Effect of Titanium and Boron Microalloying on Sulfide Stress Cracking in C110 Casing Steel
Abstract
:1. Introduction
2. Experimental Procedure
3. Results
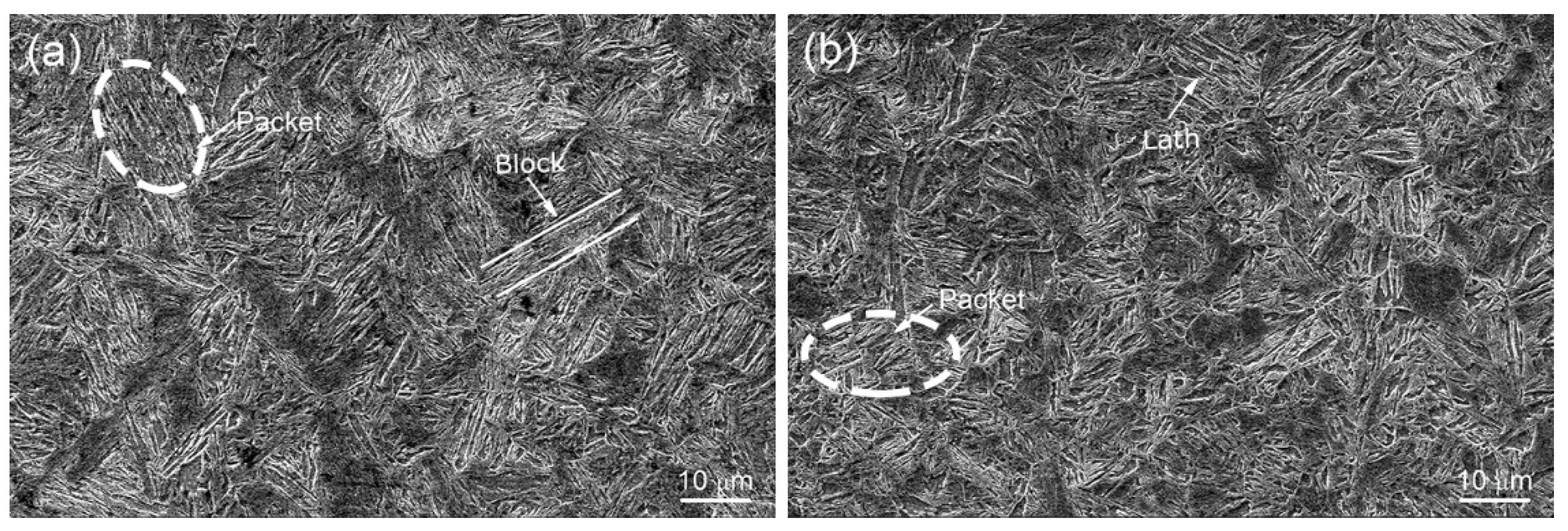
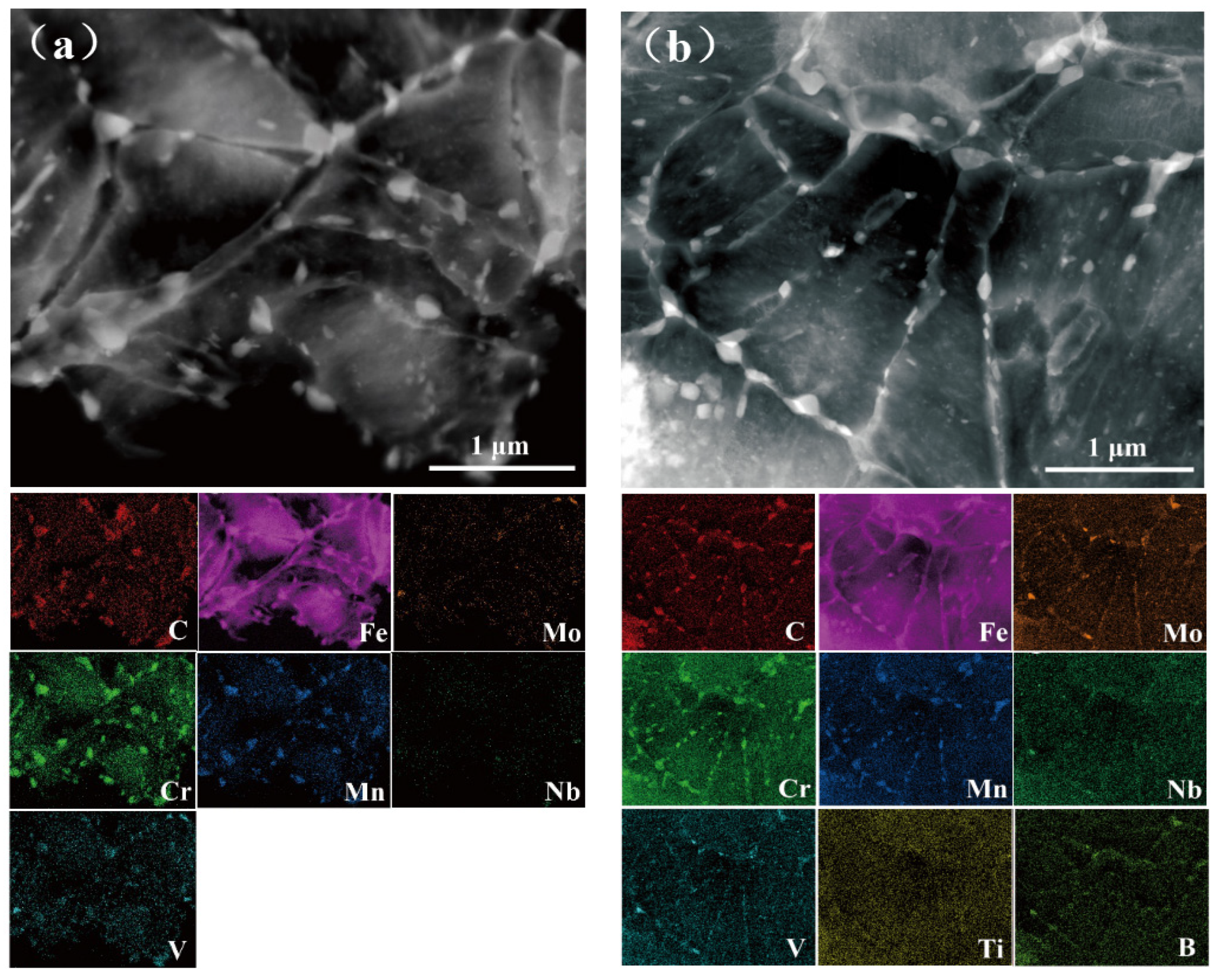
3.1. Microstructure Characterization
3.2. Steel Hardenability
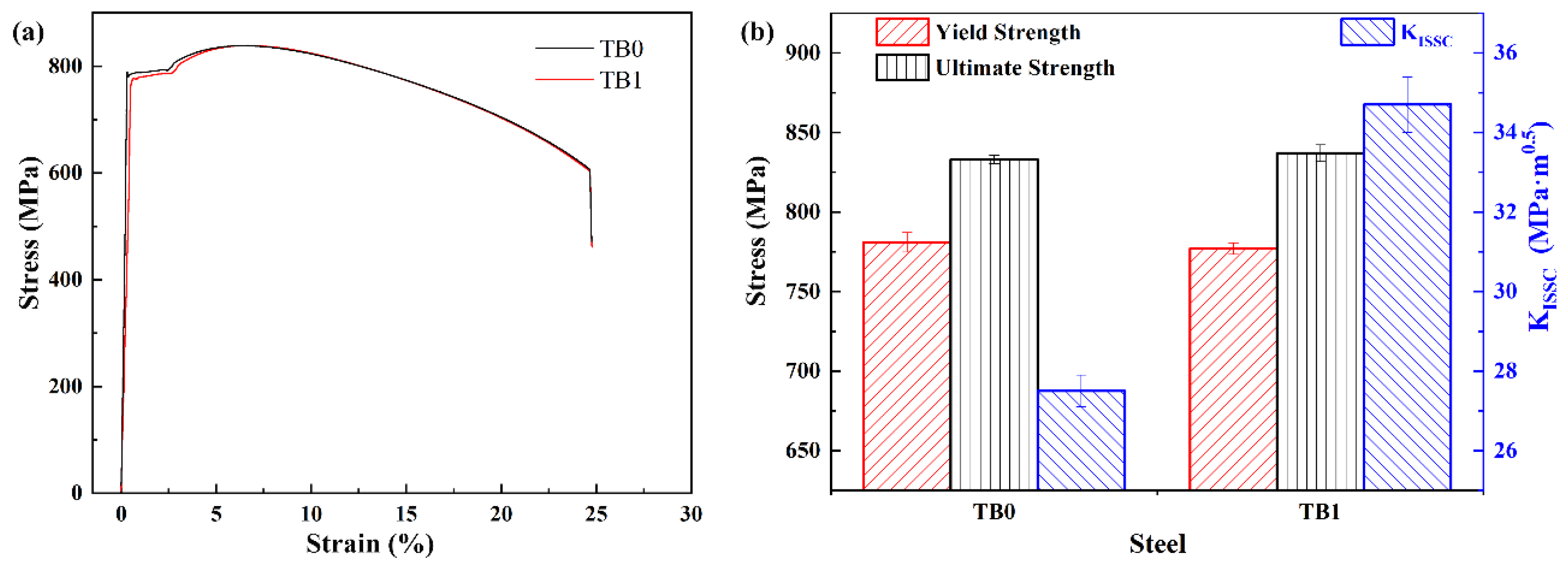
3.3. Mechanical Properties and KISSC Values for Steels
4. Discussion
5. Conclusions
- Ti and B addition increases the hardenability of the medium-carbon Fe-Cr-Mo-Nb-V steel, and the single surface quenching depth of TB1 is higher by ~6 mm than TB0. Ti and B addition enhances the quenching hardness and tempering precipitation, which leads to the same strength level for TB1 that was tempered at 710 °C and TB0 tempered at 700 °C.
- Ti and B addition refined the prior austenite grains and martensitic packets after quenching and the microstructure and precipitated carbides after tempering, which improved the sulfide stress cracking of steel. The KISSC value of TB1 (34.7 MPa·m0.5) was approximately one quarter that of TB0 (27.5 MPa·m0.5).
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Shi, X.-B.; Yan, W.; Wang, W.; Zhao, L.-Y.; Shan, Y.-Y.; Yang, K. Effect of Microstructure on Hydrogen Induced Cracking Behavior of a High Deformability Pipeline Steel. J. Iron Steel Res. Int. 2015, 22, 937–942. [Google Scholar] [CrossRef]
- Shi, X.-B.; Yan, W.; Wang, W.; Zhao, L.-Y.; Shan, Y.-Y.; Yang, K. HIC and SSC Behavior of High-Strength Pipeline Steels. Acta Metall. Sin.-Engl. Lett. 2015, 28, 799–808. [Google Scholar] [CrossRef]
- Ovchinnikov, D.V.; Sofrygina, O.A.; Zhukova, S.Y.; Pyshmintsev, I.Y.; Bityukov, S.M. Influence of microalloying with boron on the structure and properties of high-strength oil pipe. Steel Transl. 2011, 41, 356–360. [Google Scholar] [CrossRef]
