Structure and Photoluminescence Properties of Thermally Synthesized V2O5 and Al-Doped V2O5 Nanostructures
Abstract
:1. Introduction
2. Materials and Methods
2.1. Fabrication of Al-Free and Al-Doped V2O5 Nanostructures
2.2. Characterizations
3. Results
3.1. X-ray Photoelectron Spectroscopy (XPS) Analysis
3.2. X-ray Diffraction (XRD) Patterns
3.3. Field-Emission Scanning Electron Microscopy (FESEM) and High-Resolution Transmission Electron Microscopy (HRTEM) Analysis
3.4. Raman Spectra
- (1)
- The V–O2′1 bond has a length of 2.017 Å, and
- (2)
- The V–O3 bond has a length of 1.779 Å.

| Raman Scattering Modes | AV0 NSs | AV10 NSs | Deviation of the Raman Shifts (%) | ||
|---|---|---|---|---|---|
| Raman Shifts (cm−1) | Intensity | Raman Shifts (cm−1) | Intensity | ||
| Stretching (V2O2)n | 141.71 | 84,745.82 | 141.93 | 45,097.12 | 0.071 |
| Stretching (V2O2)’n | 195.26 | 3371.40 | 194.45 | 1830.32 | 0.051 |
| Bending V=O | 282.04 | 20,894.35 | 282.14 | 10,431.08 | 0.017 |
| Bending V–O | 302.64 | 9033.58 | 302.47 | 5007.98 | 0.016 |
| Bending V=O | 403.51 | 9475.78 | 403.33 | 4967.71 | 0.025 |
| Bending V–O–V | 480.45 | 6944.95 | 481.35 | 3846.47 | 0.062 |
| Stretching V–O | 524.80 | 11,097.60 | 522.07 | 11,202.89 | 0.258 |
| Stretching V–O–V | 699.93 | 12,863.33 | 701.25 | 5757.458 | 0.099 |
| Stretching V=O | 993.36 | 20,228.08 | 994.02 | 8188.87 | 0.030 |
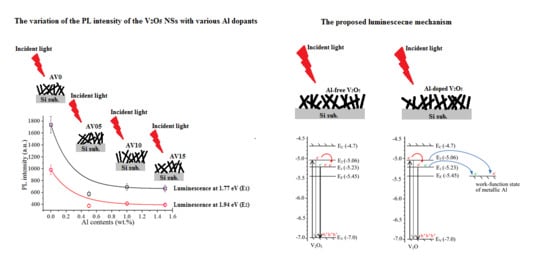
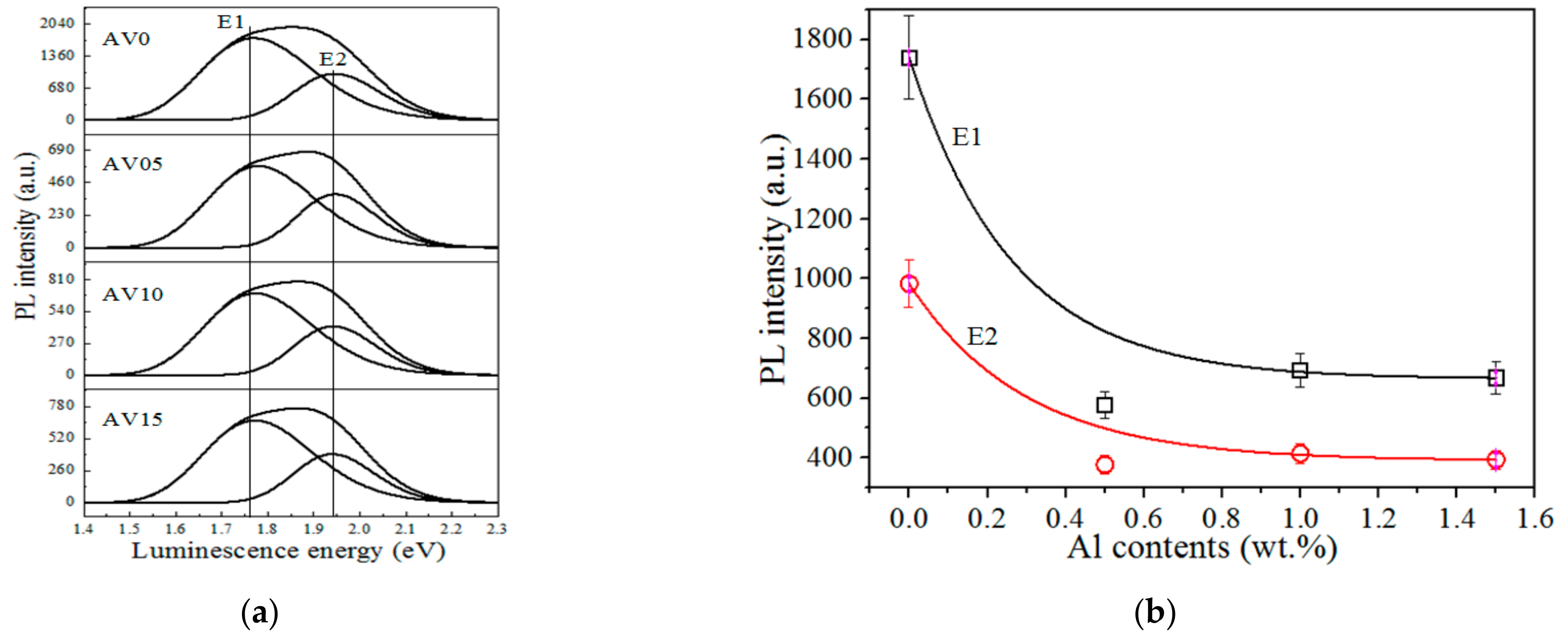
3.5. Photoluminescence (PL) Spectra
3.6. Proposed Luminescence Mechanism
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Liu, M.; Su, B.; Tang, Y.; Jiang, X.; Yu, A. Recent advances in nanostructured vanadium oxides and composites for energy conversion. Adv. Energy Mater. 2017, 7, 1–34. [Google Scholar]
- Surnev, S.; Ramsey, M.G.; Netzer, F.P. Vanadium oxide surface studies. Prog. Surf. Sci. 2003, 73, 117–165. [Google Scholar] [CrossRef]
- Lee, S.H.; Cheong, H.M.; Seong, M.J.; Liu, P.; Tracy, C.E.; Mascarenhas, A.; Pitts, J.R.; Deb, S.K. Raman spectroscopic studies of amorphous vanadium oxide thin films. Solid State Ion. 2003, 165, 111–116. [Google Scholar] [CrossRef]
- Sucharitakul, S.; Ye, G.; Lambrecht, W.R.L.; Bhandari, C.; Gross, A.; He, R.; Poelman, H.; Gao, X.P.A. V2O5: A 2D van der Waals oxide with strong in-plane electrical and optical anisotropy. ACS Appl. Mater. Interfaces 2017, 9, 23949–23956. [Google Scholar] [CrossRef] [PubMed]
- Chakrabarti, A.; Hermann, K.; Druzinic, R.; Witko, M.; Wagner, F.; Petersen, M. Geometric and electronic structure of vanadium pentoxido: A density functional bulk and surface study. Phys. Rev. B 1999, 59, 10583–10590. [Google Scholar] [CrossRef] [Green Version]
