Improving Dispersion of Recycled Discontinuous Carbon Fibres to Increase Fibre Throughput in the HiPerDiF Process
Abstract
1. Introduction
2. Methodology
2.1. Materials
2.2. Fibre Characterisation
2.2.1. Thermal Stability
2.2.2. Zeta Potential
2.3. Dissolution
2.4. Rheology
2.5. Single Fibre Fragmentation
3. Results
3.1. Fibre Characteristics
3.2. Dissolution
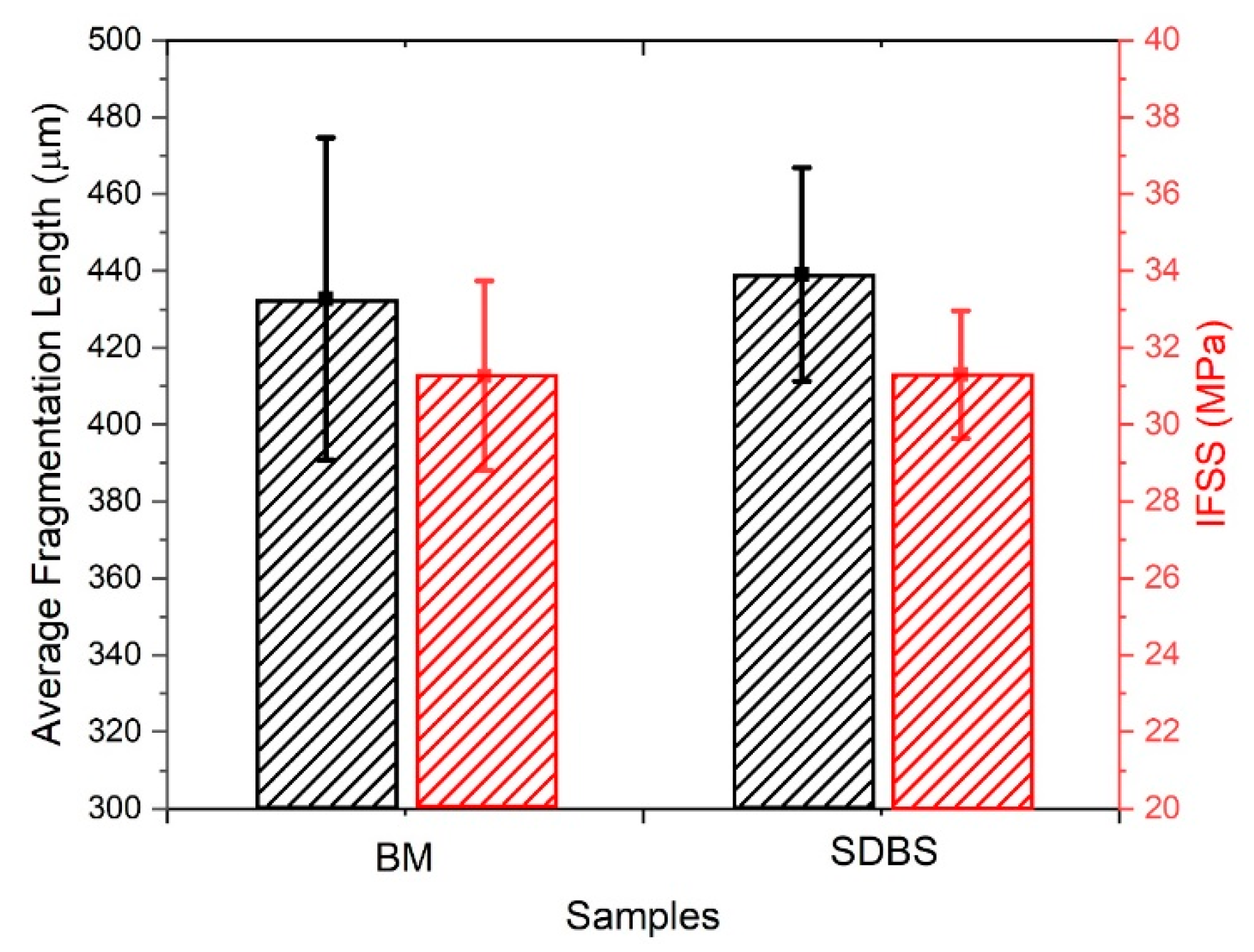
3.3. Single Fibre Fragmentation
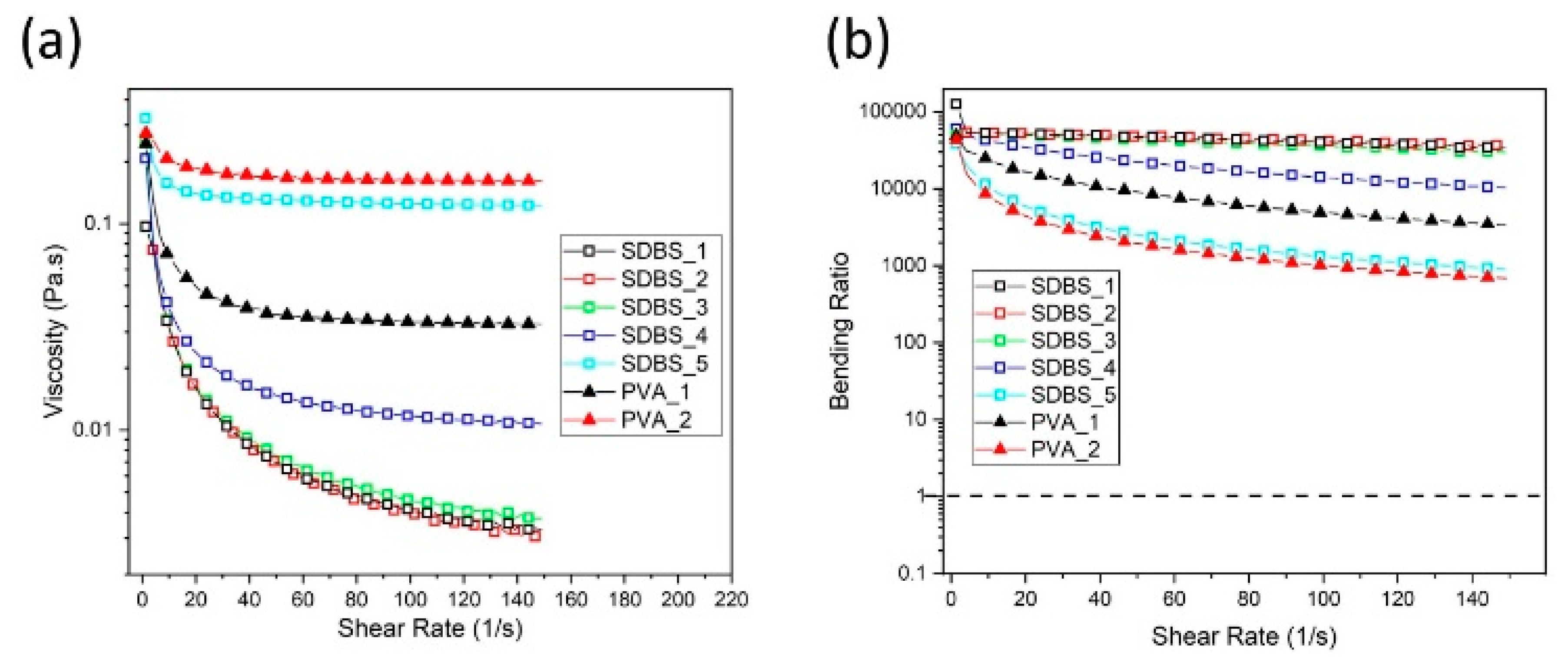
3.4. Viscosity
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Tapper, R.J.; Longana, M.L.; Norton, A.; Potter, K.D.; Hamerton, I. An evaluation of life cycle assessment and its application to the closed-loop recycling of carbon fibre reinforced polymers. Compos. Part B: Eng. 2020, 184, 107665. [Google Scholar] [CrossRef]
- Longana, M.L.; Ong, N.; Yu, H.; Potter, K. Multiple closed loop recycling of carbon fibre composites with the HiPerDiF (High Performance Discontinuous Fibre) method. Compos. Struct. 2016, 153, 271–277. [Google Scholar] [CrossRef]
- Kratmann, K.; Sutcliffe, M.; Lilleheden, L.; Pyrz, R.; Thomsen, O. A novel image analysis procedure for measuring fibre misalignment in unidirectional fibre composites. Compos. Sci. Technol. 2009, 69, 228–238. [Google Scholar] [CrossRef]
- Yu, H.; Potter, K.; Wisnom, M. A novel manufacturing method for aligned discontinuous fibre composites (High Performance-Discontinuous Fibre method). Compos. Part A: Appl. Sci. Manuf. 2014, 65, 175–185. [Google Scholar] [CrossRef]
- High Performance Discontinuous Fibre Composites—A Sustainable Route to the Next Generation of Composites; Engineering and Physical Sciences Research Council (EPSRC EP/P027393/1); Engineering and Physical Sciences Research Council: Swindon, UK; Available online: https://gow.epsrc.ukri.org/NGBOViewGrant.aspx?GrantRef=EP/P027393/1 (accessed on 26 March 2020).
