Submicron-Sized Nb-Doped Lithium Garnet for High Ionic Conductivity Solid Electrolyte and Performance of Quasi-Solid-State Lithium Battery
Abstract
1. Introduction
2. Materials and Methods
2.1. The Synthesis of LLZNO Powder and Ceramics
2.2. Fabrication of Composite Cathodes and Assembly of Quasi-Solid-State Batteries
2.3. Characterization
3. Results and Discussions
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Zhu, C.; Wei, D.; Wu, Y.; Zhang, Z.; Zhang, G.; Duan, J.; Li, L.; Zhu, H.; Zhu, Z.; Chen, Z. Controllable construction of interconnected SnOx/N-doped carbon/carbon composite for enhanced-performance lithium-ion batteries anodes. J. Alloy. Compd. 2019, 778, 731–740. [Google Scholar] [CrossRef]
- Li, X.; Zhang, K.; Mitlin, D.; Yang, Z.; Wang, M.; Tang, Y.; Jiang, F.; Du, Y.; Zheng, J. Fundamental insight into Zr modification of Li-and Mn-rich cathodes: combined transmission electron microscopy and electrochemical impedance spectroscopy study. Chem Mater 2018, 30, 2566–2573. [Google Scholar] [CrossRef]
- Chen, Z.; Xu, M.; Zhu, H.; Xie, T.; Wang, W.; Zhao, Q. Enhanced electrochemical performance of polyacene coated LiMn2O3.95F0.05 for lithium ion batteries. Appl. Surf. Sci. 2013, 286, 177–183. [Google Scholar] [CrossRef]
- Chen, Z.; Zhang, Z.; Zhao, Q.; Duan, J.; Zhu, H. Understanding the Impact of K-Doping on the Structure and Performance of LiFePO4/C Cathode Materials. J. Nanosc. Nanotechnol. 2019, 19, 119–124. [Google Scholar] [CrossRef]
- Liu, W.; Hu, G.; Du, K.; Peng, Z.; Cao, Y. Enhanced storage property of LiNi0.8Co0.15Al0.05O2 coated with LiCoO2. J. Power Sources 2013, 230, 201–206. [Google Scholar] [CrossRef]
- Li, X.; Zhang, K.; Wang, M.; Liu, Y.; Qu, M.; Zhao, W.; Zheng, J. Dual functions of zirconium modification on improving the electrochemical performance of Ni-rich LiNi0.8Co0.1Mn0.1O2. Sustain Energ Fuels 2018, 2, 413–421. [Google Scholar] [CrossRef]
