Lanthanum Ferrite Ceramic Powders: Synthesis, Characterization and Electrochemical Detection Application
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Synthesis of LaFe(C2O4)3·3H2O Oxalate Precursor and LaFeO3
2.3. Characterization of LaFe(C2O4)3·3H2O Oxalate Precursor and LaFeO3
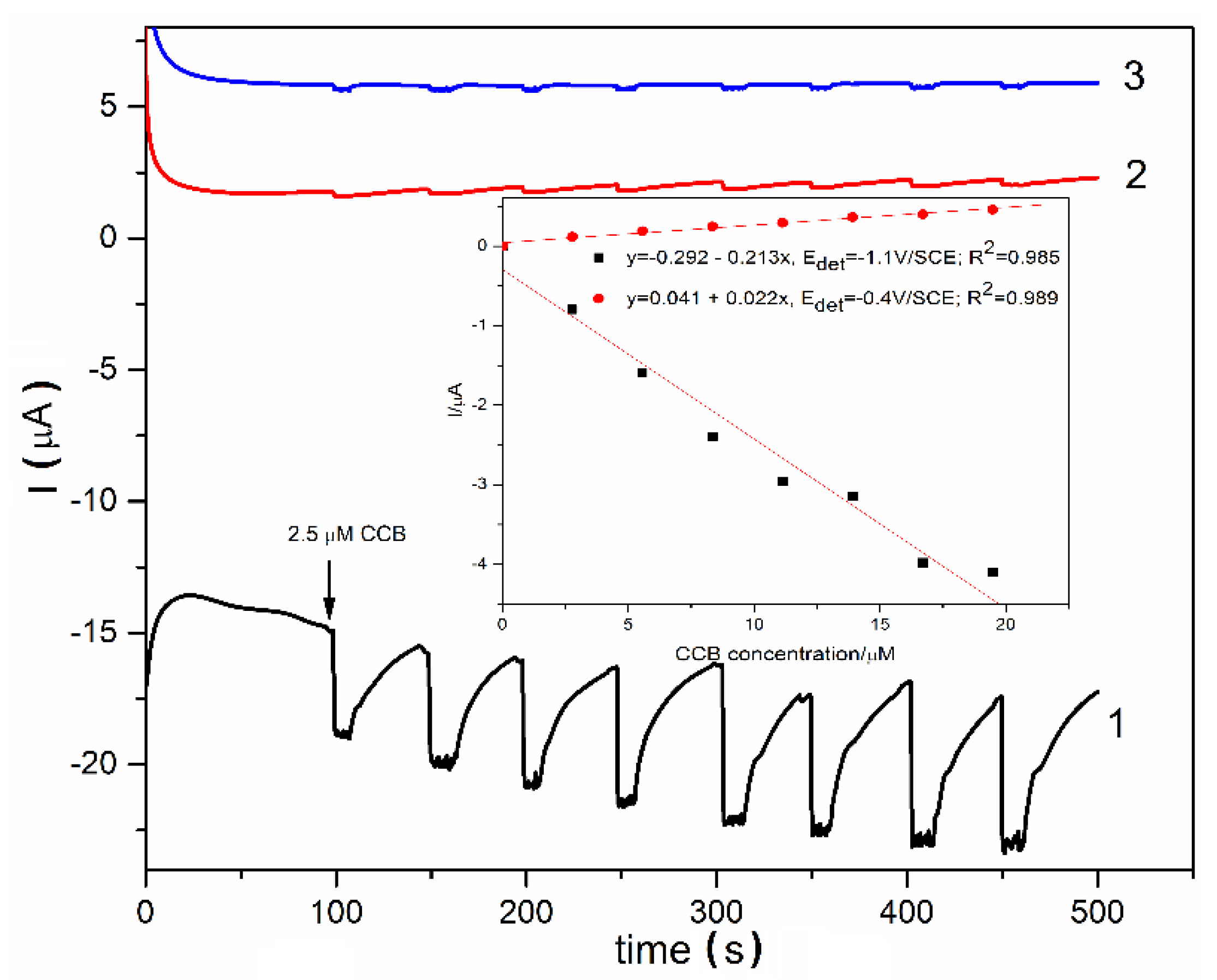
2.4. Electrochemical Detection Application
3. Results and Discussion
3.1. Characterization of LaFe(C2O4)3·3H2O Oxalate Precursor
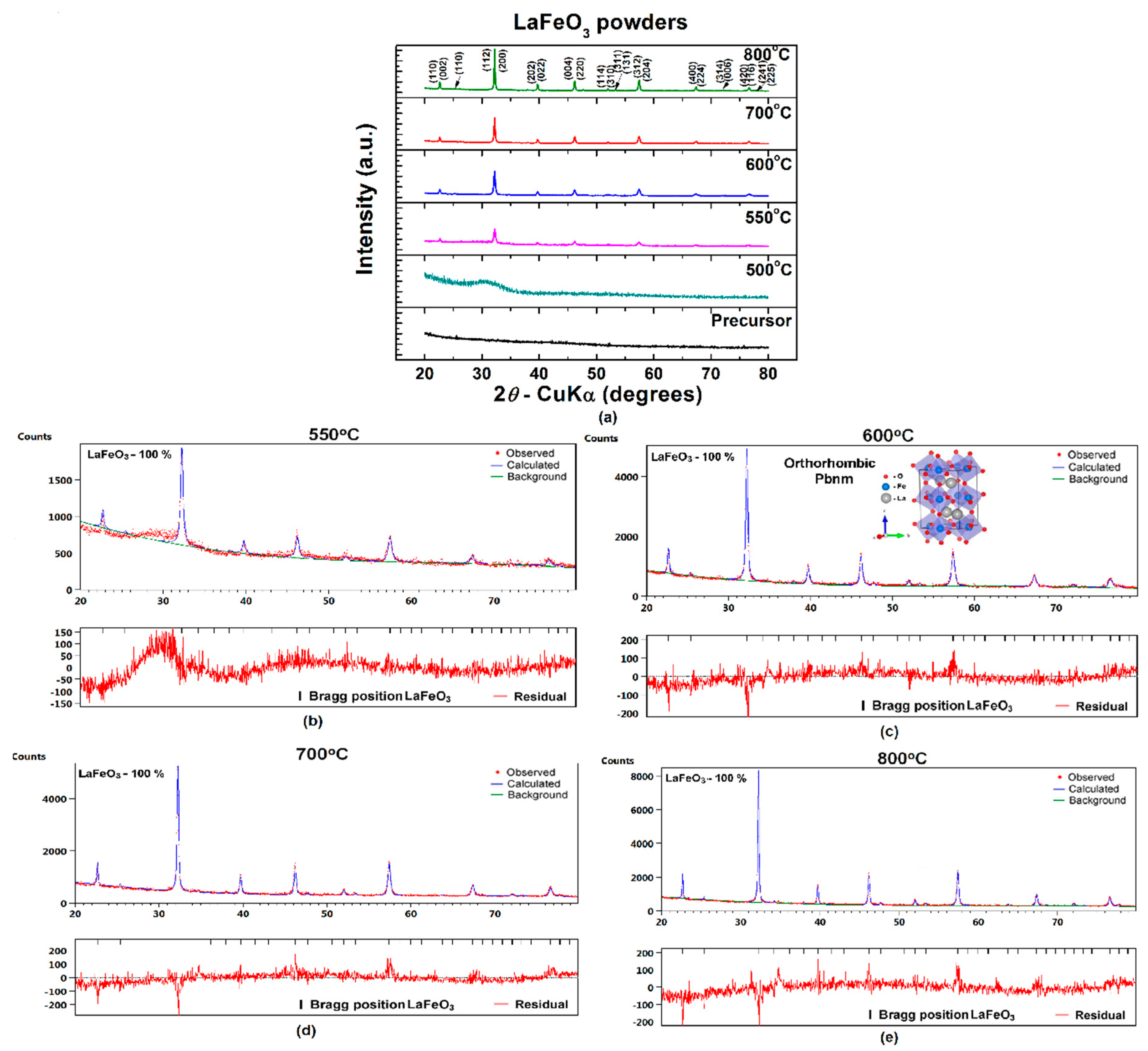
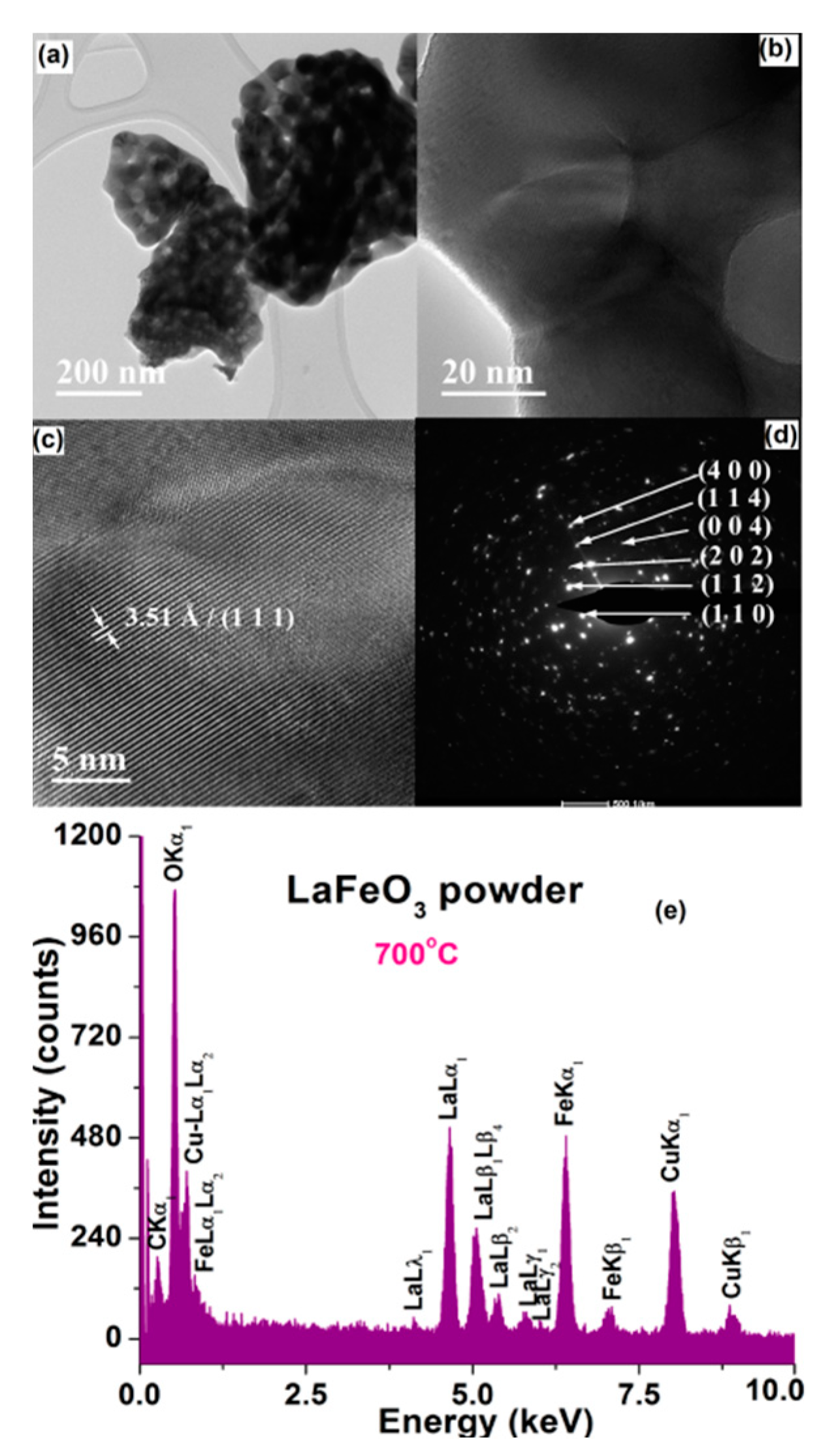
3.2. Characterization of LaFeO3 Powders
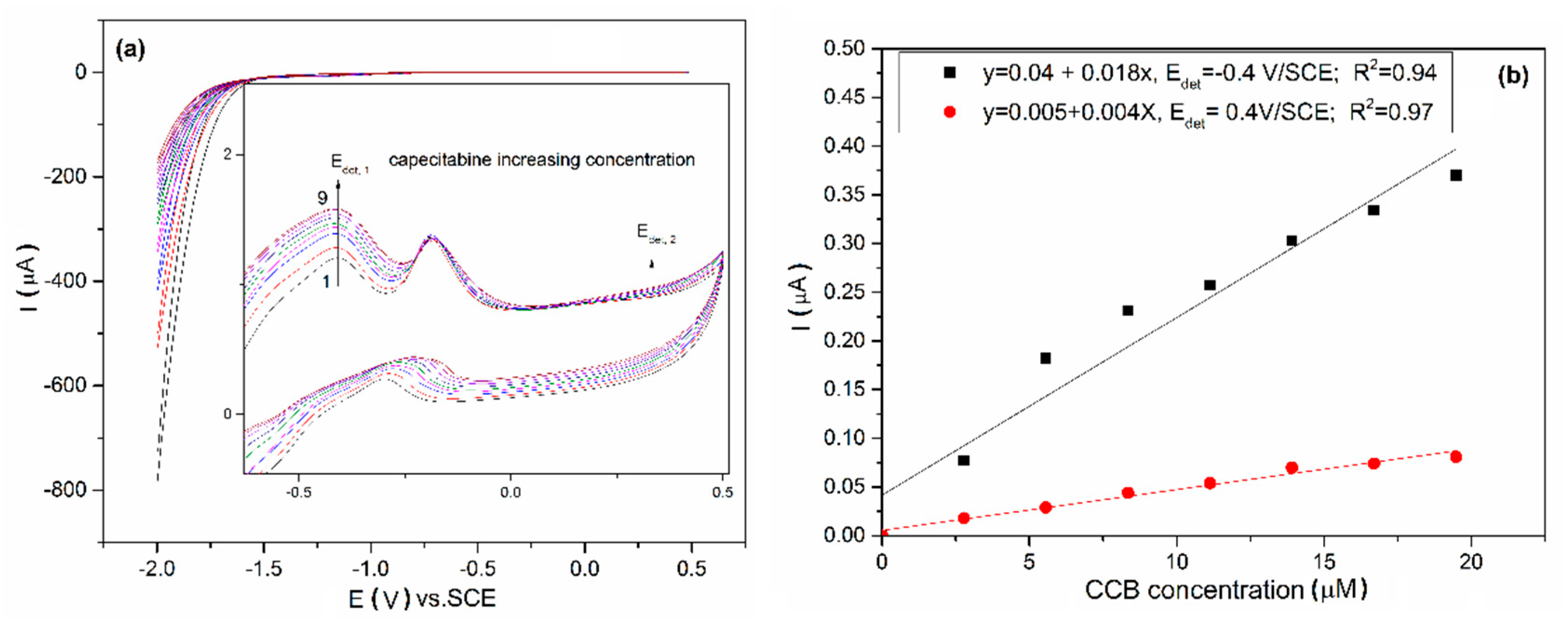
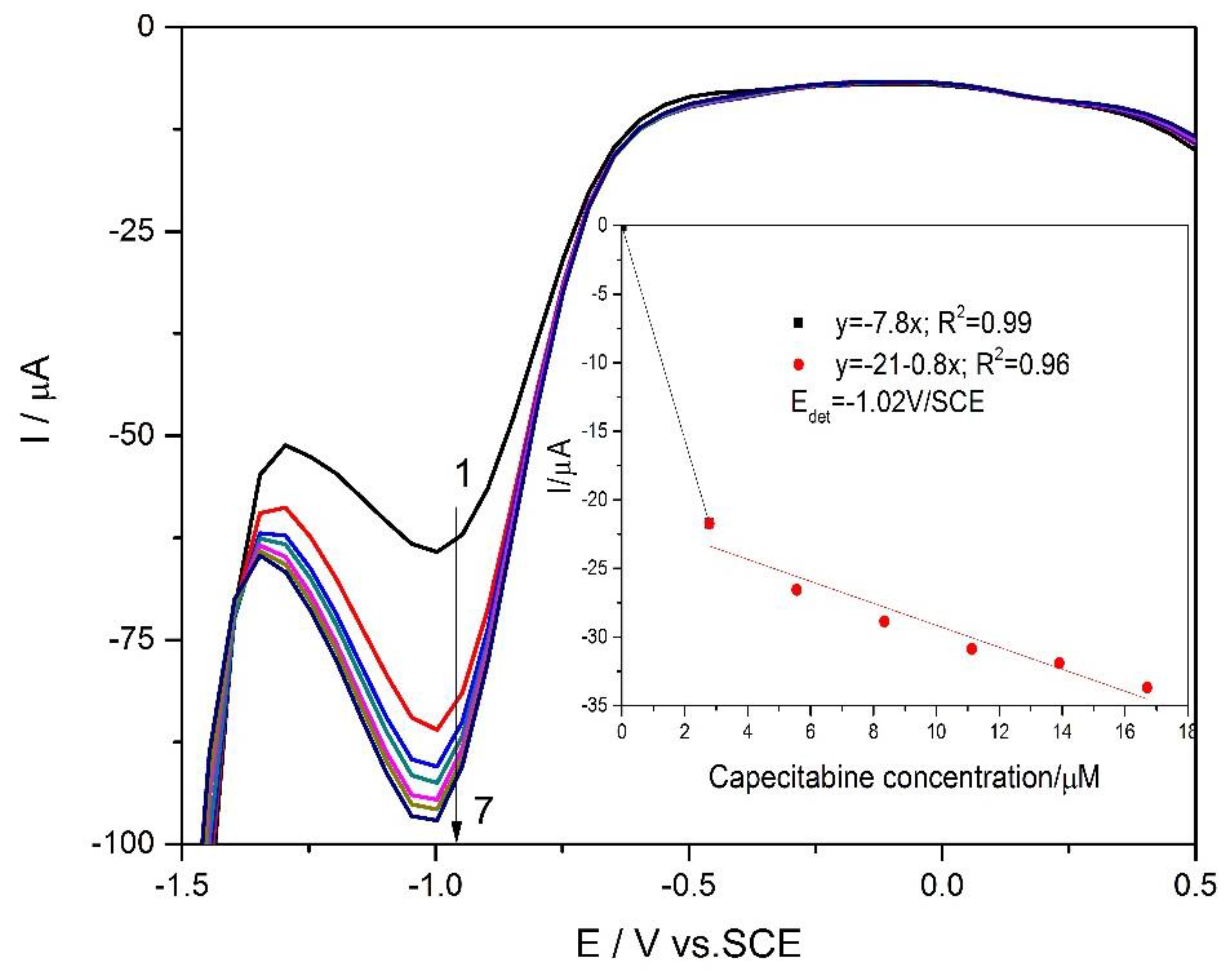
3.3. Electrochemical Characterization and Detection Application
4. Conclusions
Author Contributions
Acknowledgments
Conflicts of Interest
References
- Addabbo, T.; Bertocci, F.; Fort, A.; Gregorkiewitz, M.; Mugnaini, M.; Spinicci, R.; Vignoli, V. Gas sensing properties and modeling of YCoO3 based perovskite materials. Sens. Actuators B Chem. 2015, 221, 1137–1155. [Google Scholar] [CrossRef]
- Natile, M.M.; Ponzoni, A.; Concina, I.; Glisenti, A. Chemical tuning versus microstructure features in solid-state gas sensors: LaFe1−xGaxO3, a case study. Chem. Mater. 2014, 26, 1505–1513. [Google Scholar] [CrossRef]
- Kersen, U. Microstructural and surface characterization of solid state sensor based on LaFeO3−σ oxide for detection of NO. Analyst 2001, 126, 1377–1381. [Google Scholar] [CrossRef] [PubMed]
- Phan, T.T.N.; Nikoloski, A.N.; Bahri, P.A.; Li, D. Facile fabrication of perovskite-incorporated hierarchically mesoporous/macroporous silica for efficient photoassisted-Fenton degradation of dye. Appl. Surf. Sci. 2019, 491, 488–496. [Google Scholar] [CrossRef]
- Takalkar, G.; Bhosale, R.; AlMomani, F. Combustion synthesized A0.5Sr0.5MnO3−δ perovskites (where, A = La, Nd, Sm, Gd, Tb, Pr, Dy and Y) as redox materials for thermochemical splitting of CO2. Appl. Surf. Sci. 2019, 489, 80–91. [Google Scholar] [CrossRef]
- Wang, G.; Sun, J.; Zhang, W.; Jiao, S.; Fang, B. Simultaneous determination of dopamine, uric acid and ascorbic acid with LaFeO3 nanoparticles modified electrode. Microchim. Acta 2009, 164, 357–362. [Google Scholar] [CrossRef]