- Yu, H.; Longana, M.L.; Jalalvand, M.; Wisnom, M.R.; Potter, K.D. Hierarchical pseudo-ductile hybrid composites combining continuous and highly aligned discontinuous fibres. Compos. Part A: Appl. Sci. Manuf. 2018, 105, 40–56. [Google Scholar] [CrossRef]
- Longana, M.L.; Yu, H.; Lee, J.; Pozegic, T.; Huntley, S.; Rendall, T.; Potter, K.; Hamerton, I. Quasi-Isotropic and Pseudo-Ductile Highly Aligned Discontinuous Fibre Composites Manufactured with the HiPerDiF (High Performance Discontinuous Fibre) Technology. Mater. 2019, 12, 1794. [Google Scholar] [CrossRef] [PubMed]
- Tang, J.; Swolfs, Y.; Longana, M.L.; Yu, H.; Wisnom, M.R.; Lomov, S.V.; Gorbatikh, L. Hybrid composites of aligned discontinuous carbon fibers and self-reinforced polypropylene under tensile loading. Compos. Part A: Appl. Sci. Manuf. 2019, 123, 97–107. [Google Scholar] [CrossRef]
- Tapper, R.J.; Longana, M.L.; Yu, H.; Hamerton, I.; Potter, K. Development of a closed-loop recycling process for discontinuous carbon fibre polypropylene composites. Compos. Part B: Eng. 2018, 146, 222–231. [Google Scholar] [CrossRef]
- Tapper, R.J.; Longana, M.L.; Hamerton, I.; Potter, K.D. A closed-loop recycling process for discontinuous carbon fibre polyamide 6 composites. Compos. Part B: Eng. 2019, 179, 107418. [Google Scholar] [CrossRef]
- Yu, H.; Longana, M.L.; Jalalvand, M.; Wisnom, M.R.; Potter, K. Pseudo-ductility in intermingled carbon/glass hybrid composites with highly aligned discontinuous fibres. Compos. Part A: Appl. Sci. Manuf. 2015, 73, 35–44. [Google Scholar] [CrossRef]
- Finley, J.; Yu, H.; Longana, M.L.; Pimenta, S.; Shaffer, M.; Potter, K. Exploring the pseudo-ductility of aligned hybrid discontinuous composites using controlled fibre-type arrangements. Compos. Part A: Appl. Sci. Manuf. 2018, 107, 592–606. [Google Scholar] [CrossRef]
- Longana, M.L.; Yu, H.; Jalavand, M.; Wisnom, M.R.; Potter, K.D. Aligned discontinuous intermingled reclaimed/virgin carbon fibre composites for high performance and pseudo-ductile behaviour in interlaminated carbon-glass hybrids. Compos. Sci. Technol. 2017, 143, 13–21. [Google Scholar] [CrossRef]
- Longana, M.L.; Yu, H.; Hamerton, I.; Potter, K.D. Development and application of a quality control and property assurance methodology for reclaimed carbon fibers based on the HiPerDiF (High Performance Discontinuous Fibre) method and interlaminated hybrid specimens. Adv. Manuf. Polym. Compos. Sci. 2018, 4, 48–55. [Google Scholar] [CrossRef]
- Schmitz, J.; Frommelius, H.; Pegelow, U.; Schulte, H.-G.; Höfer, R. A new concept for dispersing agents in aqueous coatings. Prog. Org. Coatings 1999, 35, 191–196. [Google Scholar] [CrossRef]
- Ma, P.-C.; Siddiqui, N.A.; Marom, G.; Kim, J.-K. Dispersion and functionalization of carbon nanotubes for polymer-based nanocomposites: A review. Compos. Part A: Appl. Sci. Manuf. 2010, 41, 1345–1367. [Google Scholar] [CrossRef]
- Hollertz, R.; Chatterjee, S.; Gutmann, H.; Geiger, T.; A Nüesch, F.; Chu, B.T.T. Improvement of toughness and electrical properties of epoxy composites with carbon nanotubes prepared by industrially relevant processes. Nanotechnology 2011, 22, 125702. [Google Scholar] [CrossRef] [PubMed]
