Investigation and Possibilities of Reuse of Carbon Dioxide Absorbent Used in Anesthesiology
Abstract
:1. Introduction
2. Experimental
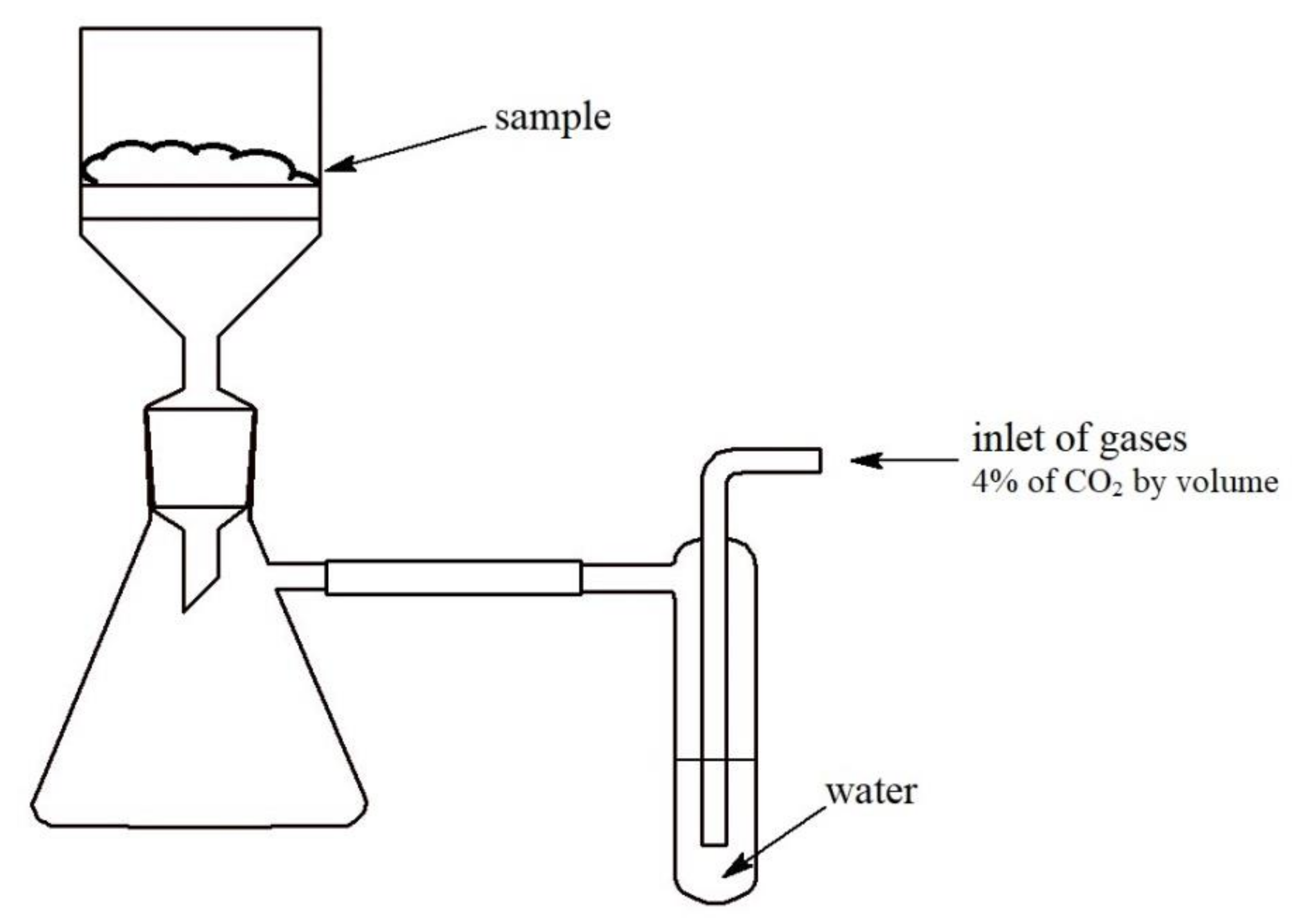
2.1. Materials and Analysis
- SL (F)—fresh soda lime sample;
- SL (U)—used soda lime sample.
- SL (5 min)—fresh soda lime sample after 5 min of carbon dioxide absorption;
- SL (15 min)—fresh soda lime sample after 15 min of carbon dioxide absorption.
- SL (30 min)—fresh soda lime sample after 30 min of carbon dioxide absorption.