- Ueno, M.; Inoue, T. Distribution of Boron at Austenite Grain Boundaries and Bainitic Transformation in Low Carbon Steels. Trans. Iron Steel Inst. Jpn. 1973, 13, 210–217. [Google Scholar] [CrossRef]
- Terzic, A.; Calcagnotto, M.; Guk, S.; Schulz, T.L.; Kawalla, R. Influence of Boron on transformation behavior during continuous cooling of low alloyed steels. Mater. Sci. Eng. A 2013, 584, 32–40. [Google Scholar] [CrossRef]
- Titova, T.I.; Shulgan, N.A.; Malykhina, I.Y. Effect of boron microalloying on the structure and hardenability of building steel. Met. Sci. Heat Treat. 2007, 49, 39–44. [Google Scholar] [CrossRef]
- Chen, C.; Yen, H.; Kao, F.; Li, W.; Huang, C.; Yang, J.; Wang, S. Precipitation hardening of high-strength low-alloy steels by nanometer-sized carbides. Mater. Sci. Eng. A 2009, 499, 162–166. [Google Scholar] [CrossRef]
- Schino, A.D.; Porcu, G.; Longobardo, M.; Turconi, G.L.; Scoppio, L. Metallurgical Design and Development of C125 Grade for Mild Sour Service Application. In Proceedings of the 61st Annual Conference & Exposition, San Diego, CA, USA, 12–16 March 2006. [Google Scholar]
- Sponseller, D.L.; Sponseller, T.E.; Bruce, E. Sulfide Fracture Toughness of Low-Alloy Steel Octg in Mild Conditions at Room Temperature and 40 °F. In Proceedings of the 63st Annual Conference & Exposition, New Orleans, LA, USA, 16–20 March 2008. [Google Scholar]
- Liu, M.; Yang, C.D.; Cao, G.H.; Russell, A.M.; Liu, Y.; Dong, X.M.; Zhang, Z.H. Effect of microstructure and crystallography on sulfide stress cracking in API-5CT-C110 casing steel. Mater. Sci. Eng. A 2016, 671, 244–253. [Google Scholar] [CrossRef] [Green Version]
- Liu, M.; Wang, C.H.; Dai, Y.C.; Li, X.; Cao, G.H.; Russell, A.M.; Liu, Y.H.; Dong, X.M.; Zhang, Z.H. Effect of quenching and tempering process on sulfide stress cracking susceptibility in API-5CT-C110 casing steel. Mater. Sci. Eng. A 2017, 688, 378–387. [Google Scholar] [CrossRef]