- Hu, G.; Liu, W.; Peng, Z.; Du, K.; Cao, Y. Synthesis and electrochemical properties of LiNi0. 8Co0.15Al0.05O2 prepared from the precursor Ni0.8Co0.15Al0.05OOH. J. Power Sources 2012, 198, 258–263. [Google Scholar] [CrossRef]
- Liu, J.; Liu, Q.; Zhu, H.; Lin, F.; Ji, Y.; Li, B.; Duan, J.; Li, L.; Chen, Z. Effect of Different Composition on Voltage Attenuation of Li-Rich Cathode Material for Lithium-ion Batteries. Materials 2020, 13, 40. [Google Scholar] [CrossRef]
- Ohta, S.; Seki, J.; Yagi, Y.; Kihira, Y.; Tani, T.; Asaoka, T. Co-sinterable lithium garnet-type oxide electrolyte with cathode for all-solid-state lithium ion battery. J. Power Sources 2014, 265, 40–44. [Google Scholar] [CrossRef]
- Sakuda, A.; Takeuchi, T.; Kobayashi, H. Electrode morphology in all-solid-state lithium secondary batteries consisting of LiNi1/3Co1/3Mn1/3O2 and Li2S-P2S5 solid electrolytes. Solid State Ionics 2016, 285, 112–117. [Google Scholar]
- Wu, B.; Wang, S.; Evans, W.J., IV; Deng, D.Z.; Yang, J.; Xiao, J. Interfacial behaviours between lithium ion conductors and electrode materials in various battery systems. J. Mater. Chem. A 2016, 4, 15266–15280. [Google Scholar] [CrossRef]
- Alpen, U.V.; Rabenau, A.; Talat, G. Ionic conductivity in Li3N single crystals. Appl. Phys. Lett. 1977, 30, 621–623. [Google Scholar] [CrossRef]
- Senevirathne, K.; Day, C.S.; Gross, M.D.; Lachgar, A.; Holzwarth, N. A new crystalline LiPON electrolyte: Synthesis, properties, and electronic structure. Solid State Ionics 2013, 233, 95–101. [Google Scholar] [CrossRef]
- Uhlmann, C.; Braun, P.; Illig, J.; Weber, A.; Ivers-Tiffée, E. Interface and grain boundary resistance of a lithium lanthanum titanate (Li3xLa2/3− xTiO3, LLTO) solid electrolyte. J. Power. Sources 2016, 307, 578–586. [Google Scholar] [CrossRef]
- Kanno, R.; Murayama, M. Lithium Ionic Conductor Thio-LISICON: The Li2S·GeS2·P2S5·System. J. Electrochem. Soc. 2001, 148, A742–A746. [Google Scholar] [CrossRef]
