3. Results and Discussion
Ba
2MgWO
6 possesses the cubic crystal structure with a rock salt lattice of which corner-shared MgO
6 and WO
6 octahedrons. The Ba cations are located in the 12-fold coordination sites. The crystal structure of BMW was described in detail in our previous study [
3]. The X-ray diffractograms of Ba
2MgWO
6: x Eu
3+ samples (x = 0.1%, 0.5%, 1%, 3%, 4%, 5%, and 7%) are shown in
Figure 2. The X-ray diffraction patterns of prepared samples match well with the Ba
2MgWO
6 pattern (ICSD card number 024-982) with the space group Fm-3m. The higher concentration of europium causes the additional diffraction peaks to appear. The alien phases belong to the barium tungstate family, including BaWO
4 (present at 2
of 26.4°), Ba
2WO
5 (28°, 28.6°, 29.6°), and Ba
3WO
6 (29.4°, 42°) these phases were also mainly found in other reports [
1,
7]. Fortunately, the alien phases did not affect the luminescence properties as well as further ceramic preparation. The positions of the XRD peaks were corrected with the aid of Si admixture (ICSD card number 026-1481). Ba
2+ is a very large ion, its crystal radius (CR) for coordination number equal 12 (CN = 12) is 175 pm. While the CR of the Eu
3+ ion for CN = 9 is-Shannon gives no data for the highest CN-only 126 pm [
6]. If europium ions have replaced barium ions, then the unit cell of BMW should shrink. Since it is growing (see
Figure 2c), we conclude that larger Eu
3+ ions (CR = 108.7 pm) replace smaller Mg
2+ ions (CR = 86 pm) in the sites with CN = 6. The replacement of W
6+ with Eu
3+ was excluded because of the large difference between the charge of these ions and their crystal radius equal to 74 and 108.7 pm for W
6+ and Eu
3+, respectively.
The microstructure of the representative sample BMW: 3% Eu
3+, and the crystallite size distribution of the obtained sample (calculated using ImageJ software) are shown in
Figure 3. In general, single grains were not observed in the SEM image. As can be seen, the morphology of the sample was heterogeneous. However, a large number of crystallites were agglomerated, forming large objects with irregular shapes. As a result, the distribution of the crystallite sizes is in a wide range from 70 nm up to 500 nm. The average crystallite size was estimated to be around 209 nm. This value is much smaller than those obtained using a conventional solid-state technique [
3,
7], demonstrating one of the advantages of the wet chemistry method. This result encourages further work to eliminate agglomeration to ease ceramic preparation.
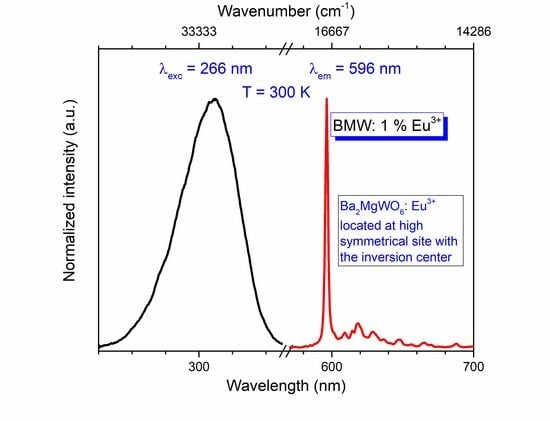
The photoluminescence of Ba
2MgWO
6 doped with a series of europium concentration from 0.1% to 7% was measured under 266 nm excitation wavelength at room temperature (see
Figure 4). The picture is uncommon to majority cases when Eu
3+ ions are located at the sites without the center of inversion. Here the
5D
0 →
7F
1 magnetic dipole transition dominates the spectrum as sharp and intense peak at 596 nm. The forced electric dipole transitions are much weaker and broadened due to vibronic coupling, assigned to the
5D
0 →
7F
J transitions (where J = 0, 2, 3, 4) are observed at about 584.4, 618.7, 665, and 721.3 nm, respectively. The integrated emission intensity in the function of the Eu
3+ concentration was depicted in the inset of
Figure 4. The luminescent intensity increases significantly with the increase of Eu
3+ concentration up to 5%. Above that point, the emission intensity decreases as a consequence of the concentration quenching process.
Due to the hypersensitive nature, the 5D0 → 7F2 electric-dipole transition it susceptible to the even small changes of the crystal field. In contrast, the 5D0–7F1 magnetic dipole transition is virtually unaffected by neither symmetry site nor host lattice-type. Hence, the asymmetry factor R, the ratio between the integrated intensity of the 5D0 → 7F2 and that of 5D0 → 7F1 transitions, is often used to determine the changes in the nearest environment of Eu3+ in the lattice. Thus, the higher the value R is, the lower the symmetry of the Eu3+ ions occupancy site. For the investigated samples, the R parameter increases gradually from 0.56 to 0.63 when dopant concentration increases. It demonstrates a slight distortion of the structure, which insignificantly decreases local symmetry of the Eu3+ ions.