- Zhou, D.M.; Wang, B.; Lu, J.Z. Selection strategies of oilwell pipe materials in sour environments. Corros. Prot. 2011, 32, 308–311. [Google Scholar]
- Gelder, K.V.; Erlings, J.G.; Damen, J.W.M.; Visser, A. The stress corrosion of duplex stainless steel in H2S/CO2/Cl- environments. Corros. Sci. 1987, 27, 1271–1279. [Google Scholar] [CrossRef]
- Zubar, T.I.; Fedosyuk, V.; Tishkevich, D.I.; Kanafyev, O.; Astapovich, K.A.; Kozlovskiy, A.L.; Zdorovets, M.V.; Vinnik, D.A.; A Gudkova, S.; Kaniukov, E.Y.; et al. The Effect of Heat Treatment on the Microstructure and Mechanical Properties of 2D Nanostructured Au/NiFe System. Nanomaterials 2020, 10, 1077. [Google Scholar] [CrossRef] [PubMed]
- NACE TM0177: Laboratory Testing of Metals for Resistance to Sulfide Stress Cracking and Stress Corrosion Cracking in H2S Environments; NACE International: Houston, TX, USA, 2005.
- API SPECIFICATION 5CT: Casing and Tubing; American Petroleum Institute: Washington, DC, USA, 2018.
- Li, W.; Zhou, Y.-J.; Xue, Y. Corrosion Behavior of 110S Tube Steel in Environments of High H2S and CO2 Content. J. Iron Steel Res. Int. 2012, 19, 59–65. [Google Scholar] [CrossRef]
- NACE MR0175: Petroleum and Natural Gas Industries—Materials for Use in H2S-Containing Environments in Oil and Gas Production; NACE International: Houston, TX, USA, 2009.
- Tsay, L.; Lee, W.; Luu, W.; Wu, J. Effect of hydrogen environment on the notched tensile properties of T-250 maraging steel annealed by laser treatment. Corros. Sci. 2002, 44, 1311–1327. [Google Scholar] [CrossRef]
- Courtney, T. Mechanical Behavior of Materials; McGraw-Hill College: New York, NY, USA, 1990; pp. 173–184. [Google Scholar]
- Jiang, B.; Zhou, L.; Wen, X.; Zhang, C.; Liu, Y. Heat Treatment Properties of 42CrMo Steel for Bearing Ring of Varisized Shield Tunneling Machine. Acta Met. Sin. Engl. Lett. 2014, 27, 383–388. [Google Scholar] [CrossRef]