- Lai, Y.; Sun, Z.; Jiang, L.; Hao, X.; Jia, M.; Wang, L.; Liu, F. Rapid sintering of ceramic solid electrolytes LiZr2(PO4)3 and Li1.2Ca0.1Zr1.9(PO4)3 using a microwave sintering process at low temperatures. Ceram. Int. 2019, 45, 11068–11072. [Google Scholar] [CrossRef]
- Murugan, R.; Thangadurai, V.; Weppner, W. Fast lithium ion conduction in garnet-type Li7La3Zr2O12. Angew. Chem. Int. Edit. 2007, 46, 7778–7781. [Google Scholar] [CrossRef]
- Famprikis, T.; Canepa, P.; Dawson, J.A.; Islam, M.S.; Masquelier, C. Fundamentals of inorganic solid-state electrolytes for batteries. Nat. Mater. 2019, 18, 1278–1291. [Google Scholar] [CrossRef]
- Inaguma, Y.; Nakashima, M. A rechargeable lithium–air battery using a lithium ion-conducting lanthanum lithium titanate ceramics as an electrolyte separator. J. Power Sources 2013, 228, 250–255. [Google Scholar] [CrossRef]
- Awaka, J.; Kijima, N.; Kataoka, K.; Hayakawa, H.; Ohshima, K.-i.; Akimoto, J. Neutron powder diffraction study of tetragonal Li7La3Hf2O12 with the garnet-related type structure. J. Solid State Chem. 2010, 183, 180–185. [Google Scholar] [CrossRef]
- Huang, Z.; Liu, K.; Chen, L.; Lu, Y.; Li, Y.; Wang, C.A. Sintering behavior of garnet-type Li6.4La3Zr1.4Ta0.6O12 in Li2CO3 atmosphere and its electrochemical property. Int. J. Appl. Ceram. Technol. 2017, 14, 921–927. [Google Scholar] [CrossRef]
- Ren, Y.; Deng, H.; Chen, R.; Shen, Y.; Lin, Y.; Nan, C.-W. Effects of Li source on microstructure and ionic conductivity of Al-contained Li6.75La3Zr1.75Ta0.25O12 ceramics. J. Eur. Ceram. Soc. 2015, 35, 561–572. [Google Scholar] [CrossRef]
- Tsai, C.-L.; Dashjav, E.; Hammer, E.-M.; Finsterbusch, M.; Tietz, F.; Uhlenbruck, S.; Buchkremer, H.P. High conductivity of mixed phase Al-substituted Li7La3Zr2O12. J. Electroceram. 2015, 35, 25–32. [Google Scholar] [CrossRef]
- Janani, N.; Deviannapoorani, C.; Dhivya, L.; Murugan, R. Influence of sintering additives on densification and Li+ conductivity of Al doped Li7La3Zr2O12 lithium garnet. RSC Adv. 2014, 4, 51228–51238. [Google Scholar] [CrossRef]
