Comparison of the Properties of Natural Sorbents for the Calcium Looping Process
Abstract
:1. Introduction
2. Materials and Methods
3. Results
3.1. Raw Sorbents
3.1.1. Dolomite
3.1.2. Saint Anne Mountain Limestone
3.1.3. Marl
3.1.4. Nephelinite
3.1.5. Magnesite
3.1.6. Serpentinite
3.2. Thermal Pretreatment of Sorbents
- Doping—aimed at postponing or avoiding sintering of sorbent in order to moderate sintering and abrasion of the sorbent (e.g., [18,19]). The effectiveness of doping depends on the concentration of the substrate used. Too low of a concentration will have no effect, while too high of a concentration may block the pores [12,20];
4. Discussion
5. Summary and Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- International Energy Agency. Energy and Climate Change—World Energy Outlook—Special Briefing for COP21; International Energy Agency: Paris, France, 2015. [Google Scholar]
- Olivier, J.G.J.; Janssens-Maenhout, G.; Muntean, M.; Peters, J.A.H.W. Trends in Global CO2 Emissions-2015 Report; No: JRC98184; PBL Netherlands Environmental Assessment Agency: The Hague, The Netherlands, 2015. [Google Scholar]
- Sanna, A.; Uibu, M.; Caramanna, G.; Kuusik, R.; Maroto-Valer, M.M. A review of mineral carbonation technologies to sequester CO2. Chem. Soc. Rev. 2014, 43, 8049. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gislason, S.R.; Wolff-Boenisch, D.; Stefansson, A.; Oelkers, E.H.; Gunnlaugsson, E.; Sigurdardottir, H.; Sigfusson, B.; Broecker, W.S.; Matter, J.M.; Stute, M.; et al. Mineral sequestration of carbon dioxide in basalt: A pre-injection overview of the carbfix project. Int. J. Greenh. Gas Contr. 2010, 4, 537–545. [Google Scholar] [CrossRef]
- McKelvy, M.J.; Chizmeshya, A.V.G.; Diefenbacher, J.; Béarat, H.; Wolf, G. Exploration of the role of heat activation in enhancing serpentine carbon sequestration reactions. Environ. Sci. Technol. 2004, 38, 6897–6903. [Google Scholar] [CrossRef] [PubMed]
- Olajire, A.A. A review of mineral carbonation technology in sequestration of CO2. J. Pet. Sci. Eng. 2013, 109, 364–392. [Google Scholar] [CrossRef]
- Werner, M.; Verduyn, M.; van Mossel, G.; Mazzotti, M. Direct flue gas CO2 mineralization using activated serpentine: Exploring the reaction kinetics by experiments and population balance modelling. Energy Procedia 2014, 4, 2043–2049. [Google Scholar] [CrossRef] [Green Version]
- Huijgen, W.J.J.; Comans, R.N.J. Carbon Dioxide Sequestration by Mineral Carbonation: Literature Review; ECN—Clean Fossil Fuels Environmental Risk Assessment: Petten, The Netherlands, 2003; p. 112. [Google Scholar]