To assign every single emission peak, and to determine the number of the Eu
3+ sites, the emission spectra were recorded at low temperature (77 K and 10 K). The intensity of the f-f lines significantly increased, while the vibronic ones almost disappeared when the temperature decreased down to 10 K (see
Figure 5). Only one narrow line at 585 nm attributed to the
5D
0 →
7F
0 transition was observed at room and low (10 and 77 K) temperature. It is apparent evidence of the europium location in the only one crystallographic site (see the inset in
Figure 5). Emission spectra were carefully studied. All detected lines were tentatively assigned either to MD or ED or vibronic transitions. Their energies are presented in
Supplementary Table S1.
The excitation spectra of the 1%, 3%, and 5% Eu
3+ doped Ba
2MgWO
6 was monitored at 596 nm, i.e.at the maximum of the
5D
0 →
7F
1 transition at room temperature (see
Figure 6). The spectra consist of a broad and intense band ranging from 250 nm to 350 nm. The broad band results from two charge transfer transitions (CTB), including the O
2− → W
6+ and O
2− → Eu
3+ transitions. There is also a small band at about 395 nm assigned to intra-configurational f-f transitions of Eu
3+ ions, weak due to the Laporte selection rule [
8]. They become partially allowed because of the lowering of the symmetry site when introducing a higher amount of Eu
3+ and Li
+ ions. Our recent investigation does not reveal any trace of f-f transitions in the excitation spectra, but there effective doping was several times smaller [
3]. The sample with 1% Eu
3+ virtually does not show the f-f transitions. Mainly CTB is observed similarly as in the solid-state method [
3].
It is not easy to find the energy of the O
2− → Eu
3+ transitions since the amount of dopant is small comparing to the tungstate, which forms the host structure. Analogously, the broad band at 318 nm results from both charge transfer transitions in Ba
3WO
6: Eu
3+ [
9]. The energy of the O
2− → Eu
3+ CTB is influenced not only by the ligand electronegativity but also by the local surroundings of the Eu
3+ ions [
8]. The location of the O
2−–W
6+ CTB has been reported around 300 nm in g-C
3N
4/Ba
2MgWO
6: Eu
3+ [
4], 301 nm in Ba
2MgWO
6: Eu
3+ [
3], 310 nm in host Ba
2MgWO
6 [
7], 325 nm [
10] in NaLaMgWO
6: Eu
3+. As noted in the preliminary work, the O
2−–Eu
3+ CTB was found at around 304 nm in CaMoO
4: Eu
3+, 293 nm in AMoO
4: Eu
3+ (A = Ba, Sr), and at 250 nm in BaNa
2W
2O
11: Eu
3+. The less intense and narrower bands appeared near the visible region (from 370 to 400 nm) and at 462.5 nm that could be ascribed to f-f transitions of the
7F
0 →
5D
4,
5G
7,
5L
7,
5L
6,
5D
3 and
7F
0 →
5D
2 transitions, respectively, [
11,
12,
13] (see inset in
Figure 6).
The absorption spectra registered at 300 K were useful to calculate the energy of the forbidden band-gap of the investigated samples. The band-gap energy E
g was determined by applying the modified Kubelka–Munk function and plotted as (F(R).h
v)
2 versus h
v, also called a Tauc method [
14]. It was found that the higher dopant content was used, the smaller band-gap energy was obtained, (see
Figure 7). For the lowest Eu
3+ concentration, the band-gap energy was 4.02 eV while for the sample doped with 5% of Eu
3+, E
g = 3.82 eV.
The 77 K decay curves of the BMW sample doped with 1% Eu
3+ was excited at 266 nm and monitored at the different wavelengths corresponding to the transitions from the
5D
1 (588 nm, 602 nm) and the
5D
0 (596 nm, 722 nm) levels (see
Figure 8). The decay curves are multi-exponential in all cases. The first component was usually connected with the nonradiative process, while the longer ones resulted from the emissions from different Eu
3+ energy levels depending on the monitored wavelength. The decay of magnetic-dipole transition consisted of two components, one with the short lifetime-related to the nonradiative process-and the longer one, τ = 5.71 ms, characteristic for the Eu
3+ located in the site with very high symmetry. The decay time of the
5D
1 level was found to be equal to 70 μs, it is much smaller than expected, probably because of the fast nonradiative transition to the lower
5D
0 level and also possible cross-relaxation mechanism—the latter will be discussed further.
The luminescence kinetics were recorded at 77 K upon excitation at 266 nm.
Figure 9 presents the
5D
0 →
7F
1 emission decay profiles of the BMW doped with 0.5%, 3%, and 7% of Eu
3+ ions. The emission decay curves exhibited the characteristics of the nonexponential function. The average decay time can be expressed as follow:
where I(t) is the emission intensity at time t, 0 < t < t
max. The emission average decay time gradually decreased from 5 ms to 2.9 ms with an increase of Eu
3+ concentration from 1% to 7%, respectively. Two factors could explain this observation, one is the nonradiative processes, which are undoubtedly present. Second the distortion of the Eu
3+ coordination polyhedra due to the increase of the dopant concentration. We recall here that the effective doping is twice as important as in the previous study [
3,
4] because of the presence of Li
+. The distortion causes a slight decrease in the symmetry, so the transition probability increases, although the emission still dominates the MD transition.