- Jin, Y.; McGinn, P.J. Al-doped Li7La3Zr2O12 synthesized by a polymerized complex method. J. Power Sources 2011, 196, 8683–8687. [Google Scholar] [CrossRef]
- Huang, M.; Shoji, M.; Shen, Y.; Nan, C.-W.; Munakata, H.; Kanamura, K. Preparation and electrochemical properties of Zr-site substituted Li7La3(Zr2− xMx)O12 (M = Ta, Nb) solid electrolytes. J. Power Sources 2014, 261, 206–211. [Google Scholar] [CrossRef]
- Ohta, S.; Kobayashi, T.; Asaoka, T. High lithium ionic conductivity in the garnet-type oxide Li7−XLa3(Zr2−X, NbX)O12 (X = 0–2). J. Power Sources 2011, 196, 3342–3345. [Google Scholar] [CrossRef]
- Cao, Z.-Z.; Ren, W.; Liu, J.-R.; Li, G.-R.; Gao, Y.-F.; Fang, M.-H.; He, W.-Y. Microstructure and ionic conductivity of Sb-doped Li7La3Zr2O12 ceramics. J. Inorg. Mater. 2014, 29, 220–224. [Google Scholar] [CrossRef]
- Deviannapoorani, C.; Shankar, L.S.; Ramakumar, S.; Murugan, R. Investigation on lithium ion conductivity and structural stability of yttrium-substituted Li7La3Zr2O12. Ionics 2016, 22, 1281–1289. [Google Scholar] [CrossRef]
- Mukhopadhyay, S.; Thompson, T.; Sakamoto, J.; Huq, A.; Wolfenstine, J.; Allen, J.L.; Bernstein, N.; Stewart, D.A.; Johannes, M. Structure and stoichiometry in supervalent doped Li7La3Zr2O12. Chem. Mater. 2015, 27, 3658–3665. [Google Scholar] [CrossRef]
- David, I.N.; Thompson, T.; Wolfenstine, J.; Allen, J.L.; Sakamoto, J. Microstructure and Li-Ion Conductivity of Hot-Pressed Cubic Li7La3Zr2O12. J. Am. Ceram. Soc. 2015, 98, 1209–1214. [Google Scholar] [CrossRef]
- Baek, S.W.; Lee, J.M.; Kim, T.Y.; Song, M.S.; Park, Y. Garnet related lithium ion conductor processed by spark plasma sintering for all solid state batteries. J. Power Sources 2014, 249, 197–206. [Google Scholar] [CrossRef]
- Amores, M.; Ashton, T.E.; Baker, P.J.; Cussen, E.J.; Corr, S.A. Fast microwave-assisted synthesis of Li-stuffed garnets and insights into Li diffusion from muon spin spectroscopy. J. Mater. Chem. A 2016, 4, 1729–1736. [Google Scholar] [CrossRef]