- Kirsch, K. CO2-Induced Metal Release from Sandstones: Implications for Geologic Carbon Sequestration. Master’s Thesis, Hydrologic Science and Engineering Faculty, School of Mines, Golden, CO, USA, 2013. [Google Scholar]
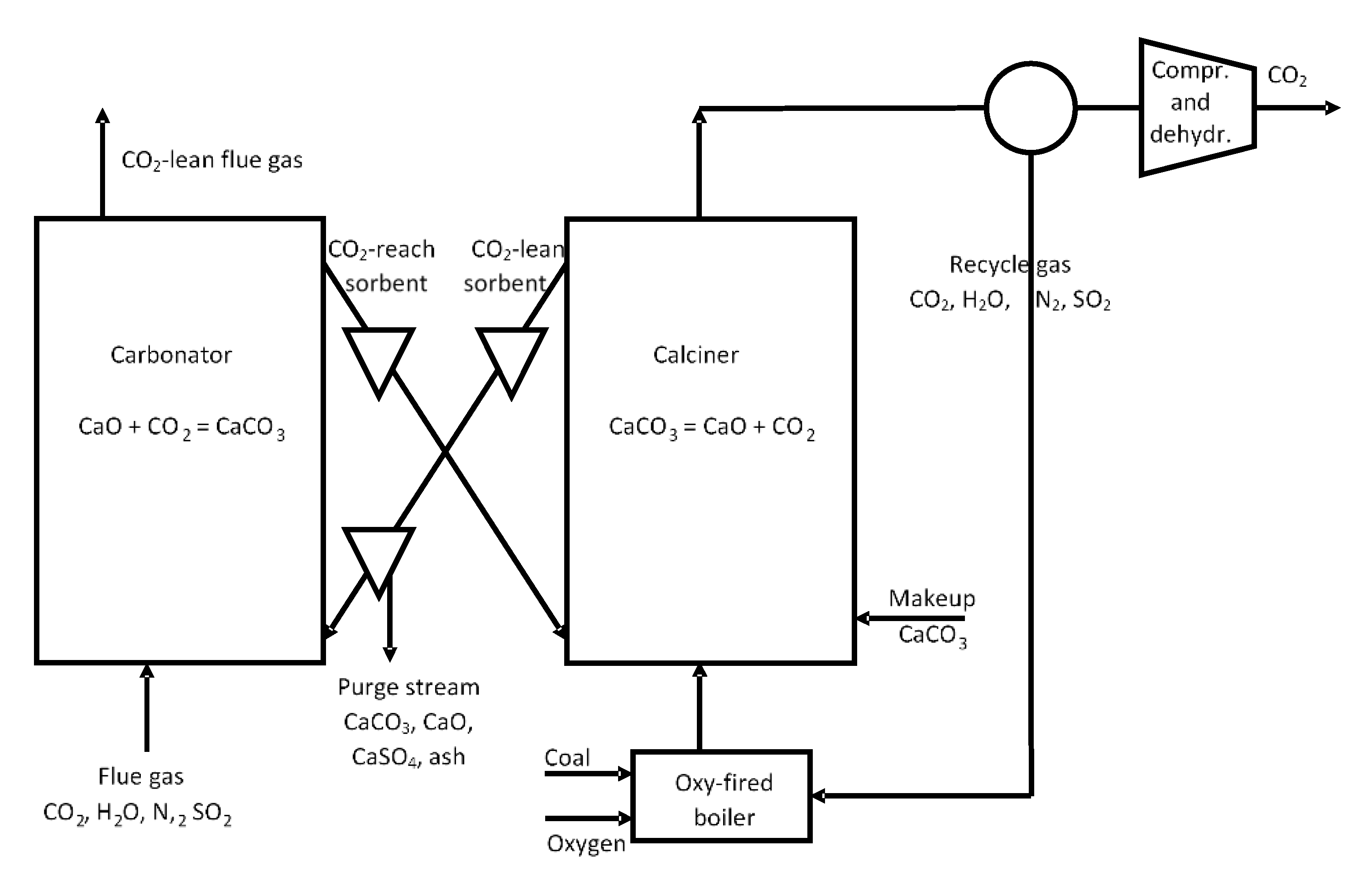
- Blamey, J.; Anthony, E.J.; Wang, J.; Fennell, P.S. The calcium looping cycle for large-scale CO2 capture. Prog. Energy Combust. Sci. 2010, 36, 260–279. [Google Scholar] [CrossRef]
- Laursen, K.; Duo, W.; Grace, J.R.; Lim, J. Sulfation and reactivation characteristics of nine limestones. Fuel 2000, 79, 153–163. [Google Scholar] [CrossRef]
- Dean, C.C.; Blamey, J.; Florin, N.H.; Al-Jeboori, M.J.; Fennell, P.S. The calcium looping cycle for CO2 capture from power generation, cement manufacture and hydrogen production. Chem. Eng. Res. Des. 2011, 89, 836–855. [Google Scholar] [CrossRef] [Green Version]
- Mantripragadaa, H.C.; Rubin, E.S. Calcium looping cycle for CO2 capture: Performance, cost and feasibility analysis. Energy Procedia 2014, 63, 2199–2206. [Google Scholar] [CrossRef] [Green Version]
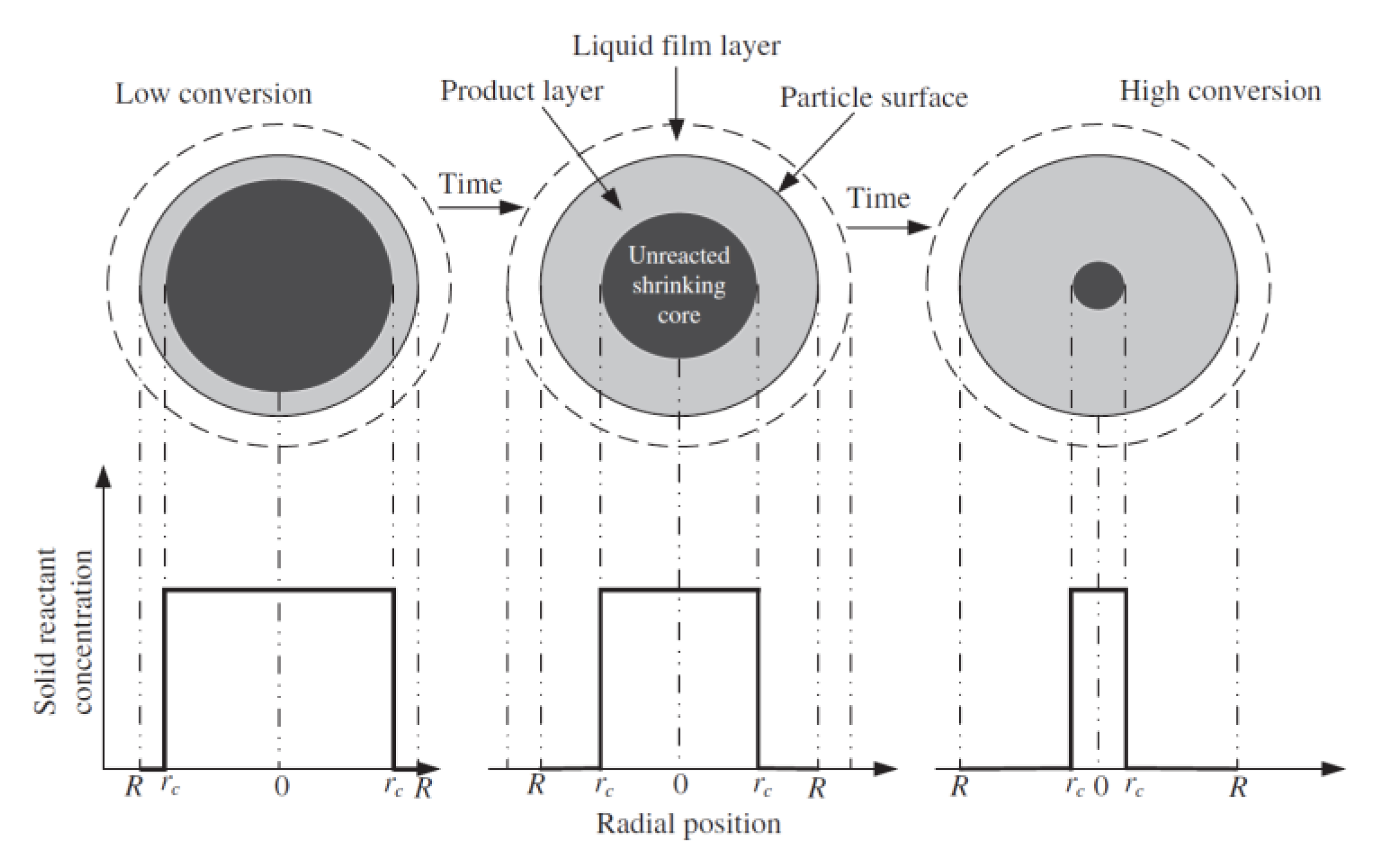
- Lee, D. An apparent kinetic model for the carbonation of calcium oxide by carbon dioxide. Chem. Eng. J. 2004, 100, 71–77. [Google Scholar] [CrossRef]
