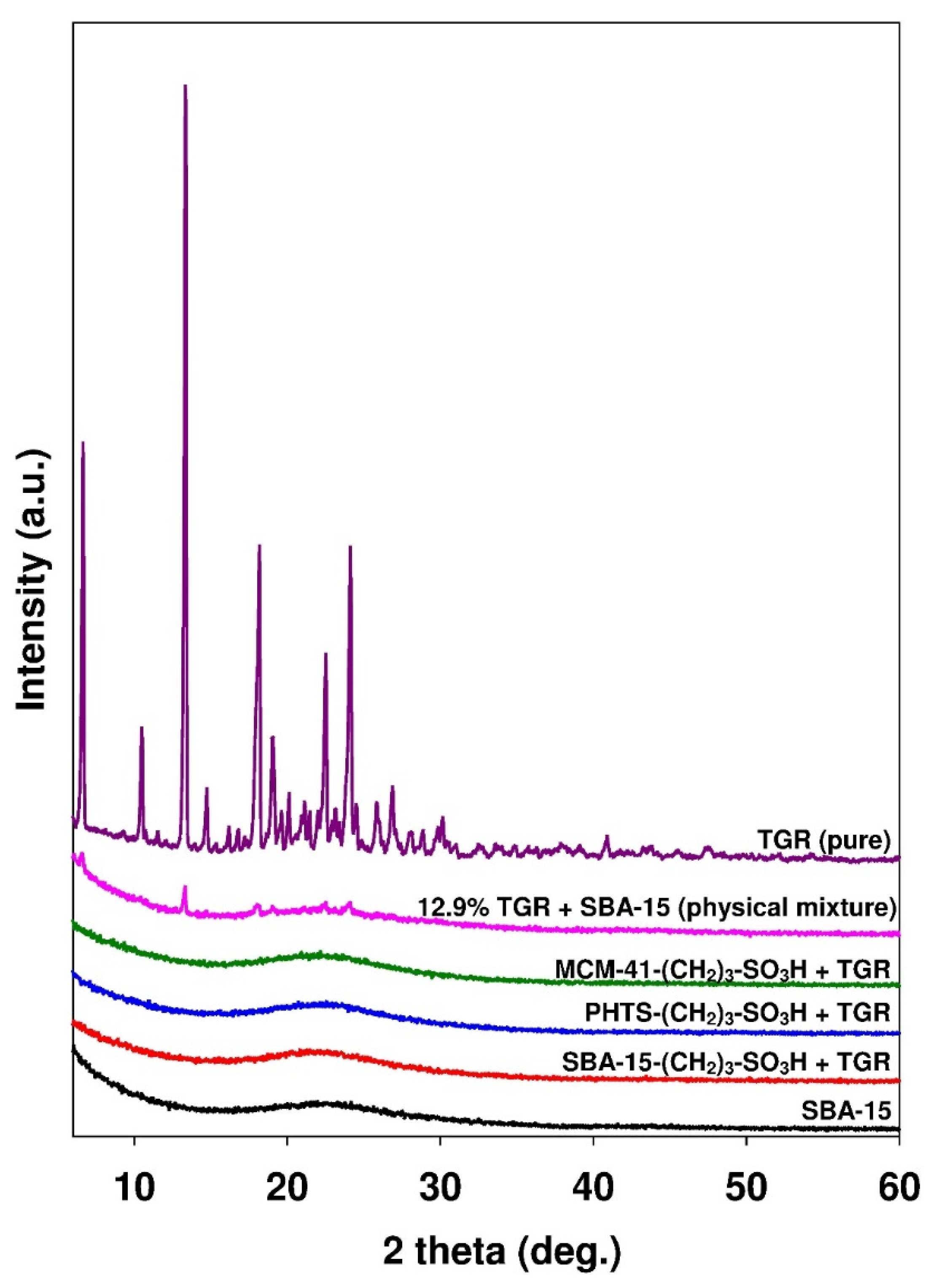
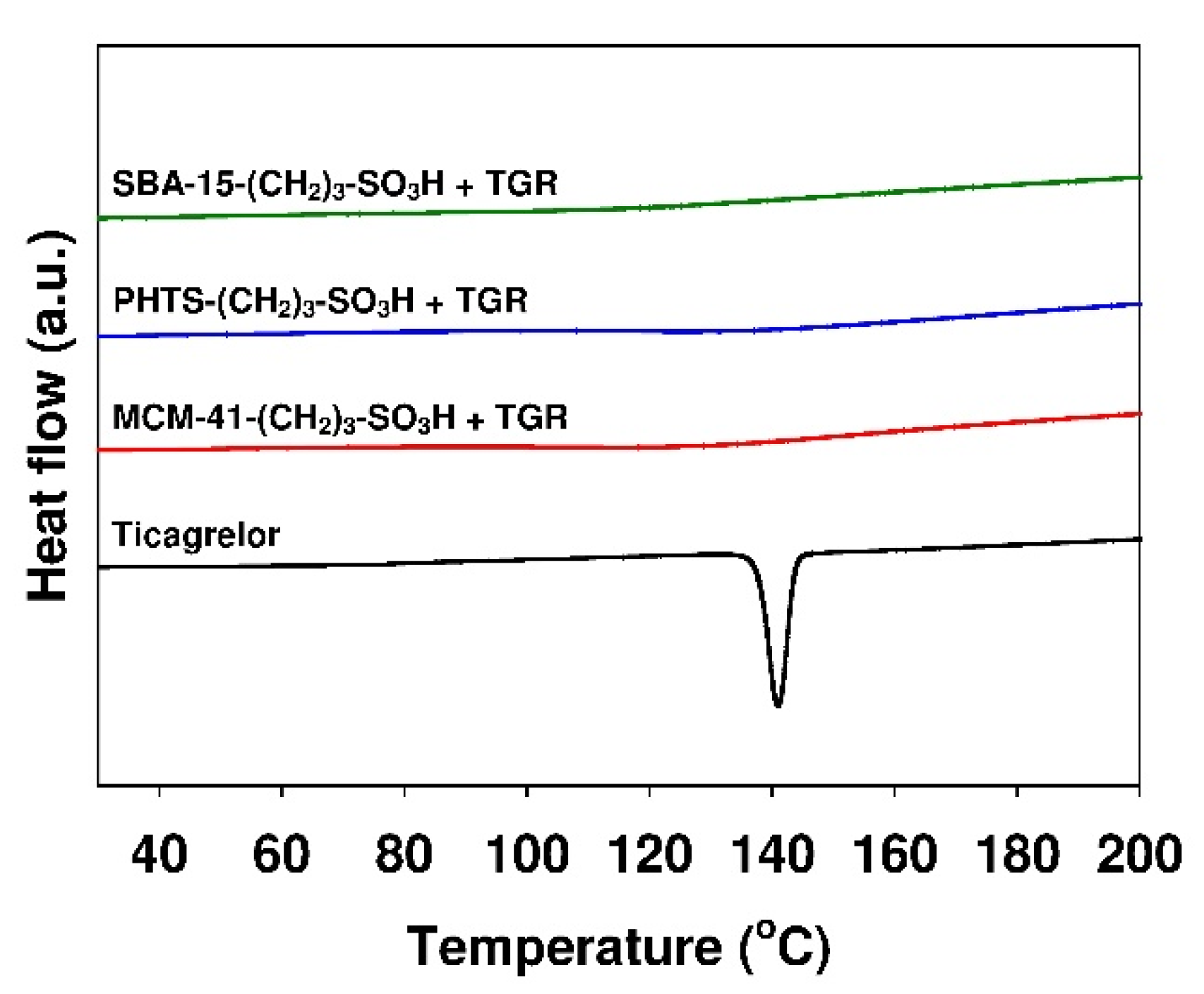

| Parameter | Adsorbent | ||
|---|---|---|---|
| SBA-15 | PHTS | MCM-41 | |
| BET surface area (m2/g) | 770 | 650 | 1160 |
| BJH pore volume (cm3/g) a | 0.97 | 0.70 | 0.65 |
| Pore diameter (nm)a | 5.9 | 5.5 | 4.0 |
| Micropore volume (cm3/g) b | 0.101 | 0.115 | - |
| Micropore area (m2/g)b | 204 | 220 | - |
| Parameter | Adsorbent | ||
|---|---|---|---|
| SBA-15-(CH2)3-SO3H | PHTS-(CH2)3-SO3H | MCM-41-(CH2)3-SO3H | |
| Amount of functional groups, Q-(CH2)3-SO3H (mol/g) a | 4.75 × 10−4 | 2.03 × 10−4 | 5.72 × 10−4 |
| BET surface area (m2/g) | 603 | 465 | 1056 |
| BJH pore volume (cm3/g)b | 0.85 | 0.55 | 0.37 |
| Pore diameter (nm) b | 5.8 | 5.1 | 3.9 |
| Micropore volume (cm3/g) c | 0.053 | 0.073 | - |
| Micropore area (m2/g) c | 105 | 139 | - |
| Adsorption Model | Parameter | Adsorbent | ||
|---|---|---|---|---|
| SBA-15-(CH2)3-SO3H | PHTS-(CH2)3-SO3H | MCM-41-(CH2)3-SO3H | ||
| Freundlich | KF (mg1-1/ndm3/n/g) | 34.85 | 26.00 | 28.34 |
| nF | 5.390 | 7.408 | 3.800 | |
| ARE (%) | 14.19 | 6.43 | 20.14 | |
| Jovanovich | QJ(max) (mg/g) | 131.1 | 70.1 | 198.4 |
