Effect of Hydrogen and Absence of Passive Layer on Corrosive Properties of Aluminum Alloys
Abstract
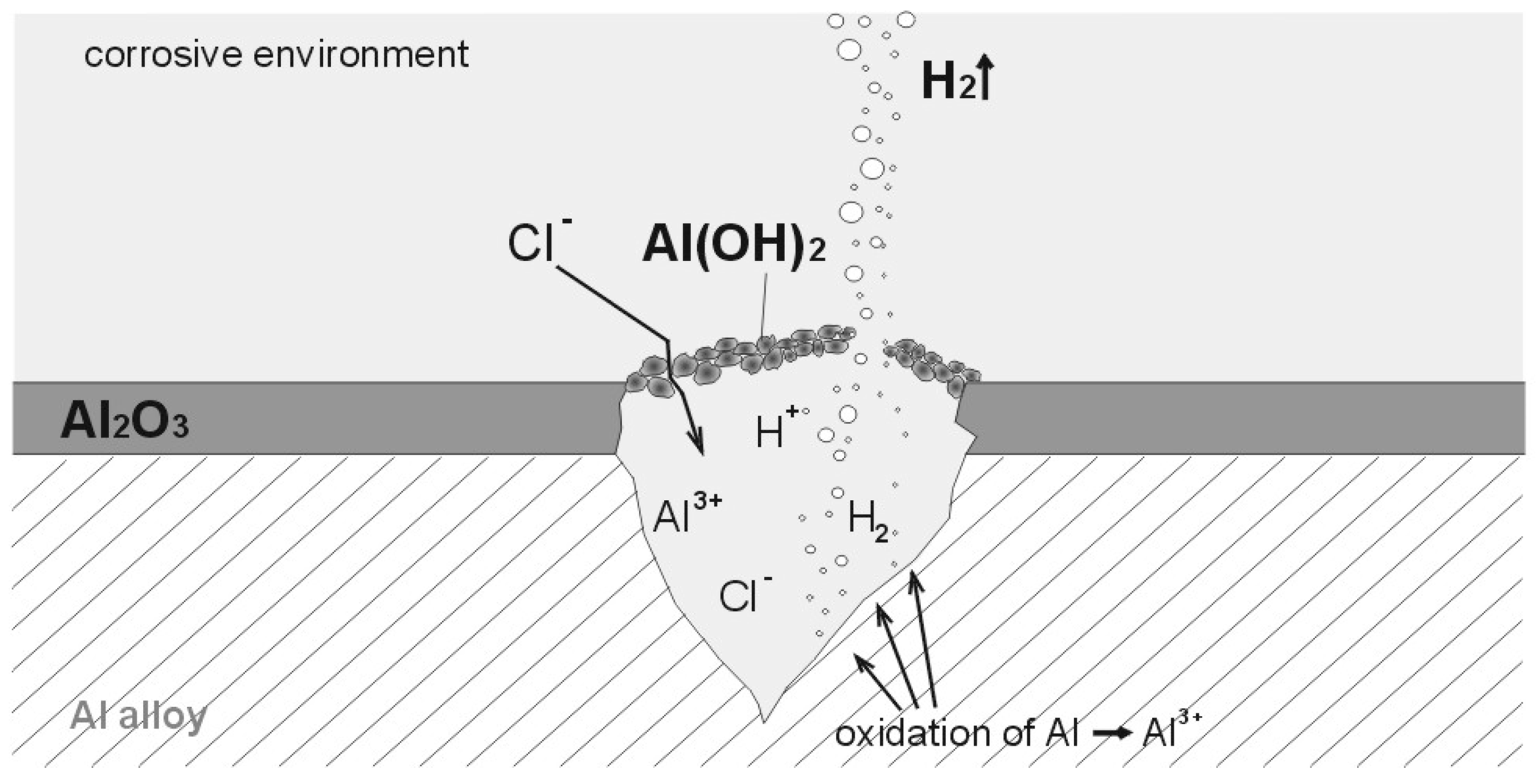
:1. Introduction
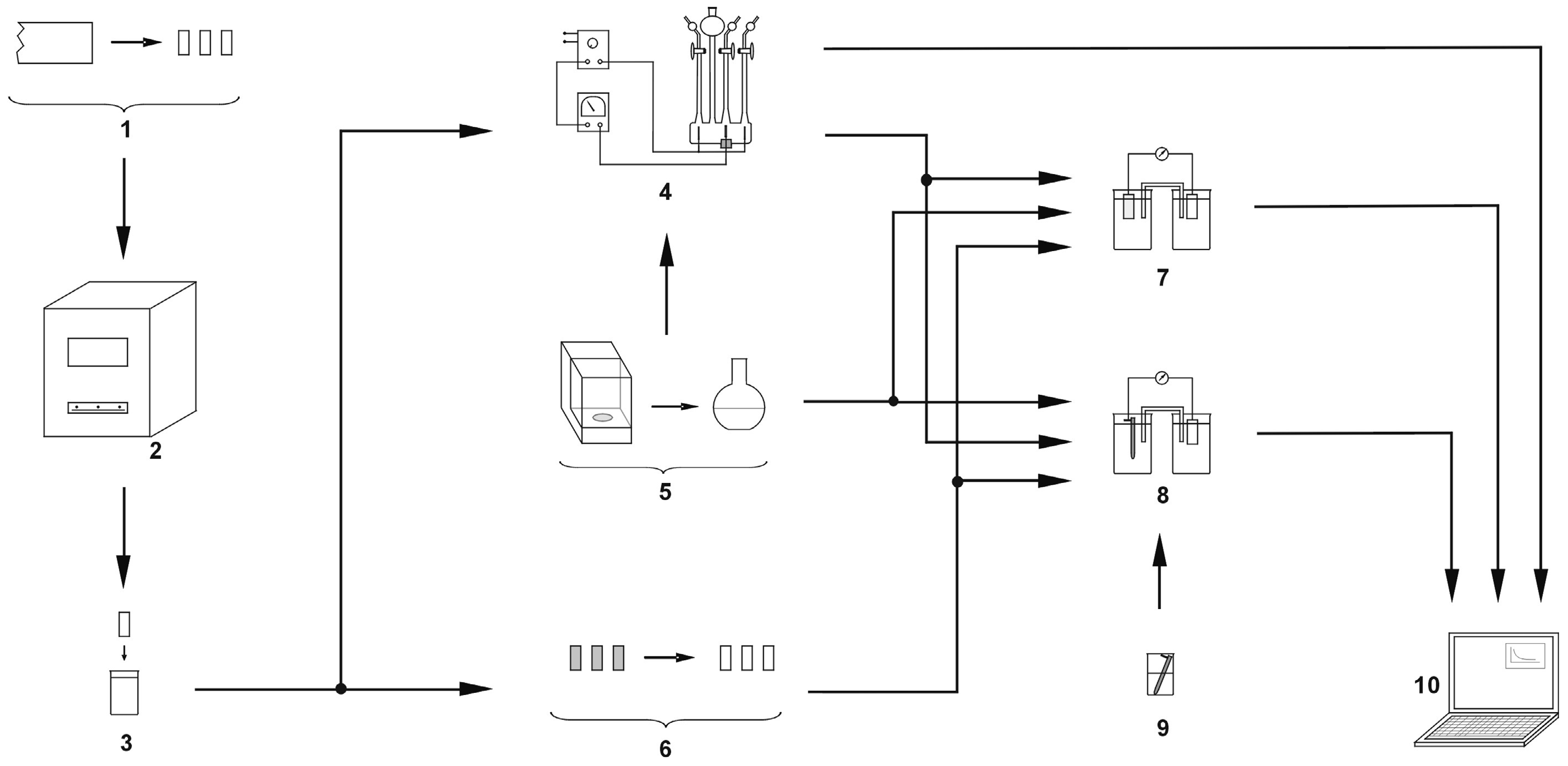
2. Materials and Methods
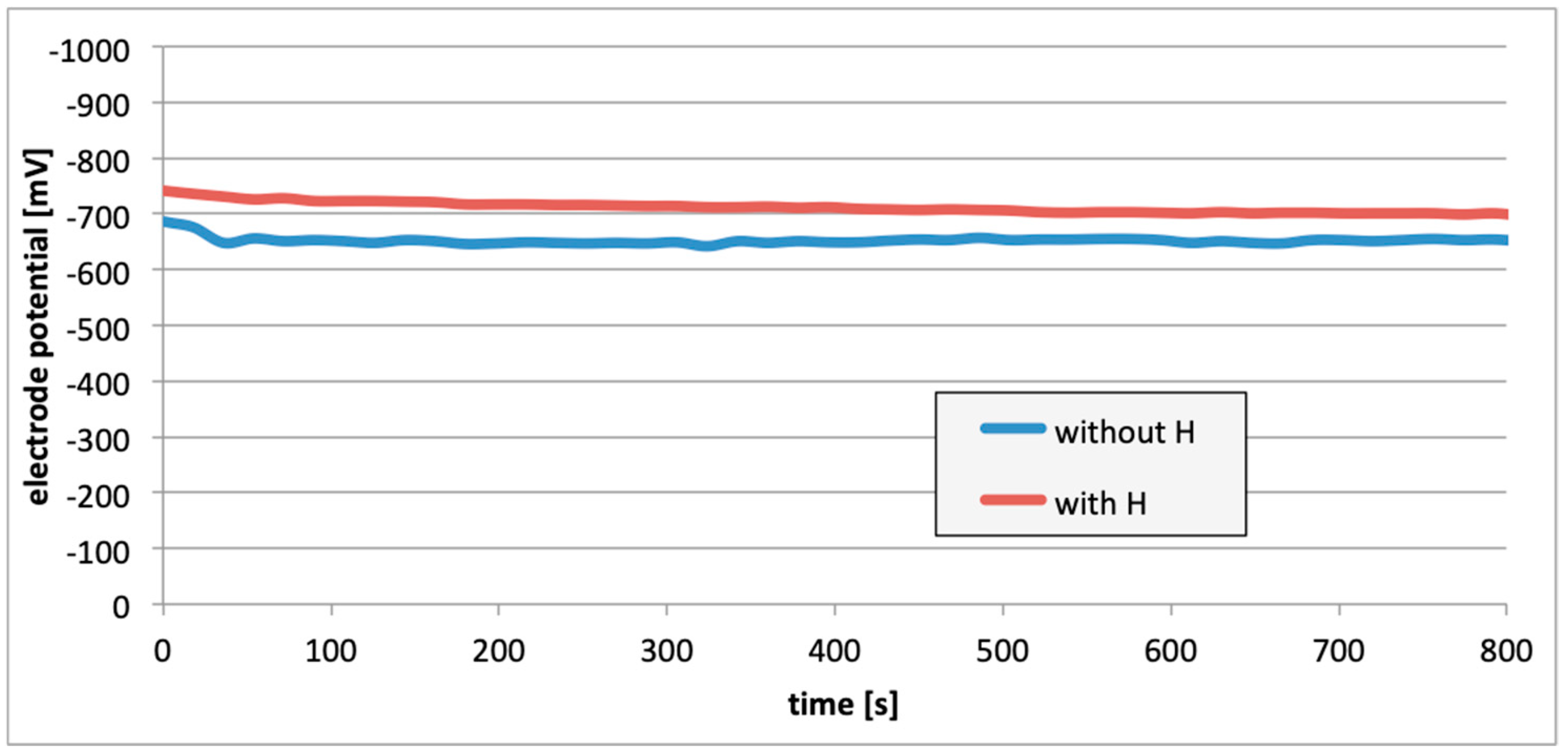
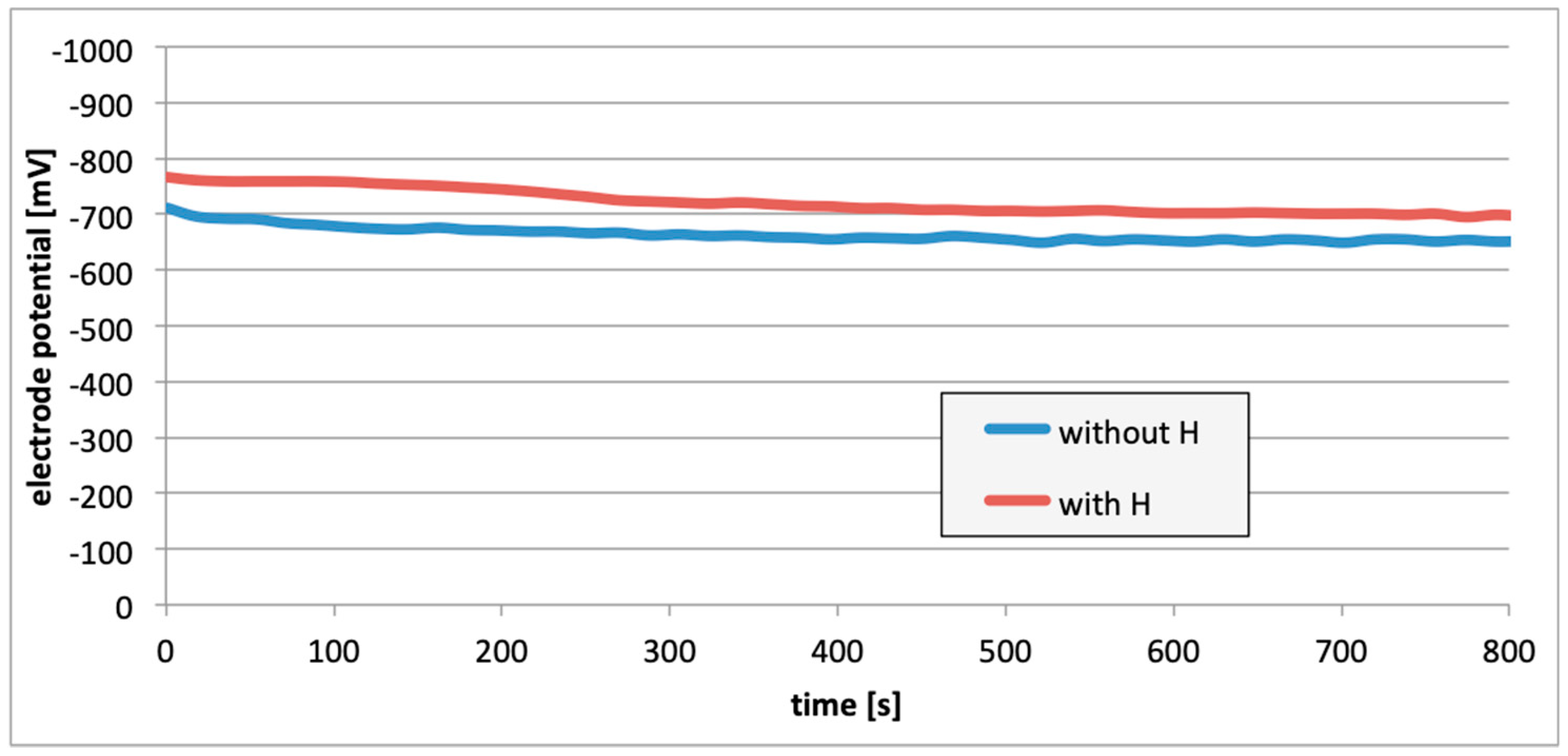
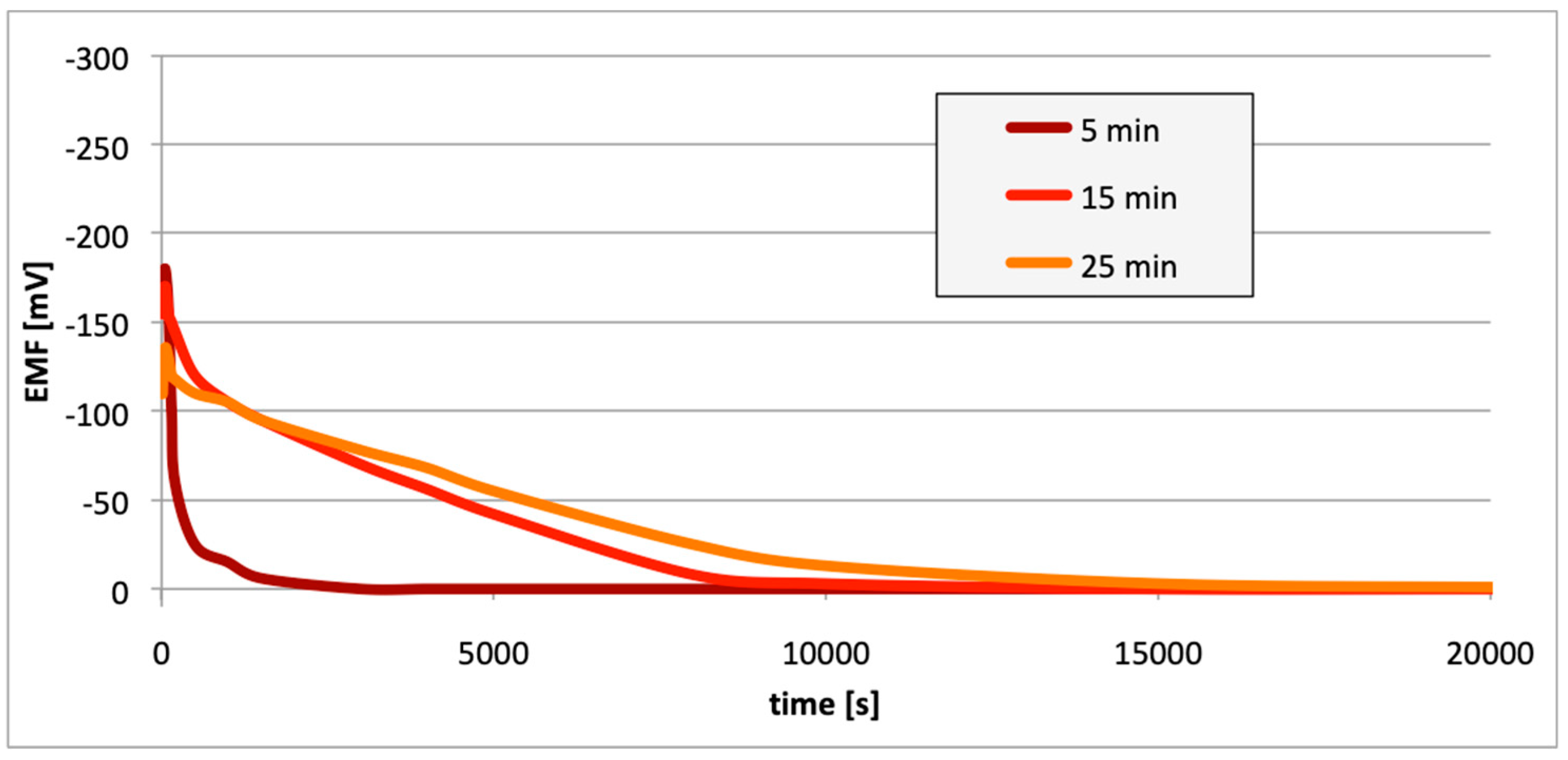
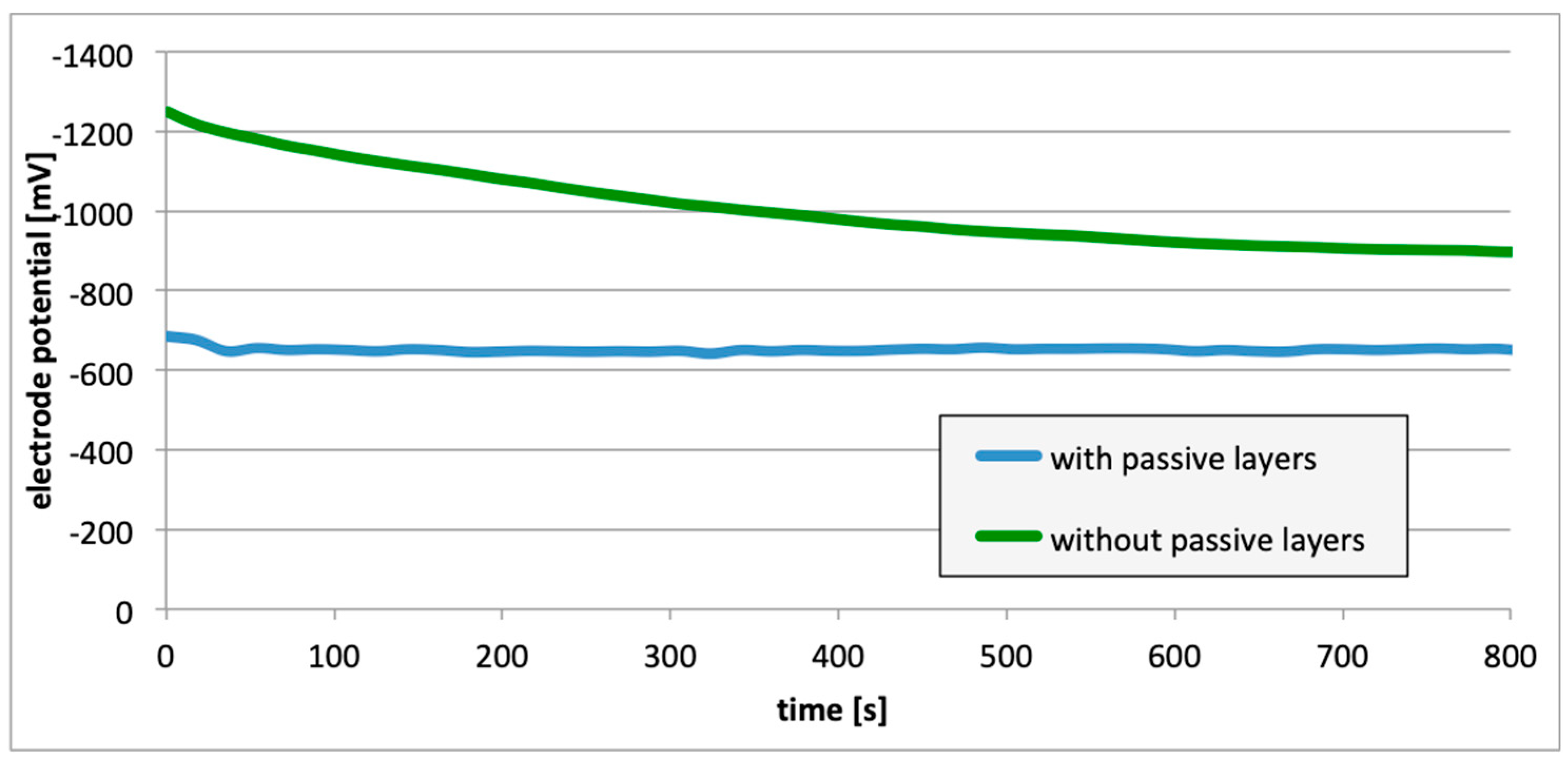
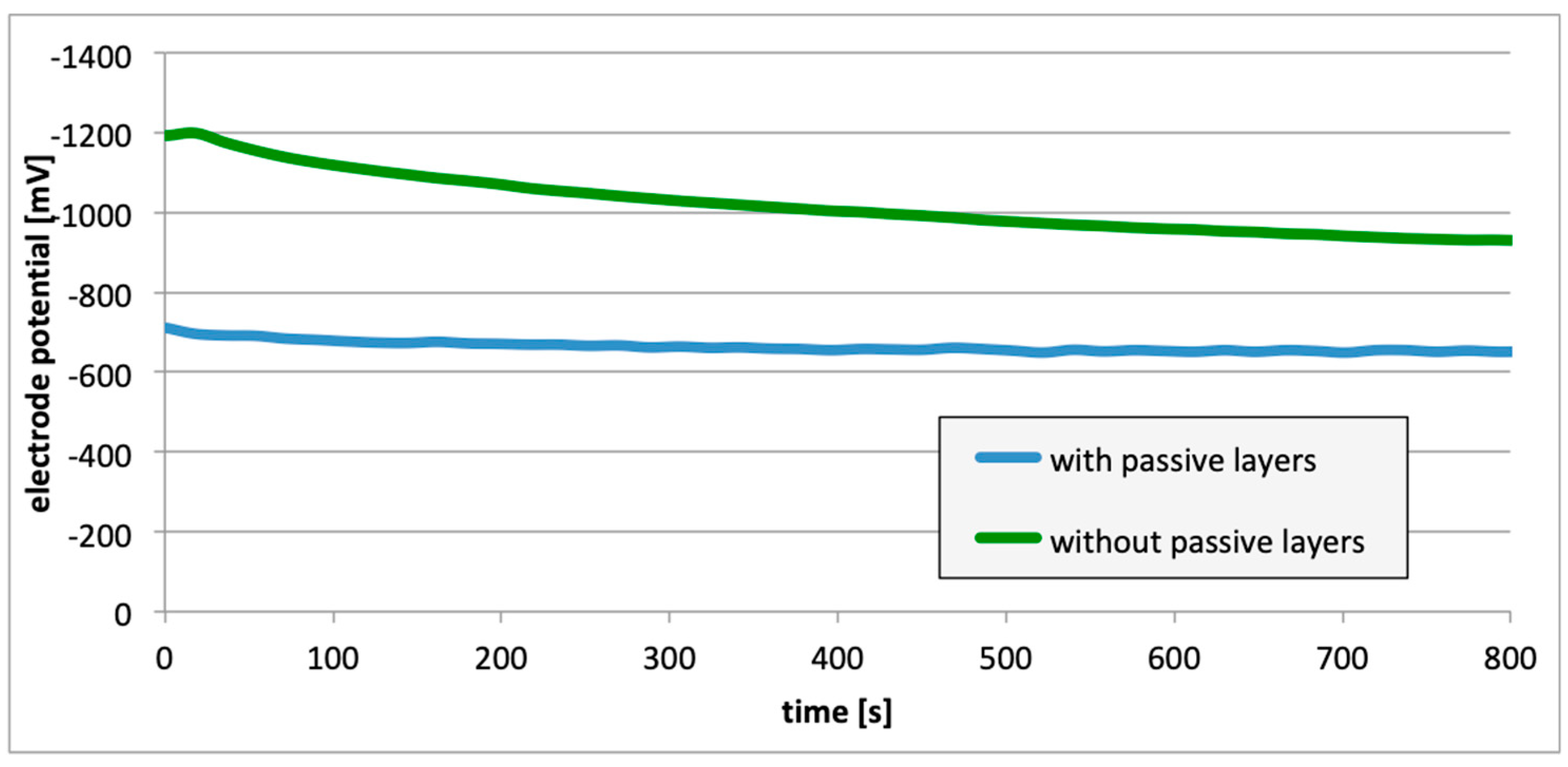
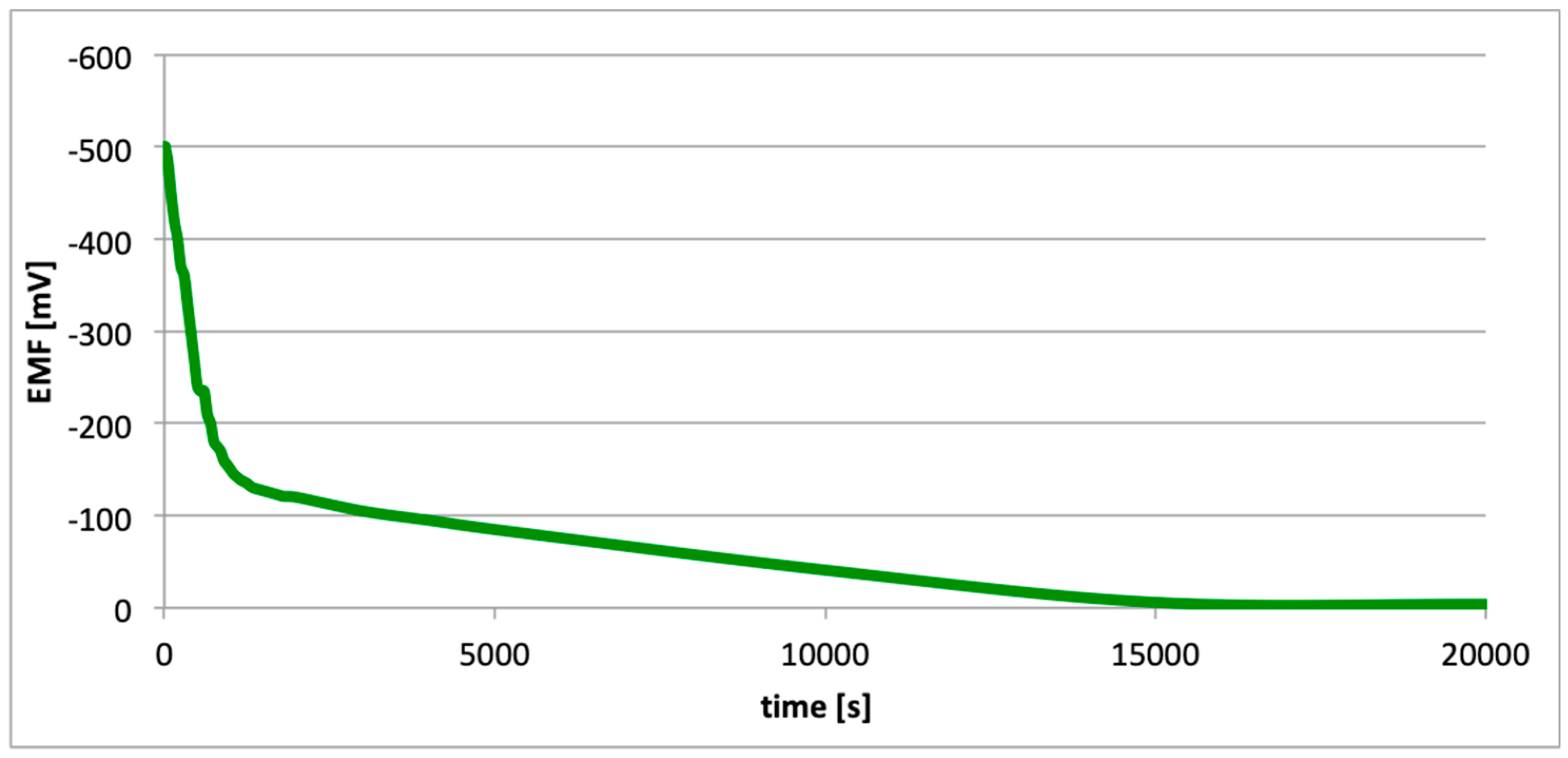
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Śmiałowski, M. Hydrogen in Steel; Pergamon Press: Oxford, UK, 1962. [Google Scholar]
- Nibur, K.A.; Bahr, D.F.; Somerday, B.P. Hydrogen effects on dislocation activity in austenitic stainless steel. Acta Mater. 2006, 54, 2677–2684. [Google Scholar] [CrossRef]
- Zhao, Y.; Seok, M.-Y.; Choi, I.-C.; Lee, Y.-H.; Park, S.-J.; Ramamurty, U.; Suh, J.-Y.; Jang, J. The role of hydrogen in hardening/softening steel: Influence of the charging process. Scr. Mater. 2015, 107, 46–49. [Google Scholar] [CrossRef]
- Dmytrakh, I.M.; Leshchak, R.L.; Syrotyuk, A.M. Effect of hydrogen concentration on strain behaviour of pipeline steel. Int. J. Hydrog. Energy 2015, 40, 4011–4018. [Google Scholar] [CrossRef]