- Oakeson, W.G.; Cutler, I.B. Effect of CO2 Pressure on the Reaction with CaO. J. Am. Ceram. Soc. 2006, 62, 556–558. [Google Scholar] [CrossRef]
- Butler, J.; Lim, J.; Grace, J. Kinetics of CO2 absorption by CaO through pressure swing cycling. Fuel 2014, 127, 78–87. [Google Scholar] [CrossRef]
- Levenspiel, O. Chemical Reaction Engineering, 2nd ed.; John Wiley and Sons: New York, NY, USA, 1972. [Google Scholar]
- Salvador, C.; Lu, D.; Anthony, E.J.; Abanades, J.C. Enhancement of CaO for CO2 capture in an FBC environment. Chem. Eng. J. 2003, 96, 187–195. [Google Scholar] [CrossRef]
- González, B.; Blamey, J.; McBride-Wright, M.; Carter, N.; Dugwell, D.; Fennell, P. Calcium looping for CO2 capture: Sorbent enhancement through doping. Energy Procedia 2011, 4, 402–409. [Google Scholar] [CrossRef] [Green Version]
- Manovic, V.; Anthony, E.J. Lime-Based sorbents for high-temperature CO2 capture—A review of sorbent modification methods. Int. J. Environ. Res. Public Health 2010, 7, 3129–3140. [Google Scholar] [CrossRef] [Green Version]
- Li, Y.J.; Zhao, C.S.; Duan, L.B.; Liang, C.; Li, Q.Z.; Zhou, W. Cyclic calcination/carbonation looping of dolomite modified with acetic acid for CO2 capture. Fuel Process. Technol. 2008, 89, 1461–1469. [Google Scholar] [CrossRef]