- Wang, Y.; Su, Q.; Chen, C.H.; Yu, M.L.; Han, G.J.; Wang, G.Q.; Xin, K.; Lan, W.; Liu, X.Q. Low temperature growth of vanadium pentoxide nanomaterials by chemical vapour deposition using VO (acac)2 as precursor. J. Phys. D Appl. Phys. 2010, 43, 185102. [Google Scholar] [CrossRef]
- Wang, C.C.; Lu, C.L.; Shieu, F.S.; Shih, H.C. Enhanced photoluminescence properties of Ga-Doped V2O5 nanorods via defect structures. Chem. Phys. Lett. 2020, 738, 136864. [Google Scholar] [CrossRef]
- Wang, C.C.; Chen, K.C.; Shieu, F.S.; Shih, H.C. Characterization and photoluminescence of V2O5@Pt core-Shell nanostructures as fabricated by atomic layer deposition. Chem. Phys. Lett. 2019, 729, 24–29. [Google Scholar] [CrossRef]
- Van, K.L.; Groult, H.; Mantoux, A.; Perrigaud, L.; Lantelme, F.; Lindstrom, R.; Badour-Hadjean, R.; Zanna, S.; Lincot, D. Amorphous vanadium oxide films synthesised by ALCVD for lithium rechargeable batteries. J. Power Sources 2006, 160, 592–601. [Google Scholar] [CrossRef]
- Venkatesan, A.; Chandar, N.R.K.; Pradeeswari, K.; Pandi, P.; Kandasamy, A.; Kumar, R.M.; Jayavel, R. Influence of Al doping on structural, luminescence and electrochemical properties of V2O5 nanostructures synthesized via non-Hydrolytic sol-Gel technique. Mater. Res. Express 2019, 6, 015017. [Google Scholar] [CrossRef]
- Díaz-Guerra, C.; Piqueras, J. Structural and cathodoluminescence assessment of V2O5 nanowires and nanotips grown by thermal deposition. J. Appl. Phys. 2007, 102, 084307. [Google Scholar] [CrossRef] [Green Version]
- Schlecht, U.; Knez, M.; Duppel, V.; Kienle, L.; Burghard, M. Boomerang-shaped VOX belts: Twinning within isolated nanocrystals. Appl. Phys. A: Mater. Sci. Process. 2004, 78, 527. [Google Scholar] [CrossRef]
- González-Rivera, Y.A.; Cervantes-Juárez, E.; Aquino-Meneses, L.; Lozada-Morales, R.; Jiménez-Sandoval, S.; Rubio-Rosas, E.; Agustín-Serrano, R.; de la Cerna, C.; Reyes-Cervantes, E.; Zelaya Angel, O.; et al. Photoluminescence in Er-Doped V2O5 and Er-doped CdV2O6. J. Lumin. 2014, 155, 119–124. [Google Scholar] [CrossRef]
- Suresh, R.; Giribabu, K.; Manigandan, R.; Munusamy, S.; Kumar, S.P.; Muthamizh, S.; Stephen, A.; Narayanan, V. Doping of Co into V2O5 nanoparticles enhances photodegradation of methylene blue. J. Alloys Compd. 2014, 598, 151–160. [Google Scholar] [CrossRef]