- Lekkerkerker, H.N.; Tuinier, R. Colloids and the Depletion Interaction; Springer Science and Business Media LLC: Berlin, Germany, 2011; Volume 833. [Google Scholar]
- Tadros, T.F. Colloid Stability: The Role of Surface Forces; Colloids and Interface Science Series; v. 1-2. 2007; Wiley-VCH Verlag: Weinheim, Germany, 2007. [Google Scholar]
- Hunter, R.J. Zeta Potential in Colloid Sicence: Principles and Applications; Academic Press: London, UK, 1988. [Google Scholar]
- Kozlowski, C.; Sherwood, P. X-ray photoelectron spectroscopic studies of carbon fiber surfaces VIII—A comparison of type I and type II fibers and their interaction with thin resin films. Carbon 1987, 25, 751–760. [Google Scholar] [CrossRef]
- Jones, F. A Review of Interphase Formation and Design in Fibre-Reinforced Composites. J. Adhes. Sci. Technol. 2010, 24, 171–202. [Google Scholar] [CrossRef]
- Loos, M.; Yang, J.; Feke, D.L.; Manas-Zloczower, I. Effect of block-copolymer dispersants on properties of carbon nanotube/epoxy systems. Compos. Sci. Technol. 2012, 72, 482–488. [Google Scholar] [CrossRef]
- Li, Q.; Zaiser, M.; Koutsos, V. Carbon nanotube/epoxy resin composites using a block copolymer as a dispersing agent. Phys. Status Solidi (a) 2004, 201, R89–R91. [Google Scholar] [CrossRef]
- Musselman, S.; Chander, S. Wetting and adsorption of acetylenic diol based nonionic surfactants on heterogeneous surfaces. Colloids Surfaces A: Physicochem. Eng. Asp. 2002, 206, 497–513. [Google Scholar] [CrossRef]
- King, S.G.; Castaldelli, E.; McCafferty, L.; Silva, S.R.P.; Stolojan, V.; McCafferty, L. Micro-Centrifugal Technique for Improved Assessment and Optimization of Nanomaterial Dispersions: The Case for Carbon Nanotubes. ACS Appl. Nano Mater. 2018, 1, 6217–6225. [Google Scholar] [CrossRef]
- Vaisman, L.; Wagner, H.D.; Marom, G. The role of surfactants in dispersion of carbon nanotubes. Adv. Colloid Interface Sci. 2006, 128, 37–46. [Google Scholar] [CrossRef]
- Gong, X.; Liu, J.; Baskaran, S.; Voise, R.D.; Young, J.S. Surfactant-Assisted Processing of Carbon Nanotube/Polymer Composites. Chem. Mater. 2000, 12, 1049–1052. [Google Scholar] [CrossRef]
- Vaisman, L.; Marom, G.; Wagner, H.D. Dispersions of Surface-Modified Carbon Nanotubes in Water-Soluble and Water-Insoluble Polymers. Adv. Funct. Mater. 2006, 16, 357–363. [Google Scholar] [CrossRef]
- White, B.; Banerjee, S.; O’Brien, S.J.; Turro, N.J.; Herman, I.P. Zeta-Potential Measurements of Surfactant-Wrapped Individual Single-Walled Carbon Nanotubes. J. Phys. Chem. C 2007, 111, 13684–13690. [Google Scholar] [CrossRef]
- Islam, M.F.; Rojas, E.; Bergey, D.M.; Johnson, A.T.; Yodh, A.G. High Weight Fraction Surfactant Solubilization of Single-Wall Carbon Nanotubes in Water. Nano Lett. 2003, 3, 269–273. [Google Scholar] [CrossRef]
- Chen, R.J.; Zhang, Y.; Wang, D.; Dai, H. Noncovalent sidewall functionalization of single-walled carbon nanotubes for protein immobilization. J. Am. Chem. Soc. 2001, 123, 3838–3839. [Google Scholar] [CrossRef]
- Chen, J.; Liu, H.; Weimer, W.A.; Halls, M.D.; Waldeck, D.H.; Walker, G. Noncovalent Engineering of Carbon Nanotube Surfaces by Rigid, Functional Conjugated Polymers. J. Am. Chem. Soc. 2002, 124, 9034–9035. [Google Scholar] [CrossRef]