- Ridha, F.N.; Manovic, V.; Macchi, A.; Anthony, E.J. The effect of SO2 on CO2 capture by CaO-based pellets prepared with a kaolin derived Al(OH)3 binder. Appl. Energy 2012, 92, 415–520. [Google Scholar] [CrossRef]
- Erans, M.; Manovic, V.; Anthony, E.J. Calcium looping sorbents for CO2 capture. Appl. Energy 2016, 180, 722–742. [Google Scholar] [CrossRef] [Green Version]
- Albrecht, K.O.; Wagenbach, K.S.; Satrio, J.A.; Shanks, B.H.; Wheelock, T.D. Development of a CaO-based sorbent with improved cyclic stability. Ind. Eng. Chem. Res. 2008, 47, 7841–7848. [Google Scholar] [CrossRef]
- Manovic, V.; Anthony, E.J. Thermal Activation of CaO-Based Sorbent and Self-Reactivation during CO2 Capture Looping Cycles. Environ. Sci. Technol. 2008, 42, 4170–4174. [Google Scholar] [CrossRef]
- Manovic, V.; Anthony, E.J.; Grasa, G.; Abanades, J.C. CO2 Looping Cycle Performance of a High-Purity Limestone after Thermal Activation/Doping. Energy Fuels 2008, 22, 3258–3264. [Google Scholar] [CrossRef]
- Lysikov, A.I.; Salanov, A.N.; Okunev, A.G. Change of CO2 carrying capacity of CaO in isothermal recarbonation-decomposition cycles. Ind. Eng. Chem. Res. 2007, 46, 4633–4638. [Google Scholar] [CrossRef]