- Aquino-Meneses, L.; Lozada-Morales, R.; del Angel-Vicente, P.; Percino-Picazo, J.C.; Zelaya-Angel, O.; Becerril, M.; Carmona-Rodriguez, J.; Rodriguez-Melgarejo, F.; Jime’nez-Sandoval, S. Photoluminescence in Nd-Doped V2O5. J. Mater. Sci. 2014, 49, 2298–2302. [Google Scholar] [CrossRef]
- Venkatesan, A.; Chandar, N.R.K.; Kandasamy, A.; Chinnu, M.K.; Marimuthu, K.N.; Kumar, R.M.; Jayavel, R. Luminescence and electrochemical properties of rare earth (Gd, Nd) doped V2O5 nanostructures synthesized by a non-Aqueous sol-Gel route. RSC Adv. 2015, 5, 21778. [Google Scholar] [CrossRef]
- Azevedo, C.F.; Balboni, R.D.C.; Cholant, C.M.; Moura, E.A.; Lemos, R.M.J.; Pawlicka, A.; Gündel, A.; Flores, W.H.; Pereira, M.; Avellaneda, C.O. New thin films of NiO doped with V2O5 for electrochromic Applications. J. Phys. Chem. Solids 2017, 110, 30–35. [Google Scholar] [CrossRef]
- Ongul, F. Solution-Processed inverted organic solar cell using V2O5 hole transport layer and vacuum free EGaIn anode. Opt. Mater. 2015, 50, 244–249. [Google Scholar] [CrossRef]
- Alsulami, A.; Griffin, J.; Alqurashi, R.; Yi, H.; Iraqi, A.; Lidzey, D.; Buckley, A. Thermally stable solution processed vanadium oxide as a hole extraction layer in organic solar cells. Materials 2016, 9, 235. [Google Scholar] [CrossRef] [Green Version]
- Zhao, W.; Zhong, Q.; Pan, Y.; Zhang, R. Defect structure and evolution mechanism of O2− radical in F-Doped V2O5/TiO2 catalysts. Colloids Surf. A: Physicochem. Eng. Asp. 2013, 436, 1013–1020. [Google Scholar] [CrossRef]
- Pandey, G.P.; Liu, T.; Brown, E.; Yang, Y.; Li, Y.; Sun, X.S.; Fang, Y.; Li, J. Mesoporous hybrids of reduced graphene oxide and vanadium pentoxide for enhanced performance in lithium-Ion batteries and electrochemical capacitors. ACS Appl. Mater. Interfaces 2016, 8, 9200–9210. [Google Scholar] [CrossRef]
- Mane, A.A.; Suryawanshi, M.P.; Kim, J.H.; Moholkar, A.V. Superior selectivity and enhanced response characteristics of palladium sensitized vanadium pentoxide nanorods for detection of nitrogen dioxide gas. J. Colloid Interface Sci. 2017, 495, 53–60. [Google Scholar] [CrossRef] [PubMed]
- Ghanei-Motlagh, M.; Taher, M.A.; Fayazi, M.; Baghayeri, M.; Hosseinifar, A. Non-Enzymatic amperometric sensing of hydrogen peroxide based on vanadium pentoxide nanostructures. J. Electrochem. Soc. 2019, 166, B367–B372. [Google Scholar] [CrossRef]
- Pradeep, I.; Kumar, E.R.; Suriyanarayanan, N.; Mohanraj, K.; Srinivas, C.; Mehar, M.V.K. Effect of Al doping concentration on the structural, optical, morphological and electrical properties of V2O5 nanostructures. New J. Chem. 2018, 42, 4278. [Google Scholar] [CrossRef]