- Laureys, A.; Van den Eeckhout, E.; Petrov, R.; Verbekena, K. Effect of deformation and charging conditions on crack and blister formation during electrochemical hydrogen charging. Acta Mater. 2017, 127, 192–202. [Google Scholar] [CrossRef]
- Hong, Y.; Zhou, C.; Zheng, Y.; Zhang, L.; Zheng, J.; Chen, X. Effect of hydrogen and strain rate on nanoindentation creep of austenitic stainless steel. Int. J. Hydrog. Energy 2019, 44, 1253–1262. [Google Scholar] [CrossRef]
- Atrens, A.; Liu, Q.; Tapia-Bastidas, C.; Gray, E.; Irwanto, B.; Venezuela, J.; Liu, Q. Influence of Hydrogen on Steel Components for Clean Energy. Corros. Mater. Degrad. 2020, 1, 3–26. [Google Scholar] [CrossRef] [Green Version]
- Kaufman, J.G.; Rooy, E.L. Aluminum Alloy Castings: Properties, Processes, and Applications; ASM International: Ohio, OH, USA, 2004. [Google Scholar]
- Nakai, M.; Eto, T. New aspect of development of high strength aluminum alloys for aerospace applications. Mater. Sci. Eng. A 2000, 285, 62–68. [Google Scholar] [CrossRef]
- Barlat, F.; Maeda, Y.; Chung, K.; Yanagawa, M.; Brem, J.C.; Hayashida, Y.; Lege, D.J.; Matsui, K.; Murtha, S.J.; Hattori, S.; et al. Yield function development for aluminum alloy sheets. J. Mech. Phys. Solids 1997, 45, 1727–1763. [Google Scholar] [CrossRef]