- Arias, B.; Grasa, G.S.; Alonso, M.; Abanades, J.C. Post-combustion calcium looping process with a highly stable sorbent activity by recarbonation. Energy Environ. Sci 2012, 5, 7353–7359. [Google Scholar] [CrossRef]
- Chen, Z.; Song, H.S.; Portillo, M.; Lim, C.J.; Grace, J.R.; Anthony, E.J. Long-term calcination/carbonation cycling and thermal pretreatment for CO2 capture by limestone and dolomite. Energy Fuels 2009, 23, 1437–1444. [Google Scholar] [CrossRef]

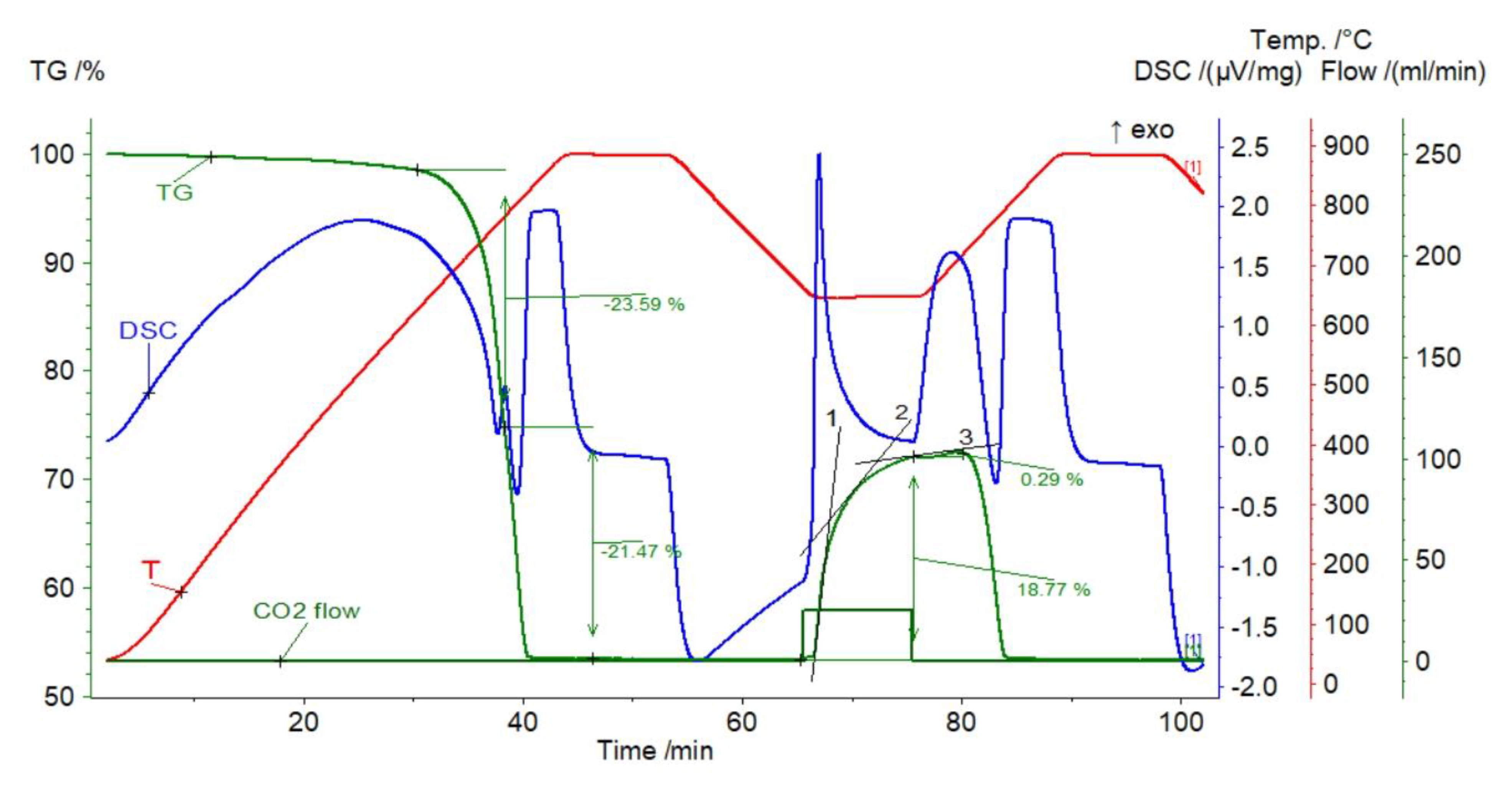
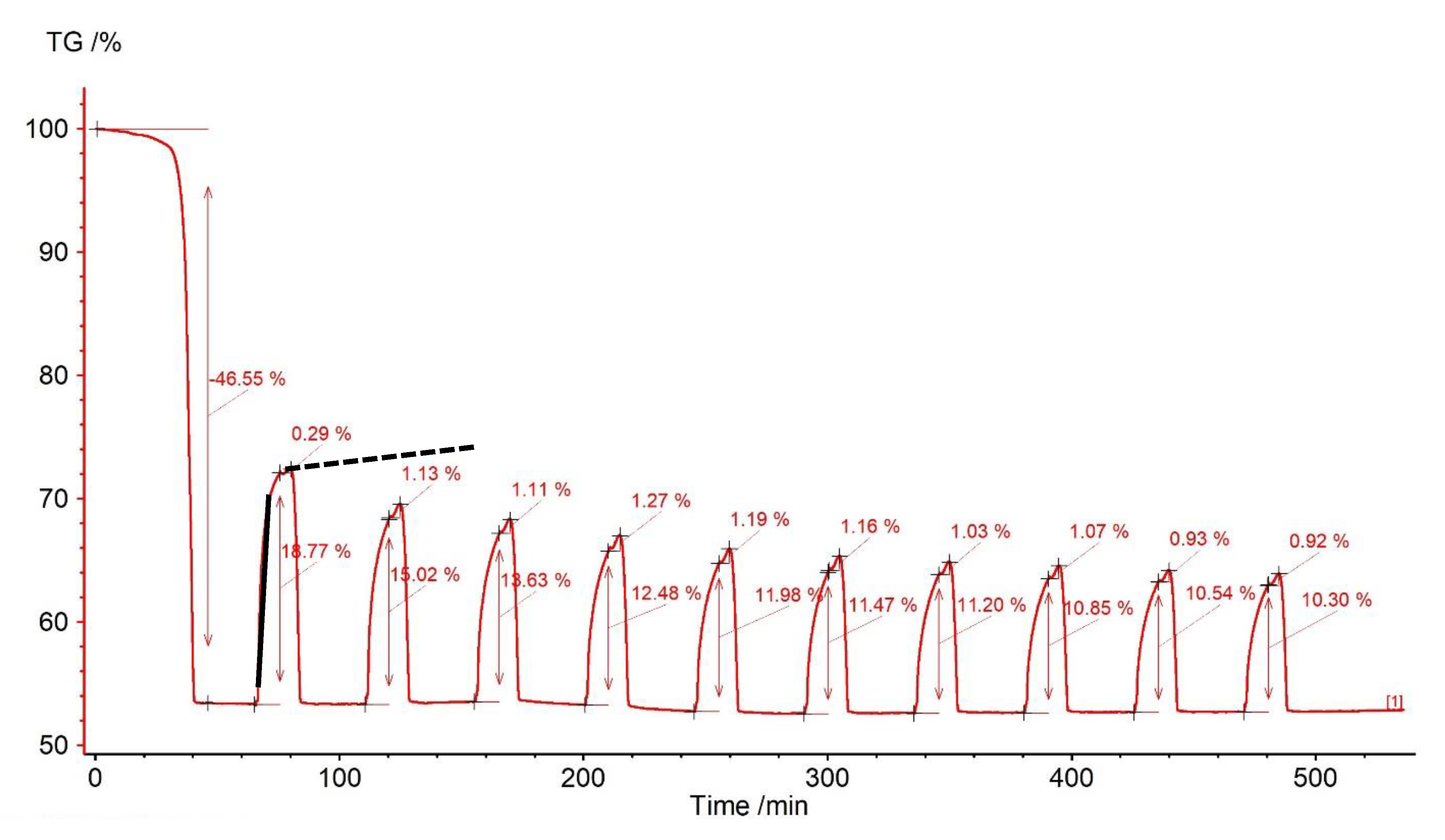
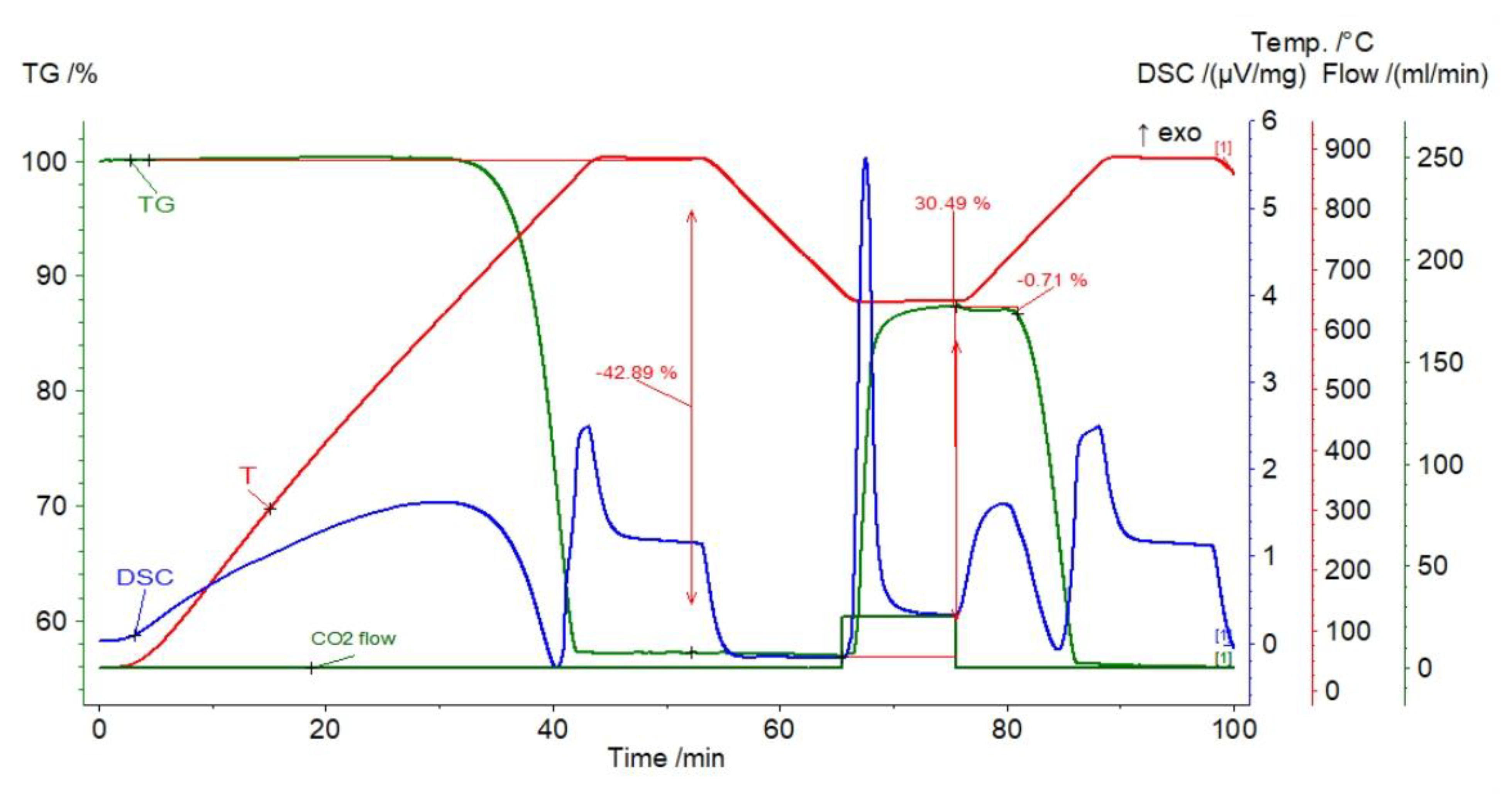

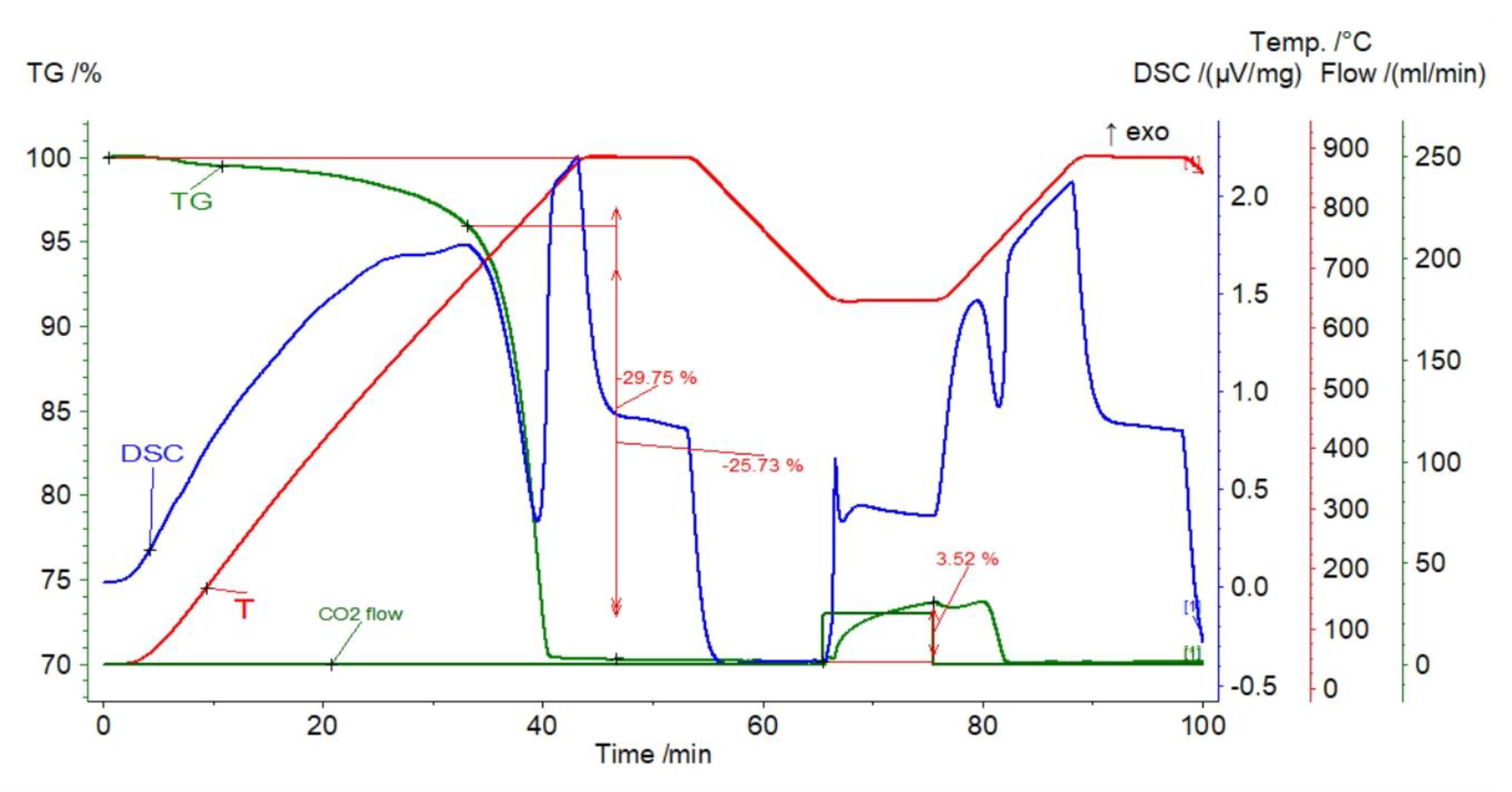

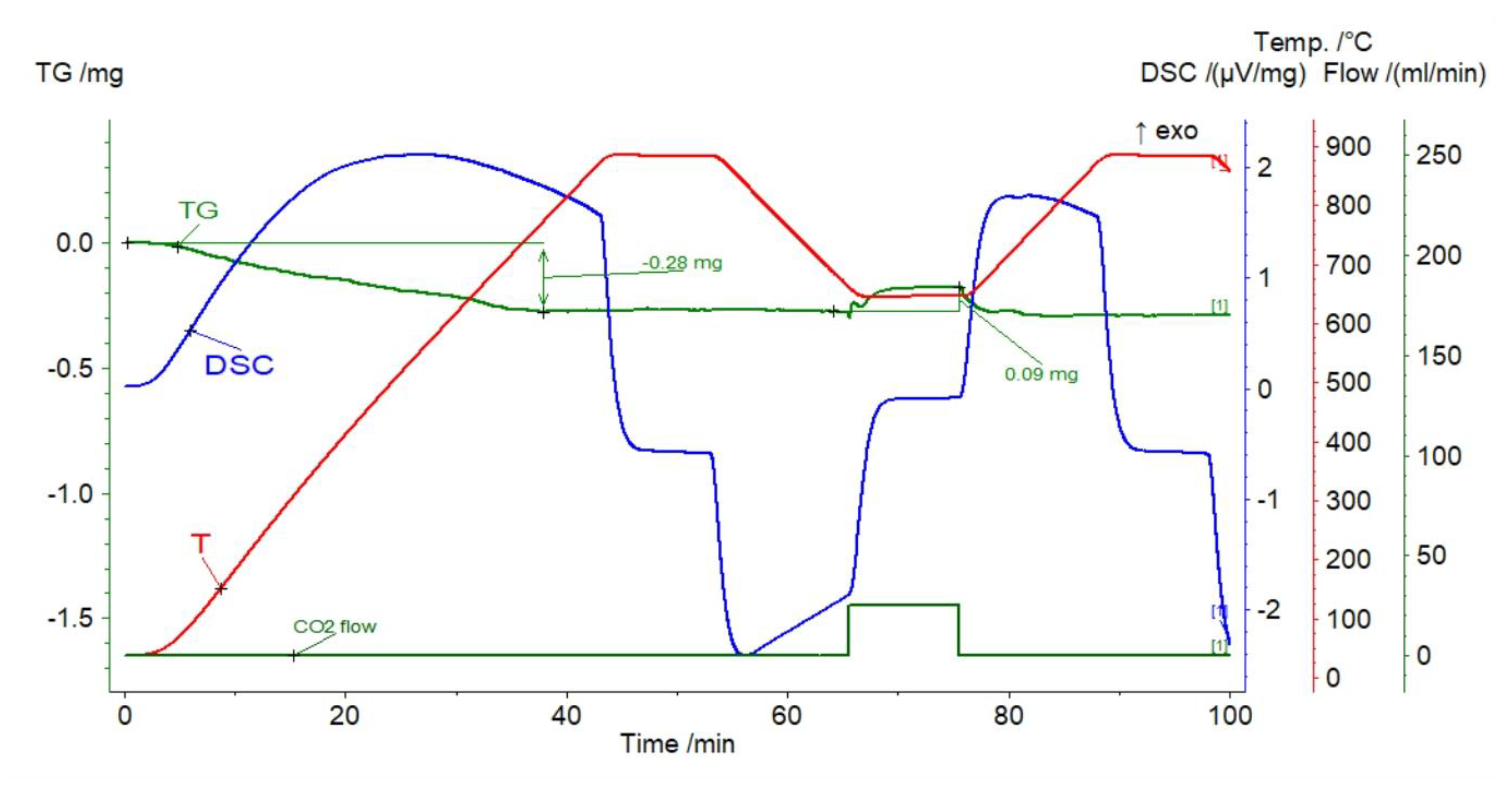
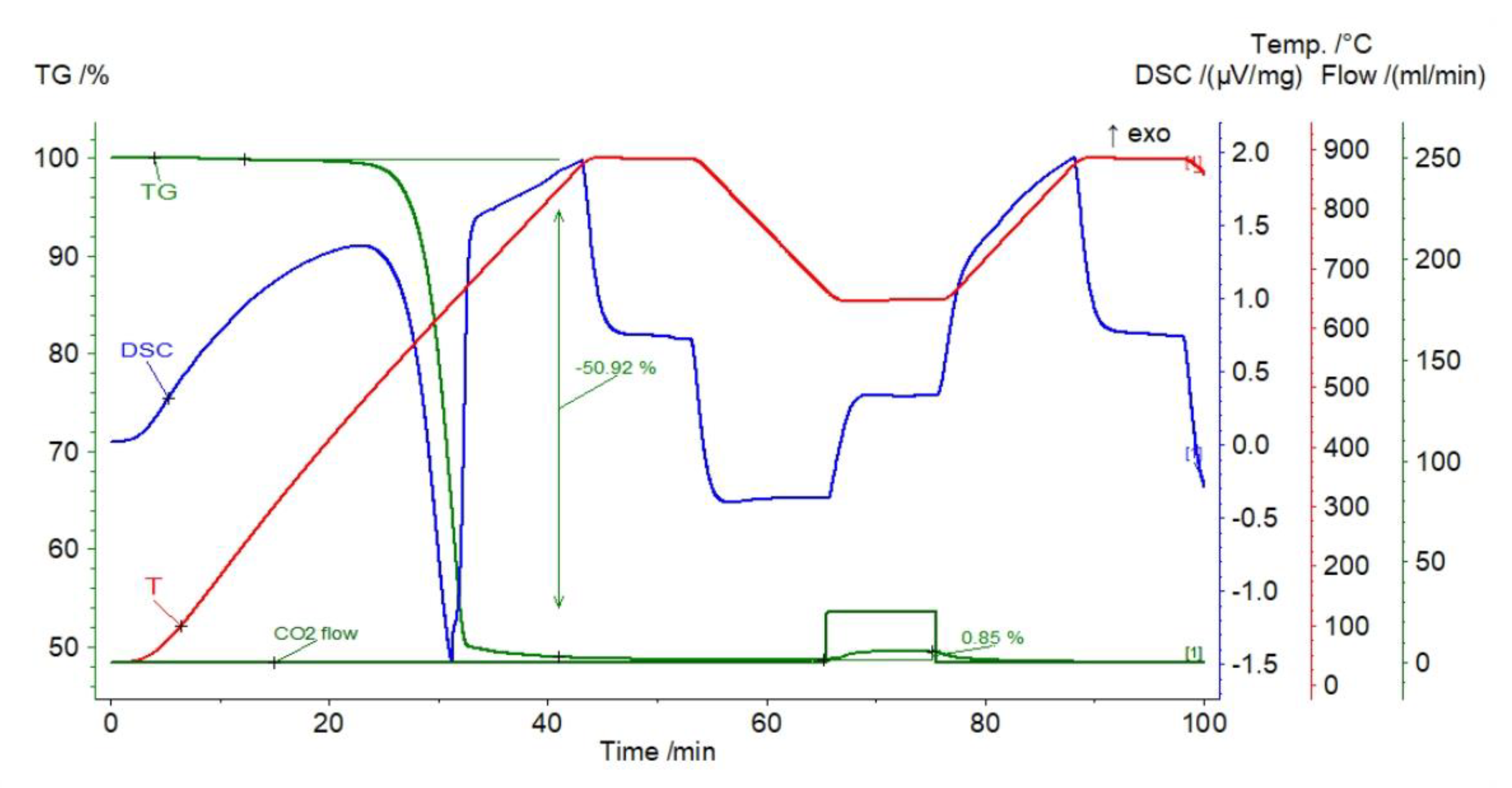
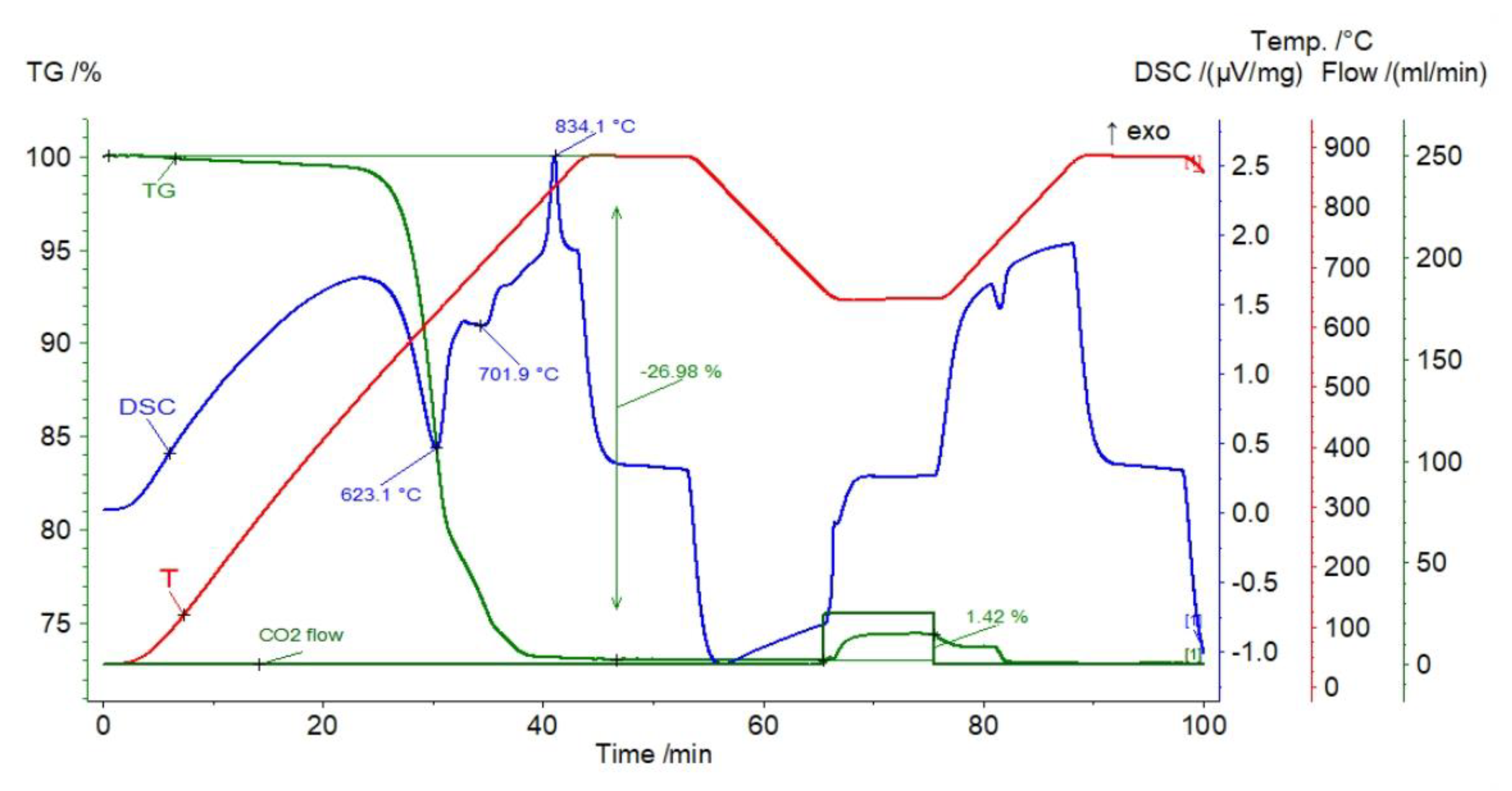
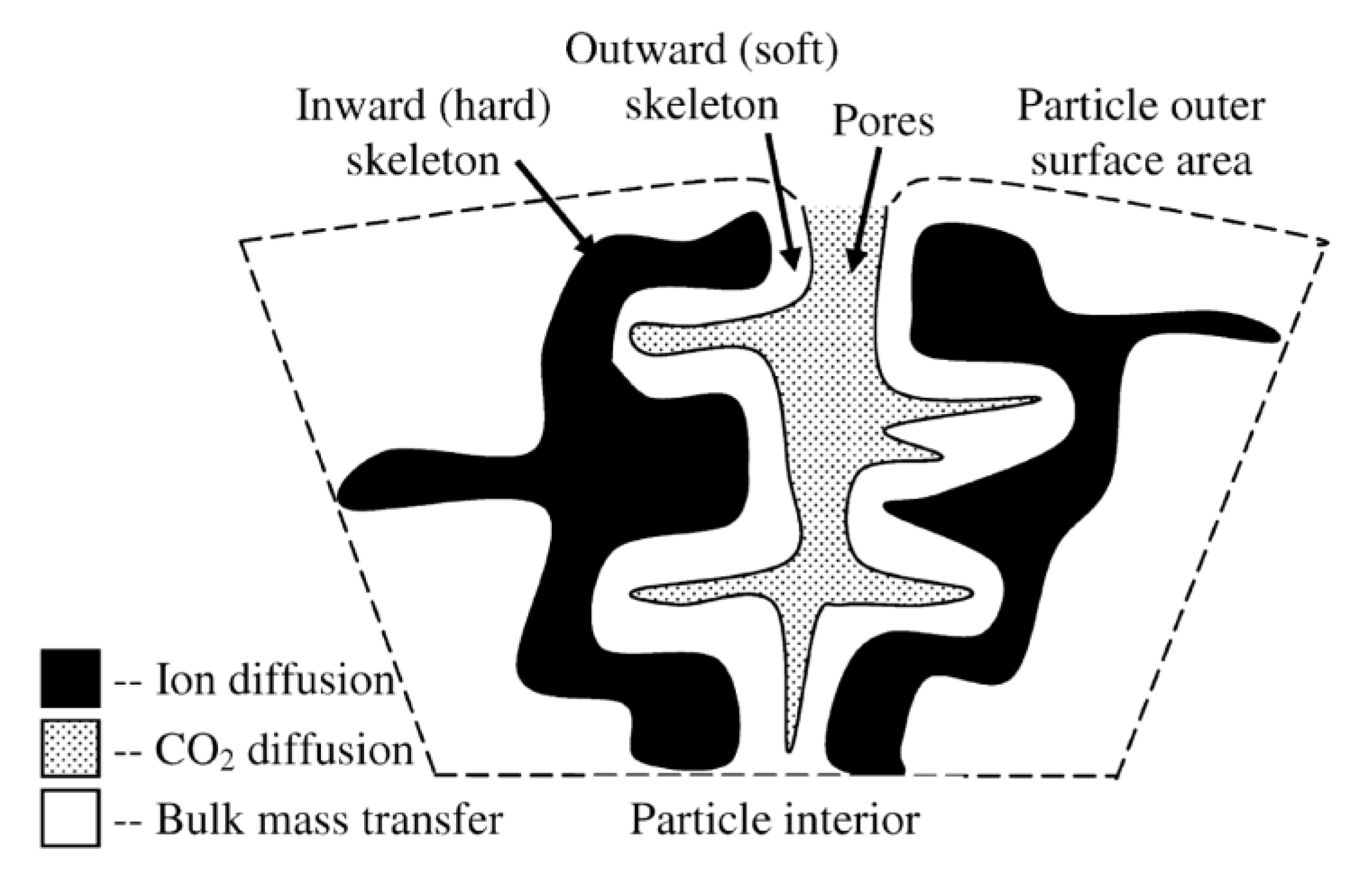
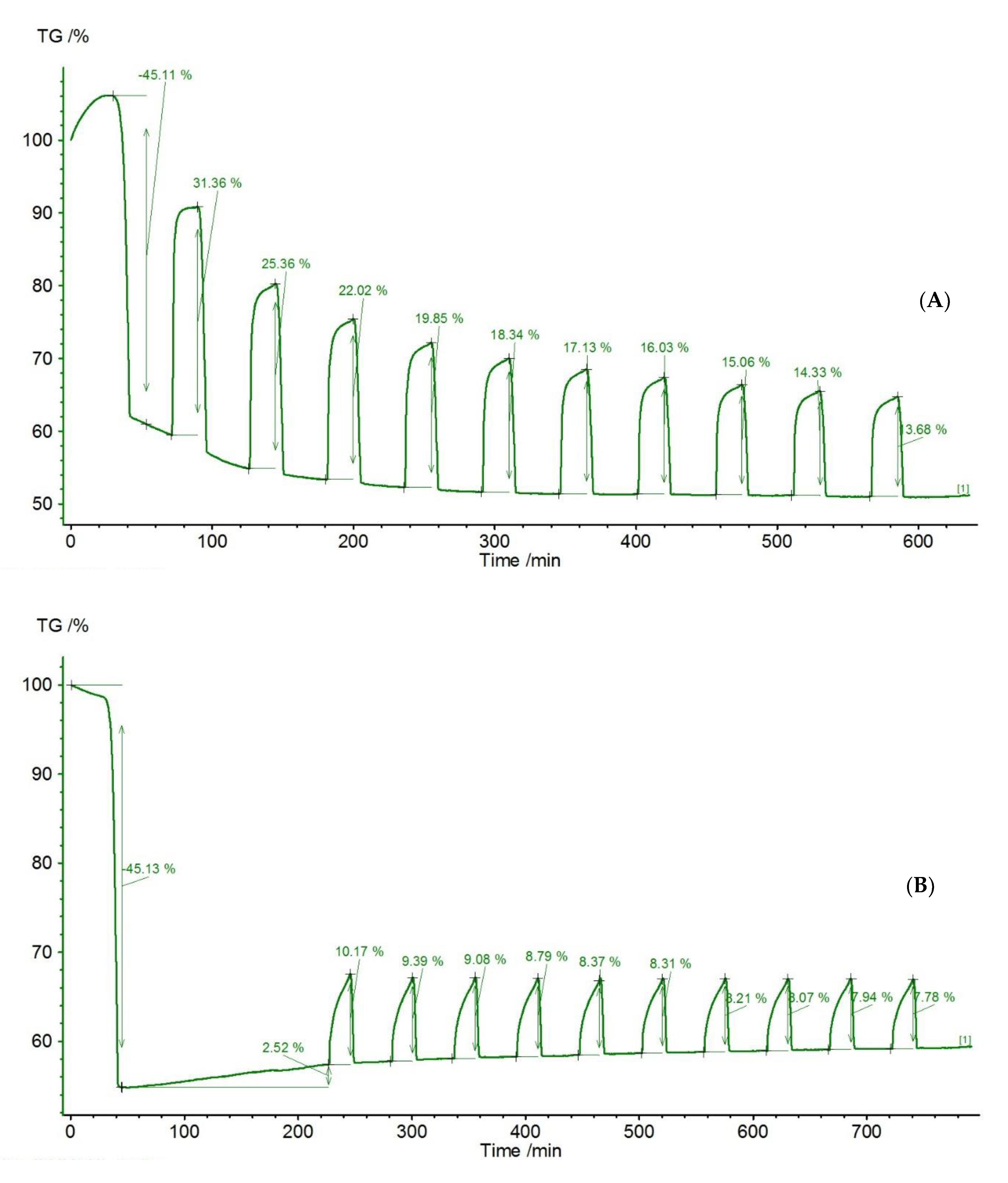

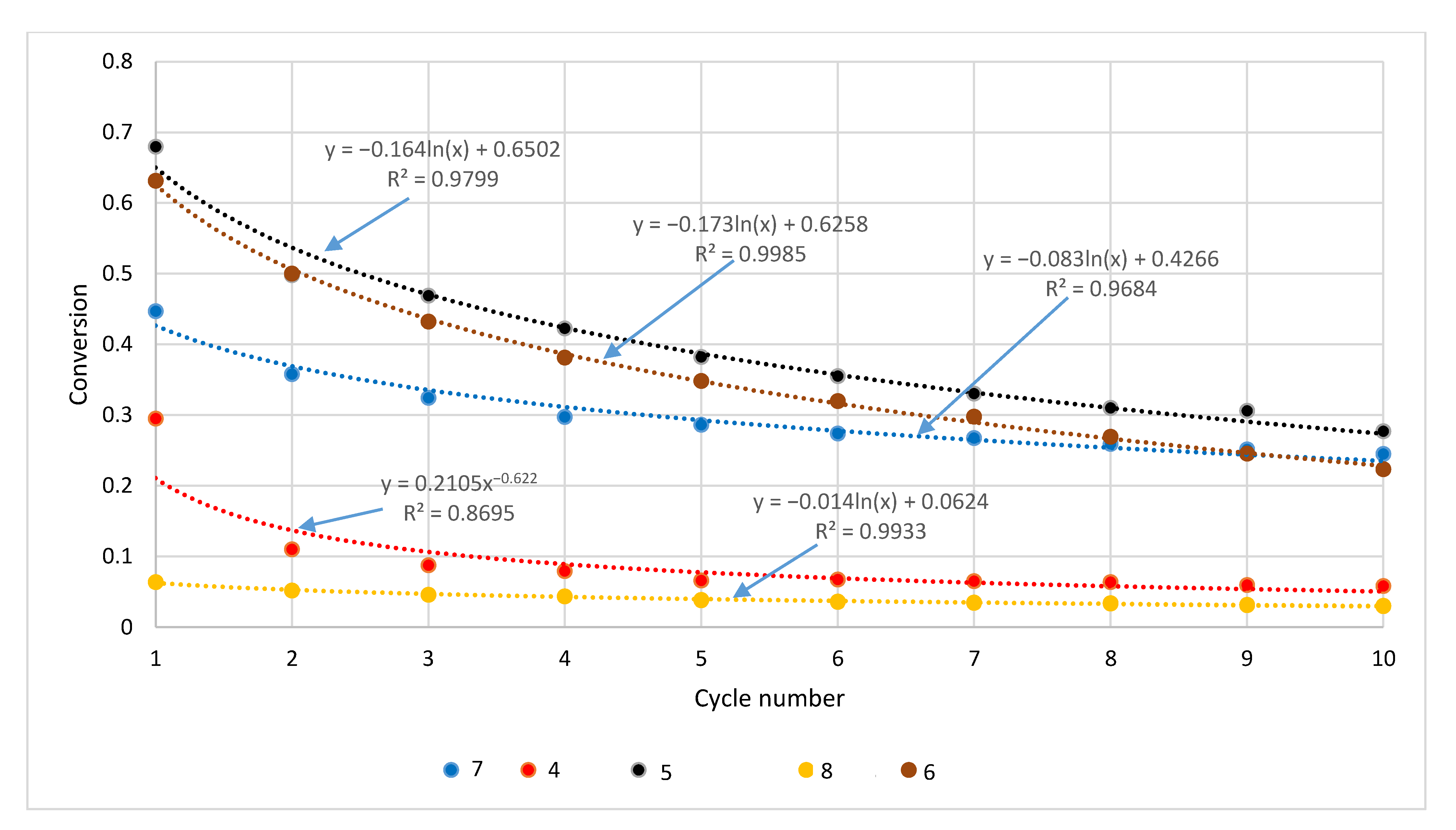
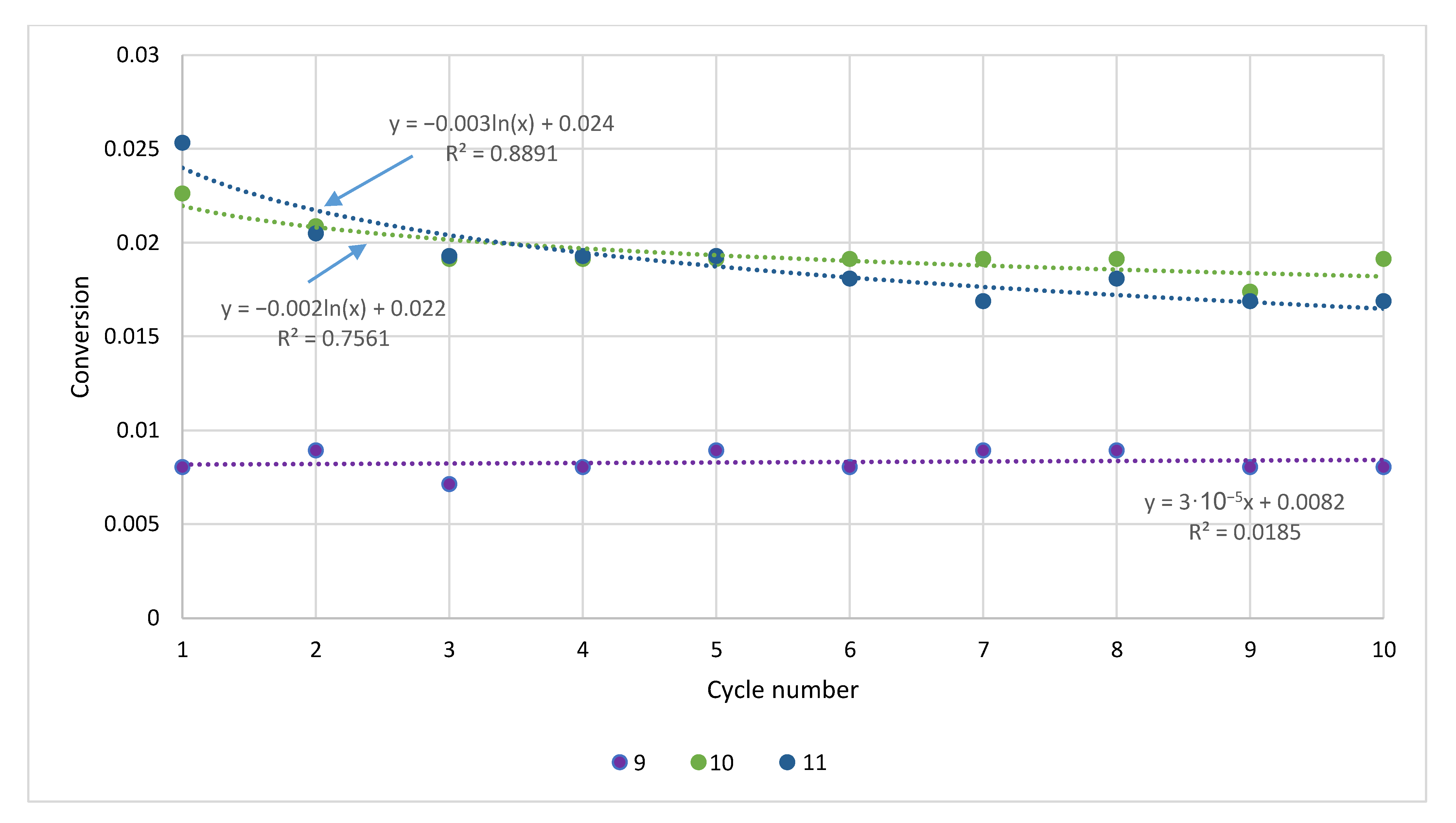
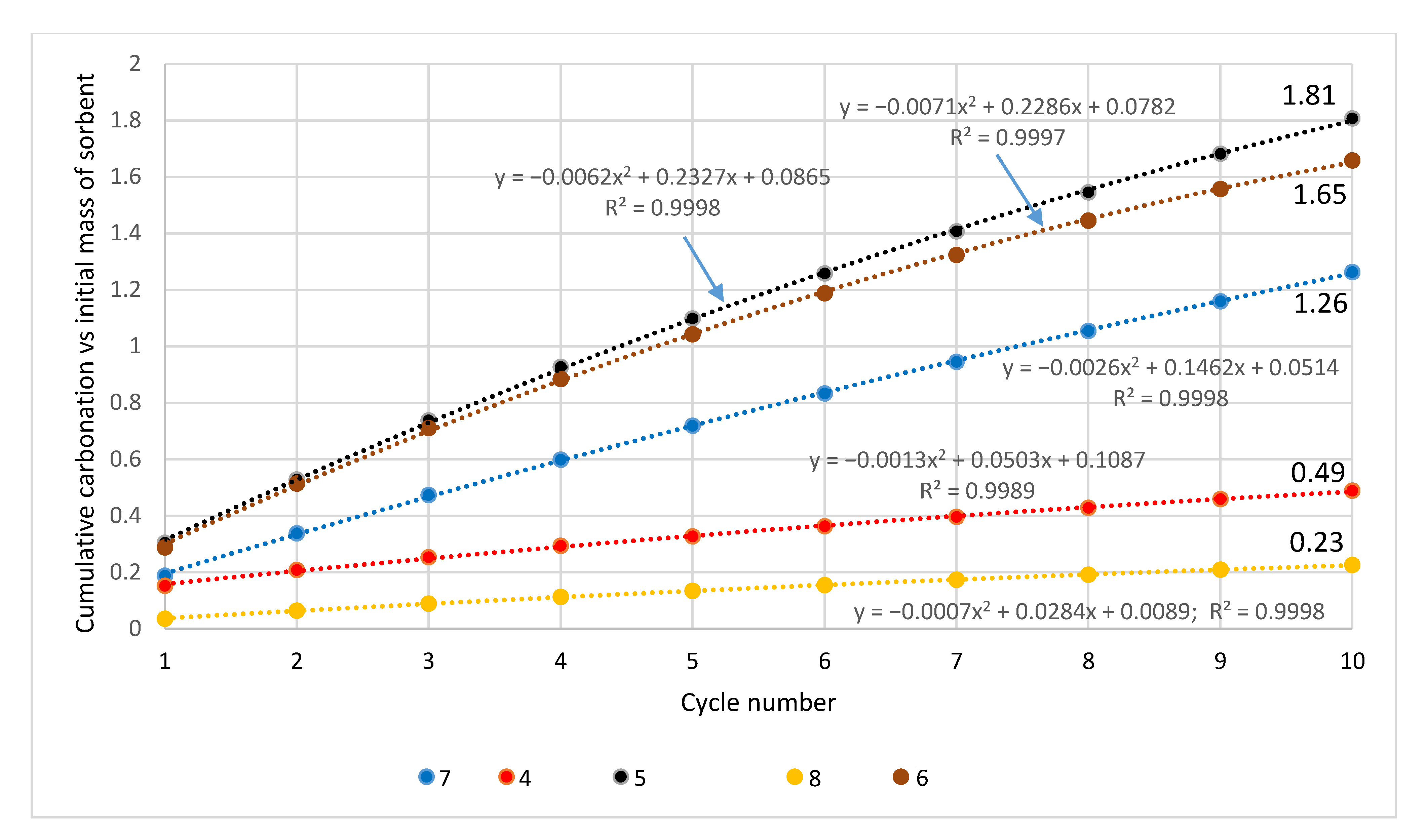
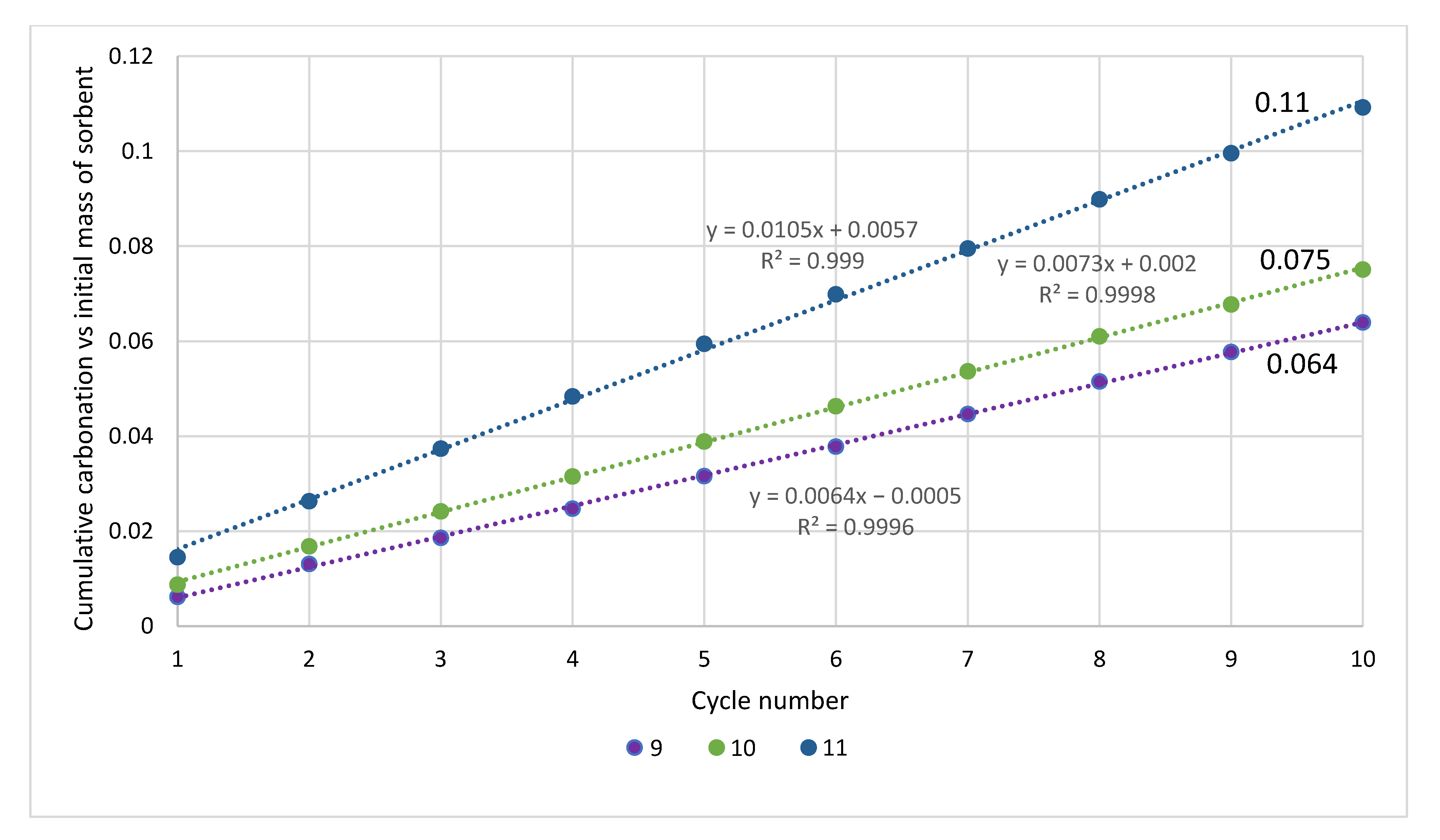
| Sample | Rock Type | Site/Age | Sample Mass [mg] | Mass Loss [%] | CaCO3 [%] |
|---|---|---|---|---|---|
| 1 | limestone | Stramberk(Czechia)/Jurassic | 20.02 | 41.57 | 90.1 |
| 2 | limestone | Podlesie (Poland)/Devonian | 19.58 | 39.00 | 84.5 |
| 3 | limestone | Butkov (Slovakia)/Cretaceous | 20.32 | 31.81 | 68.9 |