2.2. Methods and Instruments
3. Results and Discussion
3.1. Thermal Decomposition of Samples: SL (F), SL (U), Samples After Absorption: SL (5 min), SL (15 min) and SL (30 min)
3.2. Chemical Kinetics of Carbon Dioxide Absorption by SL (U) Sample
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Issa, M.C.; Yusoff, N.H.N.; Yati, M.S.D.; Muhammad, M.M.; Salleh, N.A.; Nor, M.F.M.; Minal, A.; Nain, H.; Nor, I.M. Characterisation od Carbon Dioxide Absorbent Material For Enclosed Space Applications. Def. S T Tech. Bull. Malays. 2012, 5, 1–10. [Google Scholar]
- Gai, W.-M.; Deng, Y.-F.; Du, Y. Adsorption Properties of Modified Soda Lime for Carbon Dioxide Removal within the Closed Environment of a Coal Mine Refuge Chamber. Ind. Eng. Chem. Res. 2016, 55, 10794–10802. [Google Scholar] [CrossRef]
- Fang, Z.X.; Kandel, L.; Laster, M.J.; Ionescu, P.; Eger, E.I. Factors Affecting Production of Compound A from the Interaction of Sevoflurane with Baralyme and Soda Lime. Anesth. Analg. 1996, 82, 775–781. [Google Scholar] [CrossRef]
- Morio, M.; Fujii, K.; Satoh, N.; Imai, M.; Kawakami, U.; Mizuno, T.; Kawai, Y.; Ogasawara, Y.; Tamura, T.; Negishi, A.; et al. Reaction of Sevoflurane and Its Degradation Products with Soda Lime. Toxicity of the Byproducts. Anesthesiology 1992, 77, 1155–1164. [Google Scholar] [CrossRef]
- Frink, E.J.; Malan, T.P.; Morgan, S.E.; Brown, E.A.; Malcomson, M.; Brown, B.R. Quantification of the Degradation Products of Sevoflurane in Two CO2 Absorbants during Low-flow Anesth. in Surgical Patients. Anesthesiology 1992, 77, 1064–1069. [Google Scholar] [CrossRef] [PubMed]
- Gonsowski, C.T.; Laster, M.J.; Eger, E.I.; Ferrell, L.D.; Kerschmann, R.L. Toxicity of Compound A in Rats. Effect of increasing duration of administration. Anesthesiology 1994, 80, 566–573. [Google Scholar] [CrossRef] [PubMed]
- Kandel, L.; Laster, M.J.; Ii, E.I.E.; Kerschmann, R.L.; Martin, J. Nephrotoxicity in Rats Undergoing a One-Hour Exposure to Compound A. Anesth. Analg. 1995, 81, 559–563. [Google Scholar] [CrossRef] [PubMed]
- Keller, K.A.; Callan, C.; Prokocimer, P.; Delgado-Herrera, L.; Friedman, M.B.; Hoffman, G.M.; Wooding, W.L.; Cusick, P.K.; Krasula, R.W. Inhalation Toxicity Study of a Haloalkene Degradant of Sevoflurane, Compound A (PIFE), in Sprague-Dawley Rats. Anesthesiology 1995, 83, 1220–1232. [Google Scholar] [CrossRef] [PubMed]
- Charlson, E.S.; Diamond, J.M.; Bittinger, K.; Fitzgerald, A.S.; Yadav, A.; Haas, A.R.; Bushman, F.D.; Collman, R.G. Lung-enriched Organisms and Aberrant Bacterial and Fungal Respiratory Microbiota after Lung Transplant. Am. J. Respir. Crit. Care Med. 2012, 186, 536–545. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Piters, W.A.A.D.S.; Sanders, E.A.M.; Bogaert, D. The role of the local microbial ecosystem in respiratory health and disease. Philos. Trans. R. Soc. B Biol. Sci. 2015, 370, 20140294. [Google Scholar] [CrossRef] [Green Version]
- Nguyen, L.D.N.; Viscogliosi, E.; Delhaes, L. The lung mycobiome: An emerging field of the human respiratory microbiome. Front. Microbiol. 2015, 6, 89. [Google Scholar] [CrossRef] [PubMed]
- Yi, H.; Yong, D.; Lee, K.; Cho, Y.-J.; Chun, J. Profiling bacterial community in upper respiratory tracts. BMC Infect. Dis. 2014, 14, 583. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fang, Z.X.; Eger, E.I.; Laster, M.J.; Chortkoff, B.S.; Kandel, L.; Ionescu, P. Carbon Monoxide Production from Degradation of Desflurane, Enflurane, Isoflurane, Halothane, and Sevoflurane by Soda Lime and Baralyme. Anesth. Analg. 1995, 80, 1187–1193. [Google Scholar] [CrossRef]
- Coppens, M.J.; Versichelen, L.F.M.; Rolly, G.; Mortier, E.P.; Struys, M.M.R.F. The mechanisms of carbon monoxide production by inhalational agents. Anaesth. 2006, 61, 462–468. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Holak, E.J.; Mei, D.A.; Dunning, M.B.; Gundamraj, R.; Noseir, R.; Zhang, L.; Woehlck, A.H.J. Carbon Monoxide Production from Sevoflurane Breakdown: Modeling of Exposures Under Clinical Conditions. Anesth. Analg. 2003, 96, 757–764. [Google Scholar] [CrossRef] [PubMed]