- De Almeida, I.; da Silva, L.; Hallak, S. Aluminum Alloy for Automotive Cable—A perspective on emerging cars applications. SAE Tech. Pap. 2010. [Google Scholar] [CrossRef]
- Papenberg, N.P.; Gneiger, S.; Weißensteiner, I.; Uggowitzer, P.J.; Pogatscher, S. Mg-Alloys for Forging Applications—A Review. Materials 2020, 13, 985. [Google Scholar] [CrossRef] [Green Version]
- Blatnický, M.; Sága, M.; Dižo, J.; Bruna, M. Application of Light Metal Alloy EN AW 6063 to Vehicle Frame Construction with an Innovated Steering Mechanism. Materials 2020, 13, 817. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dmitruk, A.; Naplocha, K.; Grzęda, J.; Kaczmar, J.W. Aluminum Inserts for Enhancing Heat Transfer in PCM Accumulator. Materials 2020, 13, 415. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Łunarska, E.; Chernyayeva, O. Strain induced up-hill diffusion of hydrogen in Al. Inż. Mater. 2004, 15, 1–4. [Google Scholar]
- Łunarska, E.; Chernyayeva, O. Effect of precipitates on hydrogen transport and hydrogen embrittlement of Al alloys. Int. J. Phys. Chem. Mech. Mater. 2004, 39, 88–94. [Google Scholar]