- Kumar, D.; Jayavel, R. Facile hydrothermal synthesis and characterization of LaFeO3 nanospheres for visible light photocatalytic applications. J. Mater. Sci. Mater. Electron. 2014, 25, 3953–3961. [Google Scholar] [CrossRef]
- Bai, S.L.; Shi, B.J.; Ma, L.J.; Yang, P.C.; Liu, Z.Y.; Li, D.Q.; Chen, A.F. Synthesis of LaFeO3 catalytic materials and their sensing properties. Sci. China Ser. B Chem. 2009, 52, 2106–2113. [Google Scholar] [CrossRef]
- Ciambelli, P.; Cimino, S.; Rossi, S.D. AFeO3 (A = La, Nd, Sm) and LaFe1−xMgxO3 perovskites as methane combustion and CO oxidation catalysts: Structural, redox and catalytic properties. Appl. Catal. B-Environ. 2001, 29, 239–250. [Google Scholar] [CrossRef]
- Kuščer, D.; Hrovat, M.; Holc, J.; Bernik, S.; Kolar, D. Some characteristics of Al2O3− and CaO-modified LaFeO3-based cathode materials for solid oxide fuel cells. J. Power Sources 1996, 61, 161–165. [Google Scholar] [CrossRef]
- Grygar, T. Electrochemical reactions of La(Ni,Cr)O3 in acidic aqueous solutions. J. Solid State Electrochem. 1999, 3, 412–416. [Google Scholar] [CrossRef]
- Peng, Q.; Shan, B.; Wen, Y.; Chen, R. Enhanced charge transport of LaFeO3 via transition metal (Mn, Co, Cu) doping for visible light photoelectrochemical water oxidation. Int. J. Hydrog. Energy 2015, 40, 15423–15431. [Google Scholar] [CrossRef]
- Yuan, Y.; Dong, Z.; Li, Y.; Zhang, L.; Zhao, Y.; Wang, B.; Han, S. Electrochemical properties of LaFeO3-rGO composite. Proc. Nat. Sci. Mater. 2017, 27, 88–92. [Google Scholar] [CrossRef]
- Zhang, Q.; Shan, X.; Fu, Y.; Liu, P.; Li, X.; Liu, B.; Zhang, L.; Li, D. Electrochemical determination of the anticancer drug capecitabine based on a graphene-gold nanocomposite-modified glassy carbon electrode. Int. J. Electrochem. Sci. 2017, 12, 10773–10782. [Google Scholar] [CrossRef]
- Haron, W.; Wisitsoraatb, A.; Wongnawa, S. Nanostructured perovskite oxides—LaMO3 (M=Al, Co, Fe) prepared by co-precipitation method and their ethanol-sensing characteristics. Ceram. Int. 2017, 43, 5032–5040. [Google Scholar] [CrossRef]