- Knolle, E.; Heinze, G.; Gilly, H. Carbon Monoxide Formation in Dry Soda Lime is Prolonged at Low Gas Flow. Anesth. Analg. 2001, 93, 488–493. [Google Scholar] [CrossRef]
- Pond, D.; Jaffe, R.A.; Brock-Utne, J.G. Failure to Detect CO2-absorbent Exhaustion: Seeing and Believing. Anesthesiology 2000, 92, 1196. [Google Scholar] [CrossRef]
- Lillo, R.S.; Ruby, A.; Gummin, D.D.; Porter, W.R.; Caldwel, J.M. Chemical Safety of U.S. Navy Fleet Soda Lime. Undersea Hyperb Med. 1996, 23, 43. [Google Scholar]
- Neubauer, B.; Mutzbauer, T.S.; Tetzlaff, K. Exposure to Soda-Lime Dust in Closed and Semi-Closed Diving Apparatus. Aviat Space Environ Med. 2000, 71, 1248. [Google Scholar]
- Holloway, A.M. Possible Alternatives to Soda Lime. Anaesth. Intensiv. Care 1994, 22, 359–362. [Google Scholar] [CrossRef] [PubMed]
- Shih, S.-M.; Ho, C.-S.; Song, Y.-S.; Lin, J.-P. Kinetics of the Reaction of Ca(OH)2 with CO2 at Low Temperature. Ind. Eng. Chem. Res. 1999, 38, 1316–1322. [Google Scholar] [CrossRef]
- Van Balen, K. Carbonation reaction of lime, kinetics at ambient temperature. Cem. Concr. Res. 2005, 35, 647–657. [Google Scholar] [CrossRef]
- Freeman, B.S.; Berger, J.S. Anesthesiology Core Review: Part One Basic Exam; Springer: Berlin/Heidelberg, Germany, 2014; Chapter 17. [Google Scholar]
- Samari, M.; Ridha, F.; Manovic, V.; Macchi, A.; Anthony, E.J. Direct capture of carbon dioxide from air via lime-based sorbents. Mitig. Adapt. Strat. Glob. Chang. 2019, 25, 25–41. [Google Scholar] [CrossRef] [Green Version]
- Pérez-Maqueda, L.A.; Criado, A.J.M.; Sánchez-Jiménez, P.E. Combined Kinetic Analysis of Solid-State Reactions: A Powerful Tool for the Simultaneous Determination of Kinetic Parameters and the Kinetic Model without Previous Assumptions on the Reaction Mechanism. J. Phys. Chem. A 2006, 110, 12456–12462. [Google Scholar] [CrossRef]
- Bos, M.; Kreuger, T.; Kersten, S.; Brilman, D. Study on transport phenomena and intrinsic kinetics for CO2 adsorption in solid amine sorbent. Chem. Eng. J. 2019, 377, 120374. [Google Scholar] [CrossRef]
- Singh, V.K.; Kumar, E.A. Comparative Studies on CO2 Adsorption Kinetics by Solid Adsorbents. Energy Procedia 2016, 90, 316–325. [Google Scholar] [CrossRef]
- Nikulshina, V.; Gálvez, M.; Steinfeld, A. Kinetic analysis of the carbonation reactions for the capture of CO2 from air via the Ca(OH)2–CaCO3–CaO solar thermochemical cycle. Chem. Eng. J. 2007, 129, 75–83. [Google Scholar] [CrossRef]
- Simonin, J.-P. On the comparison of pseudo-first order and pseudo-second order rate laws in the modeling of adsorption kinetics. Chem. Eng. J. 2016, 300, 254–263. [Google Scholar] [CrossRef] [Green Version]







| Sample | Percentage Content [% (m/m)] | |||
|---|---|---|---|---|
| Ca(OH)2 | CaCO3 | H2O | NaOH | |
| SL (F) | 96.78 | 0 | 0.89 | 2.50 |
| SL (U) | 35.85 | 60.50 | 1.93 | 2.10 |
| SL (5 min) | 63.23 | 34.09 | 2.08 | 2.28 |
| SL (15 min) | 42.34 | 55.09 | 2.74 | 2.15 |
| SL (30 min) | 31.41 | 64.45 | 2.95 | 2.04 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rogalewicz, B.; Czylkowska, A.; Anielak, P.; Samulkiewicz, P. Investigation and Possibilities of Reuse of Carbon Dioxide Absorbent Used in Anesthesiology. Materials 2020, 13, 5052. https://doi.org/10.3390/ma13215052
Rogalewicz B, Czylkowska A, Anielak P, Samulkiewicz P. Investigation and Possibilities of Reuse of Carbon Dioxide Absorbent Used in Anesthesiology. Materials. 2020; 13(21):5052. https://doi.org/10.3390/ma13215052
Chicago/Turabian StyleRogalewicz, Bartłomiej, Agnieszka Czylkowska, Piotr Anielak, and Paweł Samulkiewicz. 2020. "Investigation and Possibilities of Reuse of Carbon Dioxide Absorbent Used in Anesthesiology" Materials 13, no. 21: 5052. https://doi.org/10.3390/ma13215052