- Łunarska, E.; Nikiforow, K.; Sitko, E. Stress corrosion cracking of bainite 0.3C-1Cr-1Mn-1Si-1Ni type steel in acid rain simulated solution. Mater. Corros. 2004, 55, 373–380. [Google Scholar]
- Włodarczyk, P.P.; Gawdzik, A.; Pietrow, L. Wpływ elektrolitycznego wodorowania na korozję elektrochemiczną stopów glinu. Ochr. Przed Koroz. 2007, 11, 183–184. [Google Scholar]
- Włodarczyk, P.P.; Gawdzik, A.; Pietrow, L. Wpływ absorbowanego wodoru na stopy glinu jako przyczyna zmiany ich właściwości elektrochemicznych. Pr. Nauk. Politech. Warsz. Konf. 2007, 2, 687–689. [Google Scholar]
- Bala, H. Korozja Materiałów—Teoria i Praktyka; Wydawnictwo Wydziału Inżynierii Procesowej, Materiałowej i Fizyki Stosowanej Politechniki Częstochowskiej: Częstochowa, Poland, 2002. [Google Scholar]
- Kamoutsi, H.; Haidemenopoulos, G.N.; Bontozoglou, V.; Pantelakis, S. Corrosion-induced hydrogen embrittlement in aluminum alloy 2024. Corros. Sci. 2006, 48, 1209–1224. [Google Scholar] [CrossRef] [Green Version]
- Nguyen, D.; Thompson, A.W.; Bernstein, I.M. Microstructural effects on hydrogen embrittlement in a high purity 7075 aluminum alloy. Acta Metall. 1987, 35, 2417–2425. [Google Scholar] [CrossRef]