- Phokha, S.; Pinitsoontorn, S.; Maensiri, S.; Rujirawat, S. Structure, optical and magnetic properties of LaFeO3 nanoparticles prepared by polymerized complex method. J. Sol-Gel Sci. Technol. 2014, 71, 333–341. [Google Scholar] [CrossRef]
- Mitra, A.; Mahapatra, A.S.; Mallick, A.; Shaw, A.; Ghosh, M.; Chakrabarti, P.K. Simultaneous enhancement of magnetic and ferroelectric properties of LaFeO3 by co-doping with Dy3+ and Ti4+. J. Alloys Compd. 2017, 726, 1195–1204. [Google Scholar] [CrossRef]
- Dohnalová, Ž.; Šulcová, P.; Trojan, M. Synthesis and characterization of LnFeO3 pigments. J Therm. Anal. Calorim. 2008, 91, 559–563. [Google Scholar] [CrossRef]
- Anajafi, Z.; Naseri, M.; Neri, G. Optical, magnetic and gas sensing properties of LaFeO3 nanoparticles synthesized by different chemical methods. J. Electron. Mater. 2019, 48, 6503–6511. [Google Scholar] [CrossRef]
- Dai, Z.; Lee, C.S.; Kim, B.Y.; Kwak, C.H.; Yoon, J.W.; Jeong, H.M.; Lee, J.H. Honeycomb-like periodic porous LaFeO₃ thin film chemiresistors with enhanced gas-sensing performances. ACS Appl. Mater. Interfaces 2014, 6, 16217–16226. [Google Scholar] [CrossRef]
- Malik, R.; Tomer, V.K.; Mishra, Y.K.; Lin, L. Functional gas sensing nanomaterials: A panoramic view. Appl. Phys. Rev. 2020, 7, 021301. [Google Scholar] [CrossRef] [Green Version]
- Kumar, Y.; Pradhan, S.; Pramanik, S.; Bandyopadhyay, R.; Das, D.K.; Pramanik, P. Efficient electrochemical detection of guanine, uric acid and their mixture by composite of nano-particles of lanthanides ortho-ferrite XFeO3 (X = La, Gd, Pr, Dy, Sm, Ce and Tb). J. Electroanal. Chem. 2018, 830–831, 95–105. [Google Scholar] [CrossRef]
- Bidrawn, F.; Kim, G.; Aramrueang, N.; Vohs, J.M.; Gorte, R.J. Dopants to enhance SOFC cathodes based on Sr-doped LaFeO3 and LaMnO3. J. Power Sources 2010, 195, 720–728. [Google Scholar] [CrossRef]
- Murata, K.; Fukui, T.; Abe, H.; Naito, M.; Nogi, K. Morphology control of La(Sr)Fe(Co)O3−a cathodes for IT-SOFCs. J. Power Sources 2005, 145, 257–261. [Google Scholar] [CrossRef]
- Yang, Z.; Huang, Y.; Dong, B.; Li, H.L. Controlled synthesis of highly ordered LaFeO3 nanowires using a citrate-based sol-gel route. Mater. Res. Bull. 2006, 41, 274–281. [Google Scholar] [CrossRef]