| 4 | bituminous limestone | Dębnik (Poland)/Devonian | 14.72 | 34.51 | 78.5 |
| 5 | limestone | Saint Anne Mountain (Poland)/Triassic | 15.46 | 42.88 | 97.5 |
| 6 | limestone | Gorazdze (Poland)/Triassic | 13.9 | 42.09 | 95.7 |
| 7 | dolomite | Olkusz (Poland)/Triassic | 14.99 | 46.06 | 94.4 1 |
| 8 | marl | Cisownica (Poland)/Cretaceous | 15.09 | 29.75 | 67.7 |
| 9 | basalt (nefelinite) | Saint Anne Mountain (Poland)/Tertiary | 14.55 | 1.92 | - |
| 10 | magnesite | Braszowice (Poland)/Tertiary | 14.91 | 50.91 | 97.5 2 |
| 11 | serpentinite | Jordanów (Poland)/older than UpperDevonian | 14.46 | 26.97 | - |
| Cycle | Sample | |||||
|---|---|---|---|---|---|---|
| Štramberk | Podlesie | Butkov | ||||
| 90.1% CaCO3 | 84.5% CaCO3 | 68.9% CaCO3 | ||||
| Untreated | Pretreated | Untreated | Pretreated | Untreated | Pretreated | |
| 1 | 0.73 | 0.24 | 0.61 | 0.14 | 0.28 | 0.09 |
| 2 | 0.59 | 0.22 | 0.49 | 0.12 | 0.17 | 0.08 |
| 3 | 0.51 | 0.21 | 0.42 | 0.12 | 0.13 | 0.08 |
| 4 | 0.46 | 0.20 | 0.38 | 0.11 | 0.11 | 0.07 |
| 5 | 0.43 | 0.19 | 0.34 | 0.11 | 0.10 | 0.07 |
| 6 | 0.40 | 0.19 | 0.31 | 0.11 | 0.09 | 0.07 |
| 7 | 0.37 | 0.19 | 0.28 | 0.11 | 0.09 | 0.06 |
| 8 | 0.35 | 0.19 | 0.25 | 0.10 | 0.09 | 0.06 |
| 8 | 0.33 | 0.18 | 0.23 | 0.10 | 0.09 | 0.06 |