- Anyalebechi, P.N. Analysis of the effects of alloying elements on hydrogen solubility in liquid aluminum alloys. Scr. Metall. Mater. 1995, 33, 1209–1216. [Google Scholar] [CrossRef]
- Takano, N. Hydrogen diffusion and embrittlement in 7075 aluminum alloy. Mater. Sci. Eng. A 2008, 483–484, 336–339. [Google Scholar] [CrossRef]
- Song, W.; Du, J.; Xu, Y.; Long, B. A study of hydrogen permeation in aluminum alloy treated by various oxidation processes. J. Nucl. Mater. 1997, 246, 139–143. [Google Scholar] [CrossRef]
- Bala, H. Wstęp do Chemii Materiałów; WNT Wydawnictwa Naukowo-Techniczne: Warszawa, Poland, 2003. [Google Scholar]
- Włodarczyk, P.P.; Włodarczyk, B. Influence of hydrogen on shortening the operational reliability time of aluminum alloy construction. Instal 2013, 12, 32–34. [Google Scholar]
- Zhou, C.; Chen, X.; Wang, Z.; Zheng, S.; Li, X.; Zhang, L. Effects of environmental conditions on hydrogen permeation of X52 pipeline steel exposed to high H2S-containing solutions. Corros. Sci. 2014, 89, 30–37. [Google Scholar] [CrossRef]
- Paul, A.; Laurila, T.; Vuorinen, V.; Divinski, S.V. Thermodynamics, Diffusion and the Kirkendall Effect in Solids; Springer: Cham, Switzerland, 2014. [Google Scholar]