- Lebid, M.; Omari, M. Synthesis and electrochemical properties of LaFeO3 oxides prepared via sol–gel method. Arab. J. Sci. Eng. 2014, 39, 147–152. [Google Scholar] [CrossRef]
- Zhang, Q.; Saito, F. Effect of Fe2O3 crystallite size on its mechanochemical reaction with La2O3 to form LaFeO3. J. Mater. Sci. 2001, 36, 2287–2290. [Google Scholar] [CrossRef]
- Kemeng, J.; Hongxing, D.; Jiguang, D.; Song, L.; Shaohua, X.; Han, W. Glucose assisted hydrothermal preparation of porous LaFeO3 for toluene combustion. J. Solid State Chem. 2013, 199, 164–170. [Google Scholar] [CrossRef]
- Yang, J.; Li, R.; Zhou, J.; Li, X.; Zhang, Y.; Long, Y.; Li, Y. Synthesis of LaMO3 (M = Fe, Co, Ni) using nitrate or nitrite molten salts. J. Alloys Compd. 2010, 508, 301–308. [Google Scholar] [CrossRef]
- Wang, Y.; Zhu, J.; Zhang, L.; Jang, X.; Lu, L.; Wang, X. Preparation and characterization of perovskite LaFeO3 nanocrystals. Mater. Lett. 2006, 60, 1767–1770. [Google Scholar] [CrossRef]
- Zhu, C.; Nobuta, A.; Nakatsugawa, I.; Akiyama, T. Solution combustion synthesis of LaMO3 (M = Fe, Co, Mn) perovskite nanoparticles and the measurement of their electrocatalytic properties for air cathode. Int. J. Hydrog. Energy 2013, 38, 13238–13248. [Google Scholar] [CrossRef]
- Prado-Gonjal, J.; Arévalo-López, Á.M.; Morán, E. Microwave-assisted synthesis: A fast and efficient route to produce LaMO3 (M = Al, Cr, Mn, Fe, Co) perovskite materials. Mater. Res. Bull. 2011, 46, 222–230. [Google Scholar] [CrossRef]
- Fabian, F.A.; Pedra, P.P.; Filho, J.L.S.; Duque, J.G.S.; Meneses, C.T. Synthesis and characterization of La(Cr, Fe, Mn)O3 nanoparticles obtained by co-precipitation method. J. Magn. Magn. Mater. 2015, 379, 80–83. [Google Scholar] [CrossRef]
- Popa, M.; Franti, J.; Kakihama, M. Lanthanum ferrite LaFeO3+d nanopowders obtained by the polymerizable complex method. Solid State Ionics 2002, 154–155, 437–445. [Google Scholar] [CrossRef]
- Patron, L.; Budrugeac, P.; Balu, A.; Carp, O.; Diamandescu, L.; Feder, M. Thermal analysis of some polynuclear coordination compounds. Ligand tartarate, precursors of LnFeO3 perovskites. J. Therm. Anal. Calorim 2007, 88, 273–277. [Google Scholar] [CrossRef]