- Giraud, I.; Franceschi-Messant, S.; Perez, E.; Lacabanne, C.; Dantras, E. Preparation of aqueous dispersion of thermoplastic sizing agent for carbon fiber by emulsion/solvent evaporation. Appl. Surf. Sci. 2013, 266, 94–99. [Google Scholar] [CrossRef]
- Yang, W.; Badini, C.; Fuso, L.; Biamino, S.; Pavese, M.; Bolivar, C.V.; Fino, P. Preparation and prospective application of short carbon fiber/SiC multilayer composites by tape casting. World J. Eng. 2011, 8, 331–334. [Google Scholar] [CrossRef]
- Yu, D.-G.; Chatterton, N.P.; Yang, J.-H.; Wang, X.; Liao, Y.-Z. Coaxial Electrospinning with Triton X-100 Solutions as Sheath Fluids for Preparing PAN Nanofibers. Macromol. Mater. Eng. 2011, 297, 395–401. [Google Scholar] [CrossRef]
- Chuang, W.; Lei, P.; Bing-Liang, L.; Ni, G.; Li-Ping, Z.; Ke-Zhi, L. Influences of molding processes and different dispersants on the dispersion of chopped carbon fibers in cement matrix. Heliyon 2018, 4, e00868. [Google Scholar] [CrossRef] [PubMed]
- Pozegic, T.; Jayawardena, I.; Chen, J.-S.; Anguita, J.V.; Ballocchi, P.; Stolojan, V.; Silva, S.; Hamerton, I. Development of sizing-free multi-functional carbon fibre nanocomposites. Compos. Part A: Appl. Sci. Manuf. 2016, 90, 306–319. [Google Scholar] [CrossRef]
- Chen, F.; Tripathi, D.; Jones, F. Determination of the interfacial shear strength of glass-fibre-reinforced phenolic composites by a bimatrix fragmentation technique. Compos. Sci. Technol. 1996, 56, 609–622. [Google Scholar] [CrossRef]
- Kelly, A.; Tyson, W. Tensile properties of fibre-reinforced metals: Copper/tungsten and copper/molybdenum. J. Mech. Phys. Solids 1965, 13, 329–350. [Google Scholar] [CrossRef]
- Pozegic, T.; Hamerton, I.; Anguita, J.V.; Tang, W.; Ballocchi, P.; Jenkins, P.; Silva, S.R.P. Low temperature growth of carbon nanotubes on carbon fibre to create a highly networked fuzzy fibre reinforced composite with superior electrical conductivity. Carbon 2014, 74, 319–328. [Google Scholar] [CrossRef]
- Ridaoui, H.; Jada, A.; Vidal, L.; Donnet, J.-B. Effect of cationic surfactant and block copolymer on carbon black particle surface charge and size. Colloids Surfaces A: Physicochem. Eng. Asp. 2006, 278, 149–159. [Google Scholar] [CrossRef]
- Principles of Colloid and Surface Chemistry, 3rd ed.; Hiemenz, P.C., Rajagopalan, R., Eds.; Marcel Dekker: New York, NY, USA, 1997. [Google Scholar]
- Jiang, G.; Turner, T.; Pickering, S. The shear viscosity of carbon fibre suspension and its application for fibre length measurement. Rheol. Acta 2015, 55, 1–10. [Google Scholar] [CrossRef]
- Chen, T.; Liu, H.; Wang, X.; Zhang, H.; Zhang, X. Properties and fabrication of PA66/surface-modified multi-walled nanotubes composite fibers by ball milling and melt-spinning. Polymers 2018, 10, 547. [Google Scholar] [CrossRef]
- Gallardo-Moreno, A.; González-García, C.; González-Martín, M.L.; Bruque, J.M. Arrangement of SDS adsorbed layer on carbonaceous particles by zeta potential determinations. Colloids Surfaces A: Physicochem. Eng. Asp. 2004, 249, 57–62. [Google Scholar] [CrossRef]