- Callister, W.D.; Rethwisch, D.G. Fundamentals of Materials Science and Engineering: An Integrated Approach, 4th ed.; Wiley: Hoboken, NJ, USA, 2011. [Google Scholar]
- Pietrow, L.; Sopruniuk, N. Corrosion-Mechanical Destruction of Metals and Alloys (in Russian); Naukova Dumka: Kiev, Ukraine, 1991. [Google Scholar]
- Wang, X.-Z.; Luo, H.; Luo, J.-L. Effects of hydrogen and stress on the electrochemical and passivation behaviour of 304 stainless steel in simulated PEMFC environment. Electrochim. Acta 2019, 293, 60–77. [Google Scholar] [CrossRef]
- Turner, J.; Sverdrup, G.; Mann, M.K.; Maness, P.-C.; Kroposki, B.; Ghirardi, M.; Evans, R.J.; Blake, D. Renewable hydrogen production. Int. J. Energy Res. 2008, 32, 379–407. [Google Scholar] [CrossRef] [Green Version]
- Holladay, D.; Hu, J.; King, D.L.; Wang, Y. An overview of hydrogen production technologies. Catal. Today 2009, 139, 244–260. [Google Scholar] [CrossRef]
- Kapdan, I.K.; Kargi, F. Bio-hydrogen production from waste materials. Enzym. Microb. Technol. 2006, 38, 569–582. [Google Scholar] [CrossRef]