- Carp, O. Materials obtained by solid-state thermal decomposition of coordination compounds and metal-organic coordination polymers. In Reactions and Mechanisms in Thermal Analysis of Advanced Materials; Tiwari, A., Raj, B., Eds.; Scrivener Publishing LLC: Beverly, MA, USA, 2015; pp. 63–84. [Google Scholar] [CrossRef]
- Dumitru, R.; Manea, F.; Pacurariu, C.; Lupa, L.; Pop, A.; Cioabla, A.; Surdu, A.; Ianculescu, A. Synthesis, characterization of nanosized ZnCr2O4 and its photocatalytic performance in the degradation of humic acid from drinking water. Catalysts 2018, 8, 210. [Google Scholar] [CrossRef] [Green Version]
- Niculescu, M.; Birzescu, M.; Dumitru, R.; Sisu, E.; Budrugeac, P. Co(II)-Ni(II) heteropolynuclear coordination compound obtained through the reaction of 1,2-propanediol with metallic nitrates as precursor for mixed oxide of spinel type NiCo2O4. Thermochim. Acta 2009, 493, 1–5. [Google Scholar] [CrossRef]
- Stefanescu, M.; Sasca, V.; Birzescu, M. Thermal behaviour of the homopolynuclear glyoxylate complex combinations with Cu(II) and Cr(III). J. Therm. Anal. Calorim. 2003, 72, 515–524. [Google Scholar] [CrossRef]
- Caizer, C.; Stefanescu, M.; Muntean, C.; Hrinca, I. Studies and magnetic properties of Ni-Zn ferrite synthesized from the glyoxilates complex combination. J. Optoelectron. Adv. Mater. 2001, 3, 919–924. [Google Scholar]
- Bîrzescu, M.; Niculescu, M.; Dumitru, R.; Budrugeac, P.; Segal, E. Copper(II) oxalate obtained through the reaction of 1,2-ethanediol with Cu(NO3)2·3H2O. J. Therm. Anal. Calorim. 2008, 94, 297–303. [Google Scholar] [CrossRef]
- Bîrzescu, M.; Niculescu, M.; Dumitru, R.; Carp, O.; Segal, E. Synthesis, structural characterization and thermal analysis of the cobalt(II) oxalate obtained through the reaction of 1,2-ethanediol with Co(NO3)2·6H2O. J. Therm. Anal. Calorim. 2009, 96, 979–986. [Google Scholar] [CrossRef]
- Dumitru, R.; Manea, F.; Lupa, L.; Păcurariu, C.; Ianculescu, A.; Baciu, A.; Negrea, S. Synthesis, characterization of nanosized CoAl2O4 and its electrocatalytic activity for enhanced sensing application. J. Therm. Anal. Calorim. 2017, 128, 1305–1312. [Google Scholar] [CrossRef]