- Hill, M.; Kawasaki, E.P.; Kronbach, G.E. Oil Well Casing-Evidence of the Sensitivity to Rapid Failure in an H2S Environment. Mater. Prot. 1972, 11, 19–22. [Google Scholar]
- Wu, H.B.; Liu, L.F.; Wang, L.D.; Liu, Y.T. Influence of Chromium on Mechanical Properties and CO2/H2S Corrosion Behavior of P110 Grade Tube Steel. J. Iron Steel Res. Int. 2014, 21, 76–85. [Google Scholar] [CrossRef]
- Wang, B.; Liu, Z.D.; Cheng, S.C.; Liu, C.M.; Wang, J.Z. Evolution and coarsening of carbides in 2.25Cr-lMo steel weld metal during high temperature tempering. J. Iron Steel Res. Int. 2010, 17, 74–78. [Google Scholar] [CrossRef]
- Ravi, K.; Ramaswamy, V.; Namboodhiri, T. Effect of molybdenum on the resistance to H2S of high sulphur microalloyed steels. Mater. Sci. Eng. A 1993, 169, 111–118. [Google Scholar] [CrossRef]
- Chu, W.Y.; Wang, Y.B.; Guang, Y.S.; Yan, Y.L. Design of API C90 tubular steel. Acta Metall. Sin. 1998, 34, 1073–1076. [Google Scholar]
- Yu, Z.-S.; Zhang, J.-X.; Wang, H.-Z.; Zhou, R.-C.; Yuan, Y. Mechanism of Stress Relief Cracking in a Granular Bainitic Steel. Acta Met. Sin. Engl. Lett. 2016, 30, 156–163. [Google Scholar] [CrossRef]
- DePover, T.; Verbeken, K. The effect of TiC on the hydrogen induced ductility loss and trapping behavior of Fe-C-Ti alloys. Corros. Sci. 2016, 112, 308–326. [Google Scholar] [CrossRef]
- Ohnuma, M.; Suzuki, J.-I.; Wei, F.-G.; Tsuzaki, K. Direct observation of hydrogen trapped by NbC in steel using small-angle neutron scattering. Scr. Mater. 2008, 58, 142–145. [Google Scholar] [CrossRef]
- Wei, F.-G.; Hara, T.; Tsuchida, T.; Tsuzaki, K. Hydrogen Trapping in Quenched and Tempered 0.42C-0.30Ti Steel Containing Bimodally Dispersed TiC Particles. ISIJ Int. 2003, 43, 539–547. [Google Scholar] [CrossRef]