- Cheng, S.; Logan, B.E. High hydrogen production rate of microbial electrolysis cell (MEC) with reduced electrode spacing. Bioresour. Technol. 2011, 102, 3571–3574. [Google Scholar] [CrossRef]
- Aiken, D.C.; Curtis, T.P.; Heidrich, E.S. Avenues to the financial viability of microbial electrolysis cells [MEC] for domestic wastewater treatment and hydrogen production. Int. J. Hydrog. Energy 2019, 44, 2426–2434. [Google Scholar] [CrossRef]
- Lu, L.; Ren, N.; Xing, D.; Logan, B.E. Hydrogen production with effluent from an ethanol–H2-coproducing fermentation reactor using a single-chamber microbial electrolysis cell. Biosens. Bioelectron. 2009, 24, 3055–3060. [Google Scholar] [CrossRef] [PubMed]
- Soler, L.; Macanás, J.; Muñoz, M.; Casado, J. Aluminum and Aluminum Alloys as Sources of Hydrogen for Fuel Cell Applications. J. Power Sources 2007, 169, 144–149. [Google Scholar] [CrossRef]
- Wang, H.Z.; Leung, D.Y.C.; Leung, M.K.H.; Ni, M. A review on hydrogen production using aluminum and aluminum alloys. Renew. Sustain. Energy Rev. 2009, 13, 845–853. [Google Scholar] [CrossRef]
- PN-EN 573-3:2019 Aluminum and Aluminum Alloys—Chemical Composition and Types of Forged Products—Part 3: Chemical Composition and Types of Wares; Polish Committee for Standardization: Warsaw, Poland, 2019.