- Dumitru, R.; Ianculescu, A.; Păcurariu, C.; Lupa, L.; Pop, A.; Vasile, B.; Surdu, A.; Manea, F. BiFeO3-synthesis, characterization and its photocatalytic activity towards doxorubicin degradation from water. Ceram. Int. 2019, 45, 2789–2802. [Google Scholar] [CrossRef]
- Dumitru, R.; Papa, F.; Balint, I.; Culita, D.; Munteanu, C.; Stanica, N.; Ianculescu, A.; Diamandescu, L.; Carp, O. Mesoporous cobalt ferrite: A rival of platinum catalyst in methane combustion reaction. Appl. Catal. A Gen. 2013, 467, 178–186. [Google Scholar] [CrossRef]
- Patrinoiu, G.; Dumitru, R.; Culita, D.C.; Munteanu, C.; Birjega, R.; Calderon-Moreno, J.M.; Cucos, A.; Pelinescu, D.; Chifiriuc, M.C.; Bleotu, C.; et al. Self-assembled zinc oxide hierarchical structures with enhanced antibacterial properties from stacked chain-like zinc oxalate compounds. J. Colloid Interface Sci. 2019, 552, 258–270. [Google Scholar] [CrossRef]
- Kalanur, S.S.; Seetharamappa, J.; Mamatha, G.P.; Hadagali, M.D.; Kandagal, P.B. Electrochemical behavior of an anti-cancer drug at glassy carbon electrode and its determination in pharmaceutical formulations. Int. J. Electrochem. Sci. 2008, 3, 756–767. [Google Scholar]
- Barişçi, S.; Turkay, O.; Ulusoy, E.; Şeker, M.G.; Yüksel, E. Electro-oxidation of cytostatic drugs: Experimental and theoretical identification of by-products and evaluation of ecotoxicological effects. Chem. Eng. J. 2018, 334, 1820–1827. [Google Scholar] [CrossRef]
- Xie, H. Occurrence, ecotoxicology, and treatment of anticancer agents as water contaminants, environmental&analytical toxicology. J. Environ. Anal. Toxicol. 2012, S2, 002. [Google Scholar] [CrossRef] [Green Version]
- Madrakian, T.; Ghasemi, H.; Haghshenas, E.; Afkhami, A. Preparation of a ZnO nanoparticles/multiwalled carbon nantubes/carbon paste electrode a sensitive tool for capecitabine determination in real samples. RSC Adv. 2016, 6, 33851–33856. [Google Scholar] [CrossRef]
- Deng, P.; Ji, C.; Dai, X.; Zhong, D.; Ding, L.; Chen, X. Simultaneous determination of capecitabine and its three nucleoside metabolites in human plasma by high performance liquid chromatography–tandem mass spectrometry. J. Chromatogr. 2015, 989, 71–79. [Google Scholar] [CrossRef]