- Wei, F.G.; Hara, T.; Tsuzaki, K. Precise determination of the activation energy for desorption of hydrogen in two Ti-added steels by a single thermal-desorption spectrum. Met. Mater. Trans. A 2004, 35, 587–597. [Google Scholar] [CrossRef]
- Pressouyre, G. Trap theory of Hydrogen embrittlement. Acta Met. 1980, 28, 895–911. [Google Scholar] [CrossRef]
- Wang, X.T.; Liu, M.; Zhou, G.Y.; Jiang, H.; Li, X.; Luo, M.; Liu, Y.H.; Zhang, Z.H.; Cao, G.H. Effects of chromium and tungsten on sulfide stress cracking in high strength low alloy 125 ksi grade casing steel. Corros. Sci. 2019, 160, 108163. [Google Scholar] [CrossRef]



| Steel | C | Si | Mn | S | P | Cr | Mo | V | Ti | Nb | B | N | O |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| TB0 | 0.27 | 0.28 | 0.49 | 0.001 | 0.009 | 0.51 | 0.87 | 0.10 | — | 0.04 | — | 0.004 | 0.001 |
| TB1 | 0.27 | 0.28 | 0.48 | 0.001 | 0.009 | 0.53 | 0.78 | 0.11 | 0.015 | 0.04 | 0.002 | 0.004 | 0.001 |
| Steel | Ferrite | Cementite | (Nb,Ti)C | M2C | M23C6 | MC |
|---|---|---|---|---|---|---|
| TB0 | 0.9560 | 0.0223 | 0.0007 | 0.0133 | 0.0037 | 0.0040 |
| TB1 | 0.9568 | 0.0258 | 0.0010 | 0.0098 | 0.0022 | 0.0044 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Luo, M.; Zhang, Z.-H.; Liu, Y.-H.; Li, M.-C. Effect of Titanium and Boron Microalloying on Sulfide Stress Cracking in C110 Casing Steel. Materials 2020, 13, 5713. https://doi.org/10.3390/ma13245713
Luo M, Zhang Z-H, Liu Y-H, Li M-C. Effect of Titanium and Boron Microalloying on Sulfide Stress Cracking in C110 Casing Steel. Materials. 2020; 13(24):5713. https://doi.org/10.3390/ma13245713
Chicago/Turabian StyleLuo, Ming, Zhong-Hua Zhang, Yao-Heng Liu, and Mou-Cheng Li. 2020. "Effect of Titanium and Boron Microalloying on Sulfide Stress Cracking in C110 Casing Steel" Materials 13, no. 24: 5713. https://doi.org/10.3390/ma13245713