| KJ (dm3/mg) | 6.752 × 10−2 | 6.671 × 10−2 | 2.560 × 10−2 | |
| ARE (%) | 13.21 | 10.49 | 10.37 | |
| Langmuir | QL(max) (mg/g) | 136.6 | 70.6 | 204.1 |
| KL (dm3/mg) | 8.122 × 10−2 | 8.545 × 10−2 | 3.428 × 10−2 | |
| ARE (%) | 8.05 | 7.81 | 5.19 | |
| Temkin | KT (dm3/mg) | 2.829 | 2.926 | 0.637 |
| bT (J g/mol mg) | 147.4 | 288.7 | 81.38 | |
| ARE (%) | 6.27 | 4.44 | 6.07 | |
| Dubinin-Radushkevich | QDR(max) (mg/g) | 156.5 | 78.2 | 240.7 |
| KDR (mol2/J2) | 3.320 × 10−9 | 2.980 × 10−9 | 4.763 × 10−9 | |
| EDR (kJ/mol) | 12.3 | 13.0 | 10.2 | |
| ARE (%) | 6.30 | 6.03 | 9.91 | |
| Dubinin-Astakhov | QDA(max) (mg/g) | 140.1 | 75.0 | 216.3 |
| KDA (molnDA/JnDA) | 1.352 × 10−14 | 1.425 × 10−10 | 4.276 × 10−15 | |
| nDA | 3.258 | 2.313 | 3.414 | |
| EDA (kJ/mol) | 12.8 | 12.8 | 11.4 | |
| ARE (%) | 4.92 | 5.87 | 2.67 | |
| Redlich-Peterson | KRP (dm3/g) | 14.59 | 9.299 | 8.269 |
| aRP (dm3β/mgβ) | 0.185 | 0.256 | 5.475 × 10−2 | |
| β | 0.923 | 0.904 | 0.955 | |
| ARE (%) | 1.84 | 3.46 | 1.59 | |
| Parameter | Formulation (Dosage Form) | |||
|---|---|---|---|---|
| SBA-15-(CH2)3-SO3H | PHTS-(CH2)3-SO3H | MCM-41-(CH2)3-SO3H | Pure TGR | |