- Zhang, G.H.; Chen, Q.; Deng, X.Y.; Jiao, H.Y.; Wang, P.Y.; Gengzang, D.J. Synthesis and characterization of In-doped LaFeO3 hollow nanofibers with enhanced formaldehyde sensing properties. Mater. Lett. 2017, 236, 229–232. [Google Scholar] [CrossRef]
- Teixeira, P.R.S.; Teixeira, A.S.D.M.; Farias, E.A.D.; Filho, E.C.S.; Cunha, H.N.; Santos Junior, J.R.; Nunes, L.C.C.; Lima, H.R.S.; Eiras, C. Development of a low-cost electrochemical sensor based on babassu mesocarp (Orbignya phalerata) immobilized on a flexible gold electrode for applications in sensors for 5-fluorouracil chemotherapeutics. Anal. Bioanal. Chem. 2019, 411, 659–667. [Google Scholar] [CrossRef] [PubMed]
- Fujita, J.; Nakamoto, K.; Kobayshi, M. Infrared spectra of metallic complexes. II. The absorption bands of coördinated water in aquo complexes. J. Am. Chem. Soc. 1956, 78, 3963–3965. [Google Scholar] [CrossRef]
- Nakamoto, K. Infrared and Raman Spectra of Inorganic and Coordination Compounds; Wiley: New York, NY, USA, 1986. [Google Scholar]
- Bellakki, M.B.; Manivannan, V.; McCurdy, P.; Kohli, S. Synthesis, and measurement of structural and magnetic properties, of La1−xNaxFeO3 (0.0≤x≤0.3) perovskite oxides. J. Rare Earth 2009, 27, 691–697. [Google Scholar] [CrossRef]
- Dai, X.; Yu, C.; Li, R.; Wu, Q.; Shi, K.; Hao, Z. Effect of calcination temperature and reaction conditions on methane partial oxidation using lanthanum-based perovskite as oxygen donor. J. Rare Earths 2008, 26, 341–345. [Google Scholar] [CrossRef]
- Shivakumara, C. Low temperature synthesis and characterization of rare earth orthoferrites LnFeO3 (Ln = La, Pr and Nd) from molten NaOH flux. Solid State Commun. 2006, 139, 165–169. [Google Scholar] [CrossRef]
- Ljoncheva, M.; Kosjek, T.; Isidori, M.; Heath, E. 5-Fluorouracil and its prodrug capecitabine: Occurrence, fate and effect in environment. In Fate and Effect of Anticancer Drugs in the Environment; Isidori, M., Kosjek, T., Filipic, M., Eds.; Springer: Cham, Switzerland, 2020; pp. 331–375. [Google Scholar] [CrossRef]






| Compound | LaFeO3 | ||||