- Murugan, R.; Ramakumar, S.; Janani, N. High conductive yttrium doped Li7La3Zr2O12 cubic lithium garnet. Electrochem. Commun. 2011, 13, 1373–1375. [Google Scholar] [CrossRef]
- Kumazaki, S.; Iriyama, Y.; Kim, K.-H.; Murugan, R.; Tanabe, K.; Yamamoto, K.; Hirayama, T.; Ogumi, Z. High lithium ion conductive Li7La3Zr2O12 by inclusion of both Al and Si. Electrochem. Commun. 2011, 13, 509–512. [Google Scholar] [CrossRef]
- Li, Y.; Cao, Y.; Guo, X. Influence of lithium oxide additives on densification and ionic conductivity of garnet-type Li6.75La3Zr1.75Ta0.25O12 solid electrolytes. Solid State Ionics 2013, 253, 76–80. [Google Scholar] [CrossRef]
- Tadanaga, K.; Takano, R.; Ichinose, T.; Mori, S.; Hayashi, A.; Tatsumisago, M. Low temperature synthesis of highly ion conductive Li7La3Zr2O12–Li3BO3 composites. Electrochem. Commun. 2013, 33, 51–54. [Google Scholar] [CrossRef]
- Janani, N.; Ramakumar, S.; Kannan, S.; Murugan, R. Optimization of lithium content and sintering aid for maximized Li+ conductivity and density in Ta-doped Li7La3Zr2O12. J. Am. Ceram. Soc. 2015, 98, 2039–2046. [Google Scholar] [CrossRef]
- Jonson, R.A.; McGinn, P.J. Tape casting and sintering of Li7La3Zr1.75Nb0.25Al0.1O12 with Li3BO3 additions. Solid State Ionics 2018, 323, 49–55. [Google Scholar]
- Rosero-Navarro, N.C.; Yamashita, T.; Miura, A.; Higuchi, M.; Tadanaga, K. Effect of sintering additives on relative density and Li-ion conductivity of Nb-doped Li7La3Zr2O12 solid electrolyte. J. Am. Ceram. Soc. 2017, 100, 276–285. [Google Scholar] [CrossRef]
- Wu, J.-F.; Pang, W.K.; Peterson, V.K.; Wei, L.; Guo, X. Garnet-type fast Li-ion conductors with high ionic conductivities for all-solid-state batteries. ACS Appl. Mater. Interfaces 2017, 9, 12461–12468. [Google Scholar] [CrossRef] [PubMed]
- Ohta, S.; Kobayashi, T.; Seki, J.; Asaoka, T. Electrochemical performance of an all-solid-state lithium ion battery with garnet-type oxide electrolyte. J. Power Sources 2012, 202, 332–335. [Google Scholar] [CrossRef]