- Venkatesan, A.; Chandar, N.K.; Kumar, M.K.; Arjunan, S.; Kumar, R.M.; Jayavel, R. Al3+ Doped V2O5 Nanostructure: Synthesis and Structural, Morphological and Optical Characterization. In Proceedings of the AIP Conference, Mumbai, India, 3–7 December 2012; Volume 1512, p. 392. [Google Scholar]
- Ali, H.M.; Hakeem, A.M.A. Structural and optical properties of electron-Beam evapo-Rated Al2O3-Doped V2O5 thin films for various applications. Phys. Status Solidi A 2010, 207, 132–138. [Google Scholar] [CrossRef]
- De Jesus, L.R.; Horrocks, G.A.; Liang, Y.; Parija, A.; Jaye, C.; Wangoh, L.; Wang, J.; Fischer, D.A.; Piper, L.F.J.; Prendergast, D.; et al. Mapping polaronic states and lithiation gradients in individual V2O5 nanowires. Nat. Commun. 2016, 7, 12022. [Google Scholar] [CrossRef] [Green Version]
- Wu, Q.H.; Thissen, A.; Jaegermann, W.; Liu, M. Photoelectron spectroscopy study of oxygen vacancy on vanadium oxides surface. Appl. Surf. Sci. 2004, 236, 473–478. [Google Scholar] [CrossRef]
- Silversmit, G.; Depla, D.; Poelman, H.; Marin, G.B.; De Gryse, R. Determination of the V2p XPS binding energies for different vanadium oxidation states (V5+ to V0+). J. Electron Spectrosc. Relat. Phenom. 2004, 135, 167–175. [Google Scholar] [CrossRef]
- Harris, D.C. Quantitative Chemical Analysis, 7th ed.; W.H. Freeman: New York, NY, USA, 2007. [Google Scholar]
- Sun, C.; Zeng, R.; Zhang, J.; Qiu, Z.J.; Wu, D. Effects of UV-Ozone treatment on sensing behaviours of EGFETs with Al2O3 sensing film. Materials 2017, 10, 1432. [Google Scholar] [CrossRef] [Green Version]
- Wagner, C.D.; Riggs, W.M.; Davis, L.E.; Mullenberg, J.F. Handbook of X-ray Photoelectron Spectroscopy; Perkin-Elmer Corporation: Waltham, MA, USA, 1979; p. 50. [Google Scholar]
- Murugan, A.V.; Kale, B.B.; Kwon, C.W.; Campet, G.; Vijayamohanan, K. Synthesis and characterization of a new organo–inorganic poly (3,4-ethylene dioxythiophene) PEDOT/V2O5 nanocomposite by intercalation. J. Mater. Chem. 2001, 11, 2470–2475. [Google Scholar] [CrossRef]
- Singh, B.; Gupta, M.K.; Mishra, S.K.; Mittal, R.; Sastry, P.U.; Rols, S.; Chaplot, S.L. Anomalous lattice behavior of vanadium pentaoxide (V2O5): X-ray diffraction, inelastic neutron scattering and ab initio lattice dynamics. Phys. Chem. Chem. Phys. 2017, 19, 17967–17984. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shen, W.J.; Sun, K.W.; Lee, C.S. Electrical characterization and Raman spectroscopy of individual vanadium pentoxide nanowire. J. Nanoparticle Res. 2011, 13, 4929–4936. [Google Scholar] [CrossRef]