The Judd–Ofelt (J-O) intensity parameters (Ω
λ, where λ = 2, 4, 6) were calculated on the basis of the BMW: Eu
3+ (5%) emission spectrum excited at 266 nm and recorded at 300 K. Exactly the same formalism and the same set of equations were used as in our previous work [
3]. The refractive index “n” of the BMW host is 1.874 [
3]. The matrix elements U(2) = 0.0035, U(4) = 0.003 and U(6) = 0.0005 from the work of K. Binnemans [
8] were applied for the calculations.
Using the Ω
λ parameters, the A
ij transition rates, the β
ij branching ratios, were calculated for the
5D
0 →
7F
J transitions (where J = 1, 2, 4, 6) (see
Table 1 and
Table 2). A recent analysis in the frame of J-O theory for the BMW: Eu
3+ (2%) solid-state sample was carried out at 395 nm of excitation [
3]. However, there is a possibility that excitation at 395 nm will be absorbed by all Eu
3+ ions-including those which could be in the additional, foreign phases present in the sample. Consequently, this time, the emission was excited with the 4
th harmonic of the Nd: YAG laser reaching the O
2- → Eu
3+ charge transfer band, both for the solid-state and co-precipitation samples.
There is very little difference among J-O parameters between sample synthesized by co-precipitation and solid-state method (see
Table 1,
Table 2 and
Table 3). The value of Ω
2 is twofold smaller than that of Ω
6. The increase in the concentration of Eu
3+ from 2% to 5% leads to a modification of the Ω
λ (see
Table 3). These are phenomenological parameters which only depend on the matrix. An increase in the concentration of dopant ions having a larger diameter and a different charge than the replaced Mg
2+ must cause the deformation of the matrix. We must not forget either that with 5% Eu
3+, we introduce 5% Li
+.
The Ω
𝜆 values found in this work are comparable with other results obtained in similar tungstate double perovskites but with lower symmetry (Li, Na, K)LaMgWO
6 [
15]
. Due to the fact that Ω
2 is very sensitive to the angular changes while Ω
4 and Ω
6 are mostly affected by bond covalence between dopants and ligands [
16]. As a result, a distinction among various tungstate double perovskites was observed, Ω
2 values of (Li, Na, K)LaMgWO
6 with lower site symmetry are seven times higher in comparison with those of the higher site symmetry (O
h) in BMW. The Ω
4 value of (Li, Na, K)LaMgWO
6 is also fivefold higher than those of BMW while the Ω
6 value is quite similar, except for KLaMgWO
6.
Besides, the rates of total, radiative, non-radiative transition along with the theoretical radiative and experimental decay time as well as the quantum efficiency of sample BMW: Eu
3+ are presented in
Table 2. For sample prepared by co-precipitation and solid-state, the calculated radiative decay times are 4.96 and 5.31 ms and the quantum efficiencies are 90.2% and 85 %, respectively.
The temperature-dependent emission spectra under excitation at 266 nm were performed in the temperature range from 80–730 K (
Figure 10a). The emission intensity does not change much up to 200 K. Above this temperature, emission intensity significantly decreases. The temperature quenching, at which the integrated intensity of emission decreased half in comparison with the initial emission intensity (T
0.5) was at around 350 K (
Figure 10b). The emission intensity of transitions from the
5D
1 level was also quenched, along with the transitions from the
5D
0 level.
Figure 10 shows the relationship of ln(I
0/I-1) versus 1/kT, two mechanisms responsible for emission quenching took place. The activation energies for thermal quenching were calculated to be 0.09 eV and 0.23 eV. The first one is due to the process which operates in the 80–350 K temperature range. Above 350 K, the second process is switched on and is more effective in the emission quenching. The first process is of the same nature as that observed in LaAlO
3: Eu
3+ and explained in detail by Blasse [
17], but the CT state involved here is associated with the O
2− ↔ W
6+ transition. Here, the same approach was applied to support the single configurational coordinate model depicted in
Figure 10d. Upon excitation into the charge transfer state-transition A → B, some part of the excitation energy relaxes to point C and then to Eu
3+, and the rest, with thermal excitation, flows from the CTS to the Eu
3+ ground state via point D. (see
Figure 10d). The other nonradiative process is switched on above 350 K when the
5D
1 level becomes thermally populated and is due to the following cross-relaxation: [
5D
1,
7F
0] → [
5D
0,
7F
3] (see points F and E in
Figure 10d). Its activation energy (∆E
a = 0.23 eV = 1855 cm
−1) is related to the difference energy between the
5D
0 and
5D
1 and
7F
0–
7F
3 levels.