- Jin, Y.; McGinn, P.J. Li7La3Zr2O12 electrolyte stability in air and fabrication of a Li/ Li7La3Zr2O12/Cu0.1V2O5 solid-state battery. J. Power Sources 2013, 239, 326–331. [Google Scholar] [CrossRef]
- Hu, Z.; Liu, H.; Ruan, H.; Hu, R.; Su, Y.; Zhang, L. High Li-ion conductivity of Al-doped Li7La3Zr2O12 synthesized by solid-state reaction. Ceram. Int. 2016, 42, 12156–12160. [Google Scholar] [CrossRef]
- Liu, Q.; Zhu, H.; Liu, J.; Liao, X.; Tang, Z.; Zhou, C.; Yuan, M.; Duan, J.; Li, L.; Chen, Z. High-Performance Lithium-Rich Layered Oxide Material: Effects of Preparation Methods on Microstructure and Electrochemical Properties. Materials 2020, 13, 334. [Google Scholar] [CrossRef]
- Zhang, W.; Nie, J.; Li, F.; Wang, Z.L.; Sun, C. A durable and safe solid-state lithium battery with a hybrid electrolyte membrane. Nano Energy 2018, 45, 413–419. [Google Scholar] [CrossRef]
- Yu, J.; Kwok, S.C.; Lu, Z.; Effat, M.B.; Lyu, Y.Q.; Yuen, M.M.; Ciucci, F. A Ceramic-PVDF Composite Membrane with Modified Interfaces as an Ion-Conducting Electrolyte for Solid-State Lithium-Ion Batteries Operating at Room Temperature. Chem. Electro. Chem. 2018, 5, 2873–2881. [Google Scholar] [CrossRef]
- Ye, M.; Jin, X.; Nan, X.; Gao, J.; Qu, L. Paraffin wax protecting 3D non-dendritic lithium for backside-plated lithium metal anode. Energy Storage Mater. 2020, 24, 153–159. [Google Scholar] [CrossRef]
- Manzi, J.; Brutti, S. Surface chemistry on LiCoPO4 electrodes in lithium cells: SEI formation and self-discharge. Electrochim. Acta 2016, 222, 1839–1846. [Google Scholar] [CrossRef]
- Huang, X.; Xiu, T.; Badding, M.E.; Wen, Z. Two-step sintering strategy to prepare dense Li-Garnet electrolyte ceramics with high Li+ conductivity. Ceram. Int. 2018, 44, 5660–5667. [Google Scholar] [CrossRef]

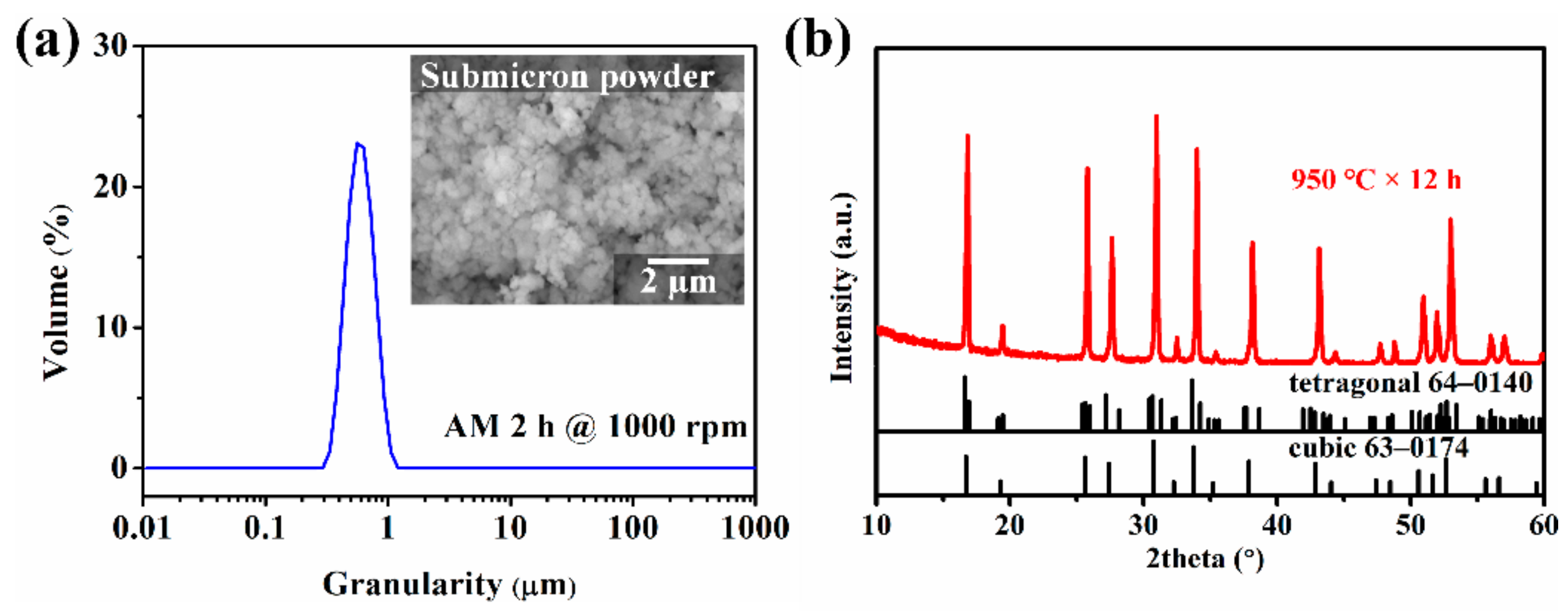
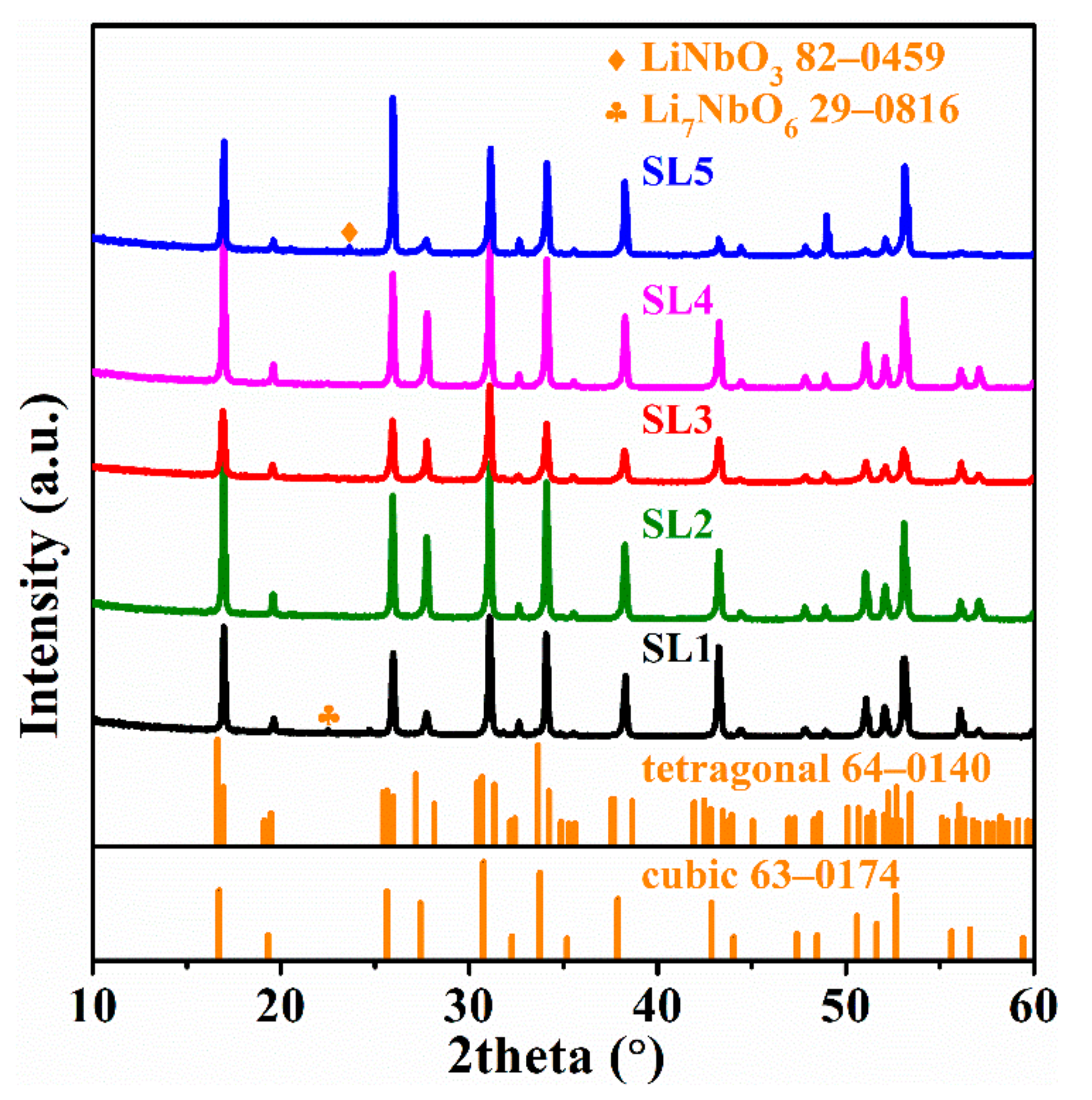