| a | 0.401 | 1.371 | 0.432 | 0.309 |
| b | 0.061 | 0.460 | 0.185 | 0.663 |
| t50% (h) | 7.72 × 10−10 | 0.896 | 1.48 × 10−3 | 0.098 |
| t90% (h) | 0.268 | 12.16 | 0.969 | 0.598 |
| ARE (%) | 0.37 | 7.55 | 1.10 | 1.59 |
| Ticagrelor amount (%) | 11.73 | 6.51 | 15.43 | - |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Moritz, M.; Geszke-Moritz, M. Sulfonic Acid Derivative-Modified SBA-15, PHTS and MCM-41 Mesoporous Silicas as Carriers for a New Antiplatelet Drug: Ticagrelor Adsorption and Release Studies. Materials 2020, 13, 2913. https://doi.org/10.3390/ma13132913
Moritz M, Geszke-Moritz M. Sulfonic Acid Derivative-Modified SBA-15, PHTS and MCM-41 Mesoporous Silicas as Carriers for a New Antiplatelet Drug: Ticagrelor Adsorption and Release Studies. Materials. 2020; 13(13):2913. https://doi.org/10.3390/ma13132913
Chicago/Turabian StyleMoritz, Michał, and Małgorzata Geszke-Moritz. 2020. "Sulfonic Acid Derivative-Modified SBA-15, PHTS and MCM-41 Mesoporous Silicas as Carriers for a New Antiplatelet Drug: Ticagrelor Adsorption and Release Studies" Materials 13, no. 13: 2913. https://doi.org/10.3390/ma13132913