| 10 | 0.32 | 0.18 | 0.21 | 0.10 | 0.08 | 0.06 |
| Cycle | Sample | |||||
|---|---|---|---|---|---|---|
| Štramberk | Podlesie | Butkov | ||||
| Untreated | Pretreated | Untreated | Pretreated | Untreated | Pretreated | |
| 1 | 0.31 | 0.10 | 0.28 | 0.06 | 0.14 | 0.05 |
| 2 | 0.57 | 0.20 | 0.50 | 0.12 | 0.23 | 0.09 |
| 3 | 0.79 | 0.29 | 0.70 | 0.17 | 0.29 | 0.13 |
| 4 | 0.99 | 0.37 | 0.87 | 0.22 | 0.35 | 0.16 |
| 5 | 1.17 | 0.46 | 1.03 | 0.27 | 0.40 | 0.20 |
| 6 | 1.34 | 0.54 | 1.17 | 0.32 | 0.45 | 0.23 |
| 7 | 1.50 | 0.62 | 1.30 | 0.37 | 0.50 | 0.26 |
| 8 | 1.65 | 0.70 | 1.41 | 0.41 | 0.54 | 0.29 |
| 8 | 1.80 | 0.78 | 1.52 | 0.46 | 0.58 | 0.32 |
| 10 | 1.93 | 0.86 | 1.62 | 0.50 | 0.62 | 0.35 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Labus, K. Comparison of the Properties of Natural Sorbents for the Calcium Looping Process. Materials 2021, 14, 548. https://doi.org/10.3390/ma14030548
Labus K. Comparison of the Properties of Natural Sorbents for the Calcium Looping Process. Materials. 2021; 14(3):548. https://doi.org/10.3390/ma14030548
Chicago/Turabian StyleLabus, Krzysztof. 2021. "Comparison of the Properties of Natural Sorbents for the Calcium Looping Process" Materials 14, no. 3: 548. https://doi.org/10.3390/ma14030548