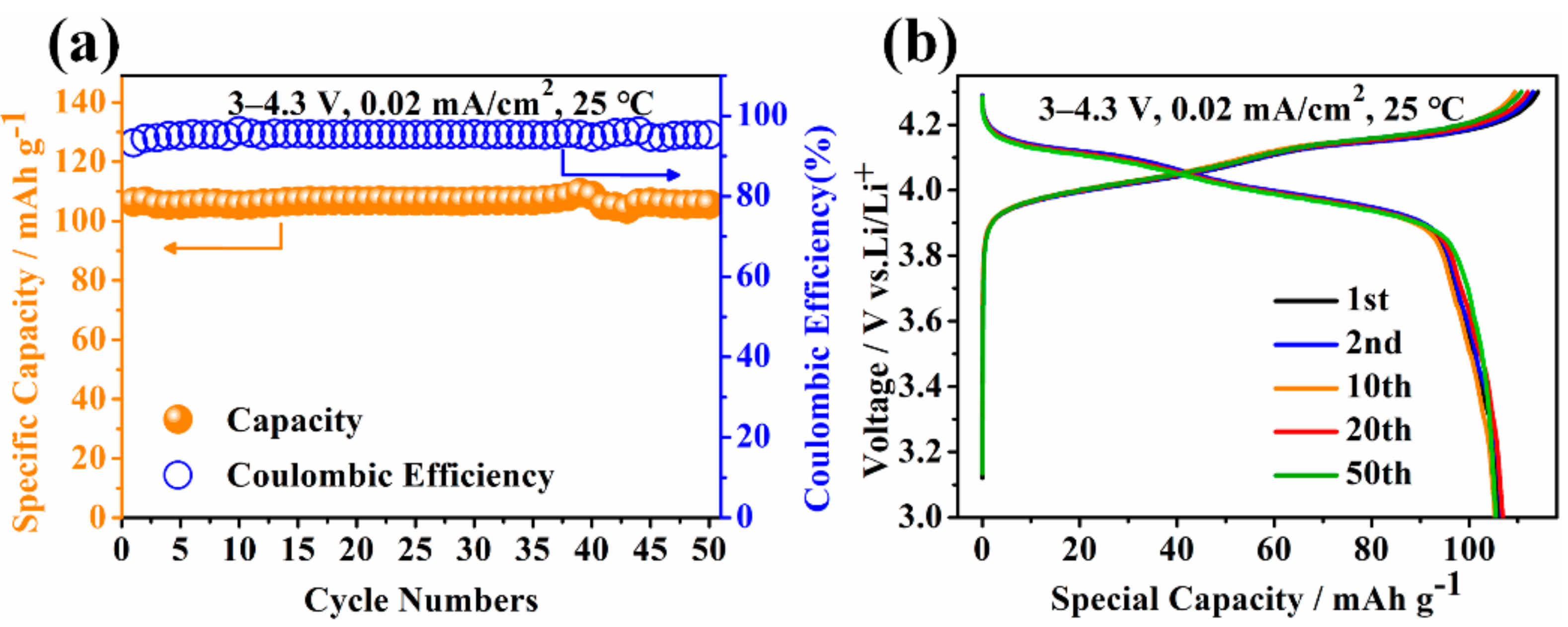
| Preparation Condition | D10 (µm) | D50 (µm) | D90 (µm) | D(3,2) (µm) | D(4,3) (µm) | Specific Surface Area (m2/kg) |
|---|---|---|---|---|---|---|
| Attrition milled 2 h @ 1000 rpm | 0.430 | 0.590 | 0.812 | 0.575 | 0.607 | 2007 |
| Sample Name | Sintering Condition | Cell Parameter (Å) | Total Ionic Conductivity (10−4 S·cm−1), 25 °C | Activation Energy (eV) | Relative Density |
|---|---|---|---|---|---|
| SL−1 | 1200 °C × 60 min | 12.8952 | 1.58 | 0.315 | 86.7% |
| SL−2 | 1200 °C × 30 min | 12.8953 | 5.09 | 0.311 | 87.3% |
| SL−3 | 1150 °C × 60 min | 12.9028 | 3.49 | 0.316 | 90.4% |
| SL−4 | 1100 °C × 60 min | 12.8916 | 0.51 | 0.319 | 90.3% |
| SL−5 | 1100 °C × 360 min | 12.8870 | 0.35 | 0.328 | 83.4% |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ji, Y.; Zhou, C.; Lin, F.; Li, B.; Yang, F.; Zhu, H.; Duan, J.; Chen, Z. Submicron-Sized Nb-Doped Lithium Garnet for High Ionic Conductivity Solid Electrolyte and Performance of Quasi-Solid-State Lithium Battery. Materials 2020, 13, 560. https://doi.org/10.3390/ma13030560
Ji Y, Zhou C, Lin F, Li B, Yang F, Zhu H, Duan J, Chen Z. Submicron-Sized Nb-Doped Lithium Garnet for High Ionic Conductivity Solid Electrolyte and Performance of Quasi-Solid-State Lithium Battery. Materials. 2020; 13(3):560. https://doi.org/10.3390/ma13030560
Chicago/Turabian StyleJi, Yan, Cankai Zhou, Feng Lin, Bingjing Li, Feifan Yang, Huali Zhu, Junfei Duan, and Zhaoyong Chen. 2020. "Submicron-Sized Nb-Doped Lithium Garnet for High Ionic Conductivity Solid Electrolyte and Performance of Quasi-Solid-State Lithium Battery" Materials 13, no. 3: 560. https://doi.org/10.3390/ma13030560
APA StyleJi, Y., Zhou, C., Lin, F., Li, B., Yang, F., Zhu, H., Duan, J., & Chen, Z. (2020). Submicron-Sized Nb-Doped Lithium Garnet for High Ionic Conductivity Solid Electrolyte and Performance of Quasi-Solid-State Lithium Battery. Materials, 13(3), 560. https://doi.org/10.3390/ma13030560