- Holtzer, M.; Staronka, A. Physical Chemistry (in Polish); AGH University of Science and Technology Press: Cracow, Poland, 2000. [Google Scholar]
- Qiao, Q.; Lu, L.; Fan, E.; Zhao, J.; Liu, Y.; Peng, G.; Huang, Y.; Li, X. Effects of Nb on stress corrosion cracking of high-strength low-alloy steel in simulated seawater. Int. J. Hydrog. Energy 2019, 44, 27962–27973. [Google Scholar] [CrossRef]










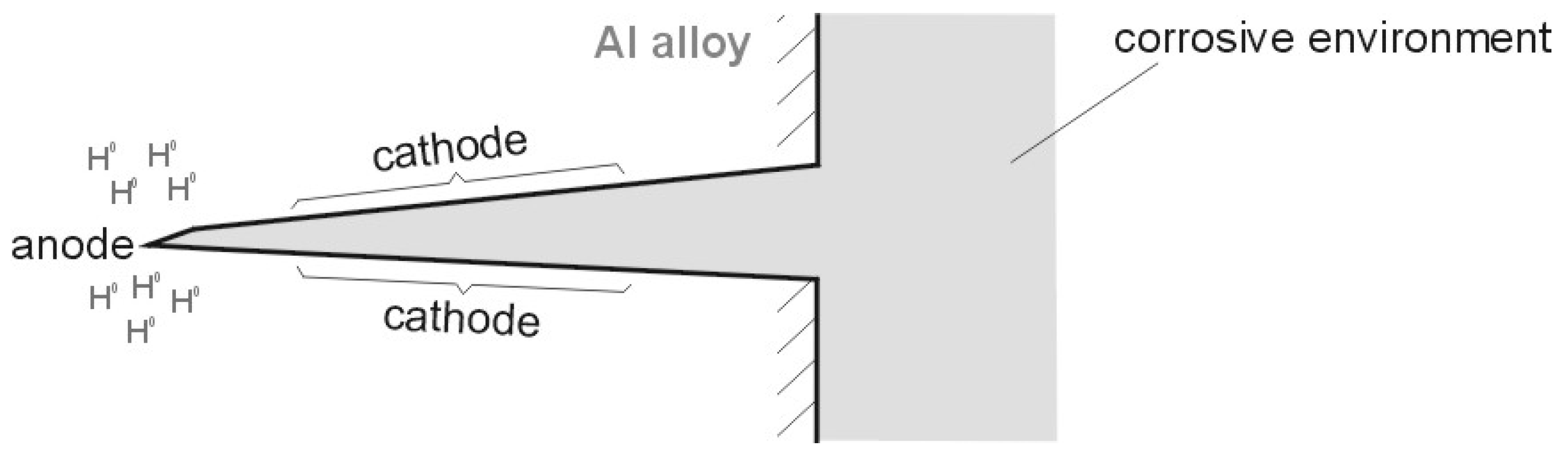
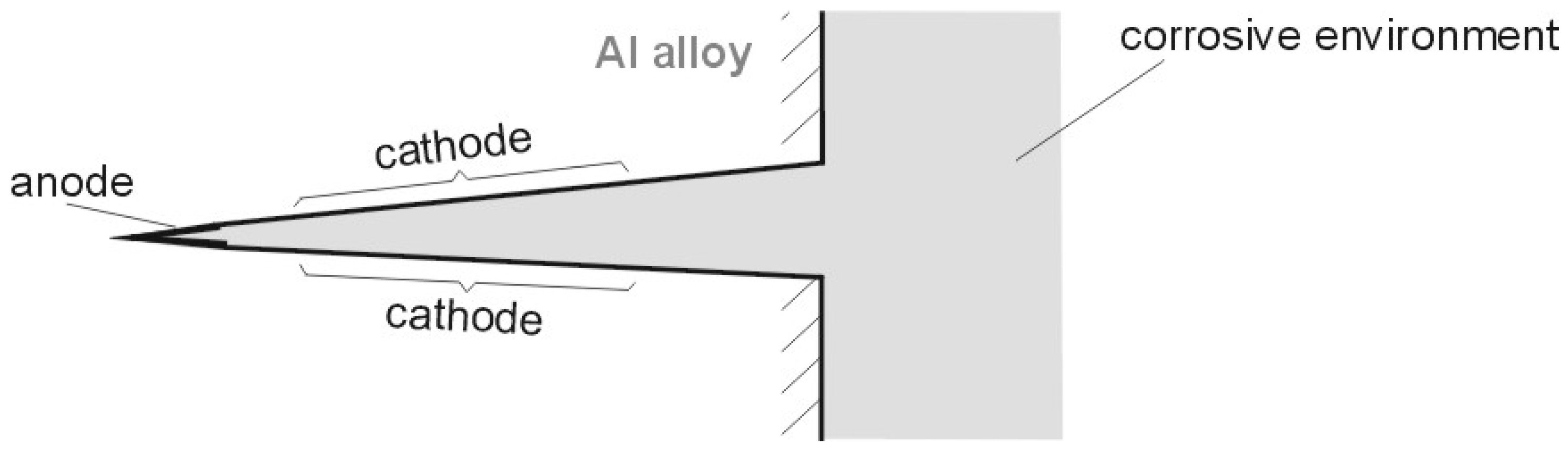
| Alloy Designation | Si | Fe | Cu | Mn | Mg | Cr | Zn | Ti | Others |
|---|---|---|---|---|---|---|---|---|---|
| EN AW-1050A | 0.25 | 0.40 | 0.05 | 0.05 | 0.05 | - | 0.07 | 0.05 | - |
| EN AW-5754 | 0.4 | 0.4 | 0.1 | 0.5 | 2.6–3.6 | 0.3 | 0.2 | 0.15 | 0.15 |
| EN AW-6060 | 0.3–0.6 | 0.1–0.3 | 0.1 | 0.1 | 0.3–0.6 | 0.05 | 0.15 | 0.1 | 0.15 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Włodarczyk, P.P.; Włodarczyk, B. Effect of Hydrogen and Absence of Passive Layer on Corrosive Properties of Aluminum Alloys. Materials 2020, 13, 1580. https://doi.org/10.3390/ma13071580
Włodarczyk PP, Włodarczyk B. Effect of Hydrogen and Absence of Passive Layer on Corrosive Properties of Aluminum Alloys. Materials. 2020; 13(7):1580. https://doi.org/10.3390/ma13071580
Chicago/Turabian StyleWłodarczyk, Paweł P., and Barbara Włodarczyk. 2020. "Effect of Hydrogen and Absence of Passive Layer on Corrosive Properties of Aluminum Alloys" Materials 13, no. 7: 1580. https://doi.org/10.3390/ma13071580