- Dewangan, K.; Sinha, N.N.; Chavan, P.G.; Sharma, P.K.; Pandey, A.C.; More, M.A.; Joag, D.S.; Munichandraiah, N.; Gajbhiye, N.S. Synthesis and characterization of self-Assembled nanofiber-bundles of V2O5: Their electrochemical and field emission properties. Nanoscale 2012, 4, 645–651. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Urena-Begar, F.; Crunteanu, A.; Raskin, J.P. Raman and XPS characterization of vanadium oxide thin films with temperature. Appl. Surf. Sci. 2017, 403, 717–727. [Google Scholar] [CrossRef]
- Baddour-Hadjean, R.; Pereira-Ramos, J.P. Raman microspectrometry applied to the study of electrode materials for lithium batteries. Chem. Rev. 2010, 110, 1278–1319. [Google Scholar] [CrossRef]
- Baddour-Hadjean, R.; Golabkan, V.; Pereira-Ramos, J.P.; Mantoux, A.; Lincot, D. A Raman study of the lithium insertion process in vanadium pentoxide thin films deposited by atomic layer deposition. J. Raman Spectrosc. 2002, 33, 631–638. [Google Scholar] [CrossRef]
- Liu, X.; Huang, C.; Qiu, J.; Wang, Y. The effect of thermal annealing and laser irradiation on the microstructure of vanadium oxide nanotubes. Appl. Surf. Sci. 2006, 253, 2747–2751. [Google Scholar] [CrossRef]
- Chen, W.; Mai, L.; Peng, J.; Xu, Q.; Zhu, Q. Raman spectroscopic study of vanadium oxide nanotubes. J. Solid State Chem. 2004, 177, 377–379. [Google Scholar] [CrossRef]
- Jung, H.; Gerasopoulos, K.; Alec Talin, A.; Ghodssi, R. A platform for in situ Raman and stress characterizations of V2O5 cathode using MEMS device. Electrochim. Acta 2017, 242, 227–239. [Google Scholar] [CrossRef]
- Al-Assiri, M.S.; El-Desoky, M.M.; Alyamani, A.; Al-Hajry, A.; Al-Mogeeth, A.; Bahgat, A.A. Structural and transport properties of Li-intercalated vanadium pentoxide nanocrystalline films. Philos. Mag. 2010, 90, 3421–3439. [Google Scholar] [CrossRef]
- Park, Y.; Kim, N.H.; Kim, J.Y.; Eom, I.Y.; Jeong, Y.U.; Kim, M.S.; Lee, S.M.; Choi, H.C.; Jung, Y.M. Surface characterization of the high voltage LiCoO2/Li cell by X-ray photoelectron spectroscopy and 2D correlation analysis. Vib. Spectrosc. 2010, 53, 60–63. [Google Scholar] [CrossRef]
- Milewska, A.; Świerczek, K.; Tobola, J.; Boudoire, F.; Hu, Y.; Bora, D.K.; Mun, B.S.; Braun, A.; Molenda, J. The nature of the nonmetal–Metal transition in LixCoO2 oxide. Solid State Ionics 2014, 263, 110–118. [Google Scholar] [CrossRef]
- Faggio, G.; Modafferi, V.; Panzera, G.; Alfieri, D.; Santangelo, S. Micro-Raman and photoluminescence analysis of composite vanadium oxide/poly-Vinyl acetate fibres synthesised by electro-Spinning. J. Raman Spectrosc. 2012, 43, 761–768. [Google Scholar] [CrossRef]