- Gupta, S.D.; Bhagwat, S.S. Adsorption of Surfactants on Carbon Black-Water Interface. J. Dispers. Sci. Technol. 2005, 26, 111–120. [Google Scholar] [CrossRef]
- Sis, H.; Birinci, M. Effect of nonionic and ionic surfactants on zeta potential and dispersion properties of carbon black powders. Colloids Surfaces A: Physicochem. Eng. Asp. 2009, 341, 60–67. [Google Scholar] [CrossRef]
- Hait, S.K.; Majhi, P.R.; Blume, A.; Moulik, S.P. A Critical Assessment of Micellization of Sodium Dodecyl Benzene Sulfonate (SDBS) and Its Interaction with Poly (vinyl pyrrolidone) and Hydrophobically Modified Polymers, JR 400 and LM 200. J. Phys. Chem. B 2003, 107, 3650–3658. [Google Scholar] [CrossRef]
- Forgacs, O.; Mason, S. Particle motions in sheared suspensions. J. Colloid Sci. 1959, 14, 457–472. [Google Scholar] [CrossRef]
- Goldsmith, H.L.; Mason, S.G. Chapter 2—The microrheology of dispersions, in Rheology; Eirich, F.R., Ed.; Academic Press: Cambridge, MA, USA, 1967; pp. 85–250. [Google Scholar]






| Fibre Property | High Modulus Carbon Fibre | Polymer Matrix |
|---|---|---|
| Commercial Name | Granoc XN-90 | PRIME(TM) 27 |
| Density (g/cm3) | 2.21 | 1.139 (Cured) |
| Diameter (µm) | 10 | – |
| Young’s modulus (GPa) | 860 | 3.47 |
| Tensile strength (MPa) | 3430 | 73.3 |
| Failure Strain (%) | 0.398 | 4.5 |
| Surfactant | Chemical Name | Molecular Weight (g/mol.) | Surfactant Type |
|---|---|---|---|
| Brij® 58 | Poly(ethylene glycol) hexadecyl ether | 1124 | Non-ionic |
| Pluronic F127 | Poloxamer 407 | 12500 | Non-ionic |
| SDS | sodium dodecyl sulphate | 288 | Anionic |
| SDBS | sodium dodecylbenzene sulphonate | 348 | Anionic |
| Triton™ X-100 | Poly(ethylene glycol) tert-octylphenyl ether | 625 | Non-ionic |
| Triton™ X-405 | Poly(ethylene glycol) tert-octylphenyl ether | 1968 | Non-ionic |
| Tween® 80 | Poly(ethylene glycol) sorbitan monooleate | 1310 | Non-ionic |
| Concentration (mg/mL) | Identifier |
|---|---|
| 13.92 | SDBS_1 |
| 34.80 | SDBS_2 |
| 69.60 | SDBS_3 |
| 104.40 | SDBS_4 |
| 139.20 | SDBS_5 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pozegic, T.R.; Huntley, S.; Longana, M.L.; He, S.; Bandara, R.M.I.; King, S.G.; Hamerton, I. Improving Dispersion of Recycled Discontinuous Carbon Fibres to Increase Fibre Throughput in the HiPerDiF Process. Materials 2020, 13, 1544. https://doi.org/10.3390/ma13071544
Pozegic TR, Huntley S, Longana ML, He S, Bandara RMI, King SG, Hamerton I. Improving Dispersion of Recycled Discontinuous Carbon Fibres to Increase Fibre Throughput in the HiPerDiF Process. Materials. 2020; 13(7):1544. https://doi.org/10.3390/ma13071544
Chicago/Turabian StylePozegic, Thomas R., Samantha Huntley, Marco L. Longana, Suihua He, R. M. Indrachapa Bandara, Simon G. King, and Ian Hamerton. 2020. "Improving Dispersion of Recycled Discontinuous Carbon Fibres to Increase Fibre Throughput in the HiPerDiF Process" Materials 13, no. 7: 1544. https://doi.org/10.3390/ma13071544
APA StylePozegic, T. R., Huntley, S., Longana, M. L., He, S., Bandara, R. M. I., King, S. G., & Hamerton, I. (2020). Improving Dispersion of Recycled Discontinuous Carbon Fibres to Increase Fibre Throughput in the HiPerDiF Process. Materials, 13(7), 1544. https://doi.org/10.3390/ma13071544