|---|---|---|---|---|---|
| Annealing Temperature (°C) | 550 | 600 | 700 | 800 | |
| Crystal System | Orthorhombic (ICCD no. 04-013-6775) | ||||
| Space Group | Pbnm (62) | ||||
| Lattice parameters (Å) | A | 5.562 ± 0.011 | 5.562 ± 0.003 | 5.561 ± 0.002 | 5.561 ± 0.002 |
| B | 7.856 ± 0.016 | 7.856± 0.004 | 7.856 ± 0.003 | 7.856 ± 0.002 | |
| C | 5.558 ± 0.012 | 5.558 ± 0.003 | 5.558 ± 0.002 | 5.558 ± 0.001 | |
| Unit cell volume, V (Å3) | 242.9 | 242.8 | 242.8 | 242.8 | |
| R factors (%) | Rexp | 5.520 | 5.612 | 5.892 | 5.664 |
| Rp | 5.244 | 4.814 | 5.488 | 5.041 | |
| Rwp | 6.416 | 5.845 | 6.578 | 6.057 | |
| χ2 | 1.351 | 1.085 | 1.246 | 1.143 | |
| Technique | Mechanism Detection | Detection Potential/ V vs. SCE | Sensitivity/ µA µM−1 cm−2 | Correlation Coefficient/R2 | LOD */µM | LQ/µM | RSD ** % |
|---|---|---|---|---|---|---|---|
| CV | CCB oxidation | −0.40 | 0.257 | 0.971 | 0.021 | 0.038 | 0.10 |
| 0.40 | 0.050 | 0.966 | 0.590 | 1.970 | 1.02 | ||
| DPV | CCB reduction | −1.02 | 111.0 (CCB concentration lower than 2.5 µM); | 0.990 | 0.010 | 0.250 | 2.50 |
| 11.43 (CCB concentration higher than 2.5 µM) | 0.960 | 0.734 | 2.446 | 2.50 | |||
| MPA | CCB reduction and oxidation | −1.1 | 3.040 | 0.985 | 0.158 | 0.527 | 0.84 |
| −0.4 | 0.314 | 0.989 | 0.103 | 0.343 | 0.68 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dumitru, R.; Negrea, S.; Ianculescu, A.; Păcurariu, C.; Vasile, B.; Surdu, A.; Manea, F. Lanthanum Ferrite Ceramic Powders: Synthesis, Characterization and Electrochemical Detection Application. Materials 2020, 13, 2061. https://doi.org/10.3390/ma13092061
Dumitru R, Negrea S, Ianculescu A, Păcurariu C, Vasile B, Surdu A, Manea F. Lanthanum Ferrite Ceramic Powders: Synthesis, Characterization and Electrochemical Detection Application. Materials. 2020; 13(9):2061. https://doi.org/10.3390/ma13092061
Chicago/Turabian StyleDumitru, Raluca, Sorina Negrea, Adelina Ianculescu, Cornelia Păcurariu, Bogdan Vasile, Adrian Surdu, and Florica Manea. 2020. "Lanthanum Ferrite Ceramic Powders: Synthesis, Characterization and Electrochemical Detection Application" Materials 13, no. 9: 2061. https://doi.org/10.3390/ma13092061