- Gurulakshmi, M.; Selvaraj, M.; Selvamani, A.; Vijayan, P.; Sasi Rekha, N.R.; Shanthi, K. Enhanced visible-Light photocatalytic activity of V2O5/S-TiO2 nanocomposites. Appl. Catal. A Gen. 2012, 449, 31–46. [Google Scholar] [CrossRef]
- Kumar, B.; Kaushik, B.K.; Negi, Y.S. Perspectives and challenges for organic thin film transistors: Materials, devices, processes and applications. J. Mater. Sci: Mater. Electron. 2014, 25, 1–30. [Google Scholar]




| Samples | AV0 | AV05 | AV10 | AV15 |
|---|---|---|---|---|
| Al contents(g) | 0 | 0.0015 | 0.003 | 0.0045 |
| Al contents (wt.%) | 0 | 0.5 | 1 | 1.5 |
| Al contents (at.%) | 0 | 4.45 | 8.64 | 11.31 |
| Al Contents (wt.%) | Diffraction Angle (2θ) | Lattice Constants of V2O5 (Å) | c/a | c/b | ||||
|---|---|---|---|---|---|---|---|---|
| V2O5 (001) | V2O5 (110) | V2O5(400) | a | b | c | |||
| 0 | 20.36 | 26.17 | 31.07 | 11.504 | 3.562 | 4.357 | 0.3787 | 1.2234 |
| 0.5 | 20.42 | 26.28 | 31.19 | 11.461 | 3.546 | 4.345 | 0.3791 | 1.2253 |
| 1.0 | 20.35 | 26.19 | 31.10 | 11.492 | 3.558 | 4.361 | 0.3795 | 1.2256 |
| 1.5 | 20.33 | 26.19 | 31.09 | 11.494 | 3.558 | 4.363 | 0.3842 | 1.2263 |
| Dopant Precursors | Dopant Type | PL Intensity of the V2O5 | Ref. |
|---|---|---|---|
| metallic Al | Al0 | Decreases with the increasing precursor contents | This work |
| metallic Ga | Ga3+ | Enhancement at proper precursor contents | [7] |
| Al(NO3)3·9H2O | Al3+ | Decreases with the increasing precursor contents | [10] |
| Er(NO3)3·5H2O | Er3+ | No data | [13] |
| CoSO4·7H2O | Co2+ | Decreases with the increasing precursor contents | [14] |
| Gd2O3 | Gd3+ | Decreases with the adding of precursor | [16] |
| Nd2O3 | Nd3+ | Decreases with the adding of precursor | [16] |
| Al(NO3)3 | Al3+ | Decreases with the adding of precursor | [24] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, C.-C.; Lu, C.-L.; Shieu, F.-S.; Shih, H.C. Structure and Photoluminescence Properties of Thermally Synthesized V2O5 and Al-Doped V2O5 Nanostructures. Materials 2021, 14, 359. https://doi.org/10.3390/ma14020359
Wang C-C, Lu C-L, Shieu F-S, Shih HC. Structure and Photoluminescence Properties of Thermally Synthesized V2O5 and Al-Doped V2O5 Nanostructures. Materials. 2021; 14(2):359. https://doi.org/10.3390/ma14020359
Chicago/Turabian StyleWang, Chih-Chiang, Chia-Lun Lu, Fuh-Sheng Shieu, and Han C. Shih. 2021. "Structure and Photoluminescence Properties of Thermally Synthesized V2O5 and Al-Doped V2O5 Nanostructures" Materials 14, no. 2: 359. https://doi.org/10.3390/ma14020359