Assessment of Mechanical, Chemical, and Biological Properties of Ti-Nb-Zr Alloy for Medical Applications
Abstract
:1. Introduction
2. Materials and Methods
2.1. Material Properties Analyses
2.2. X-ray Photoelectron Spectroscopy
2.3. Mechanical Properties Analyses
2.4. Electrochemical Properties Analyses
2.5. Biological Properties Analyses
3. Results
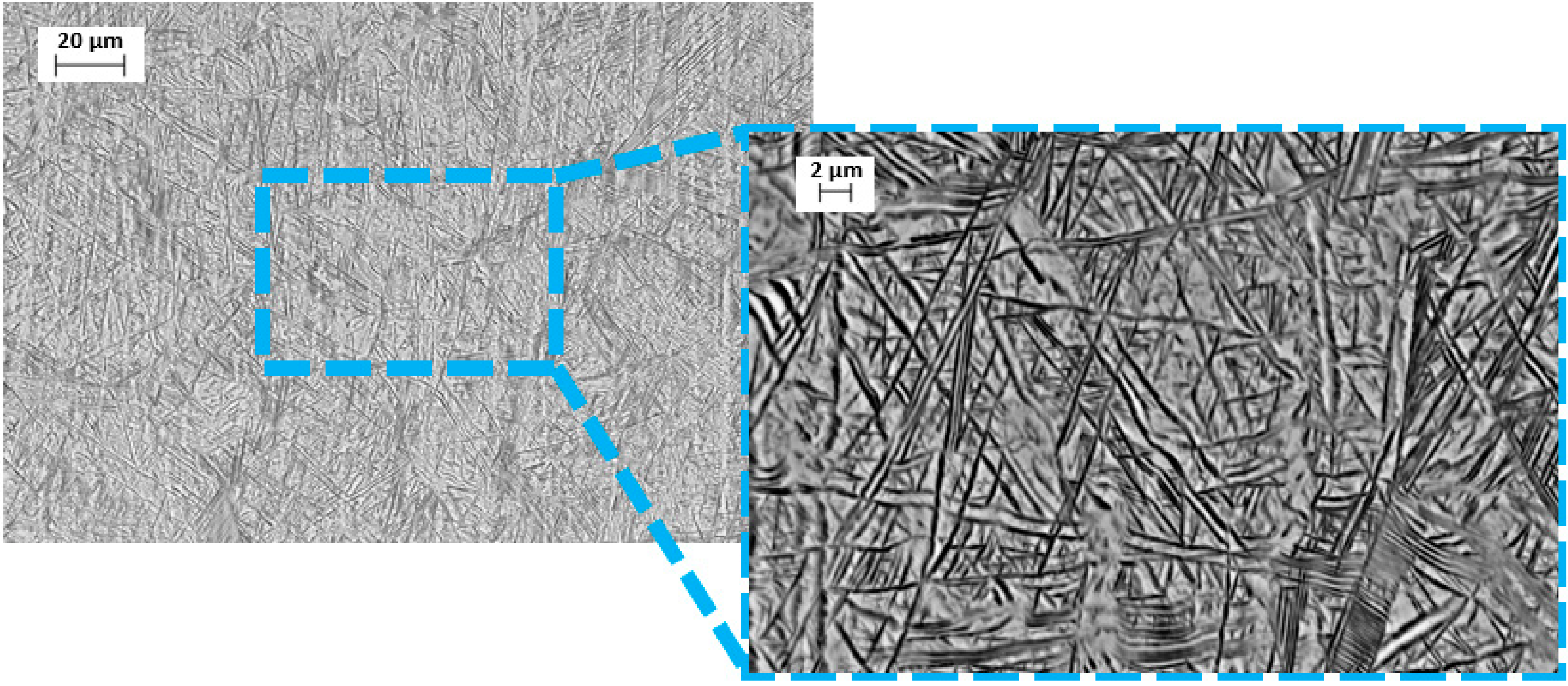
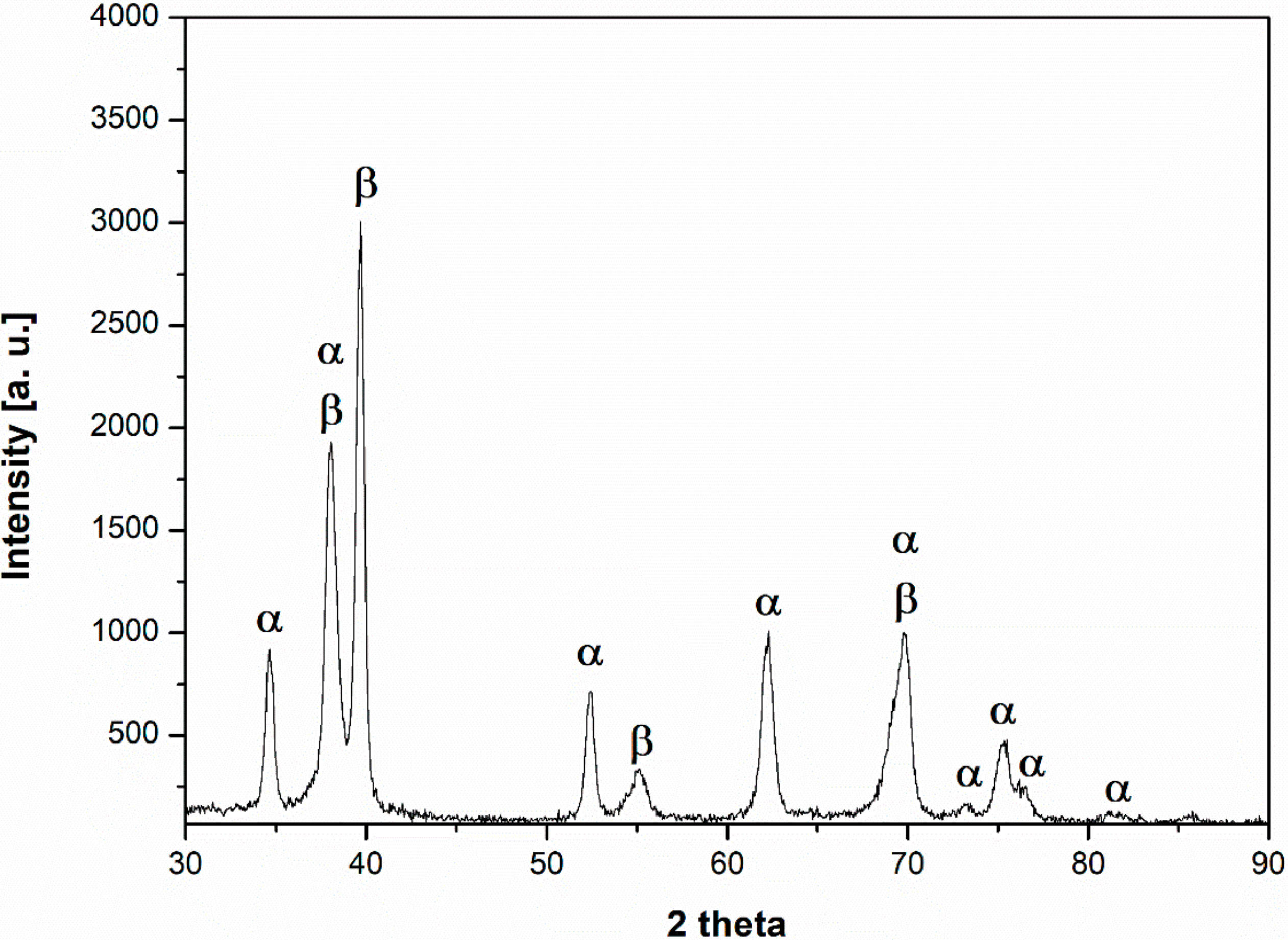
3.1. Material Properties Analyses
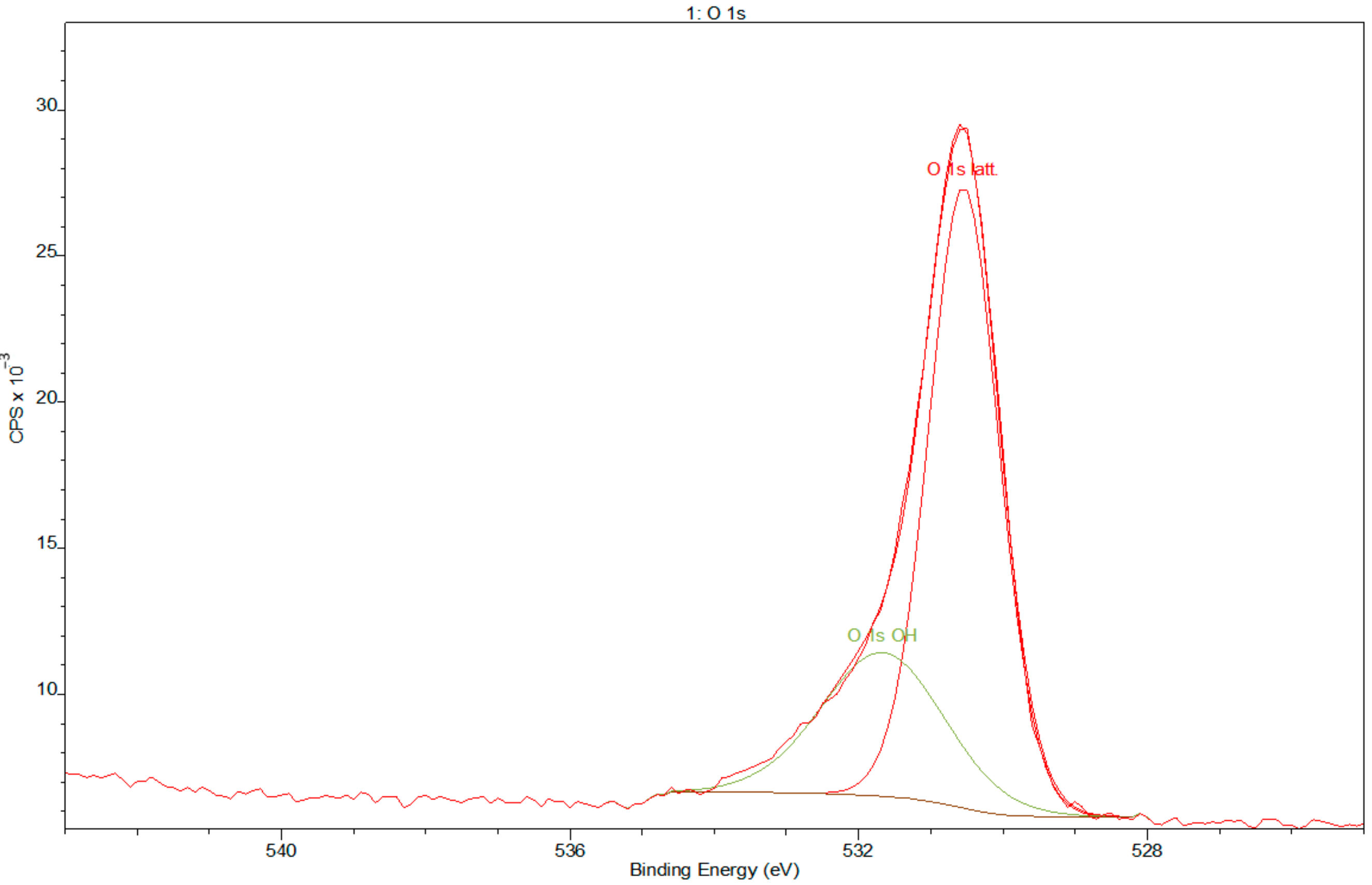
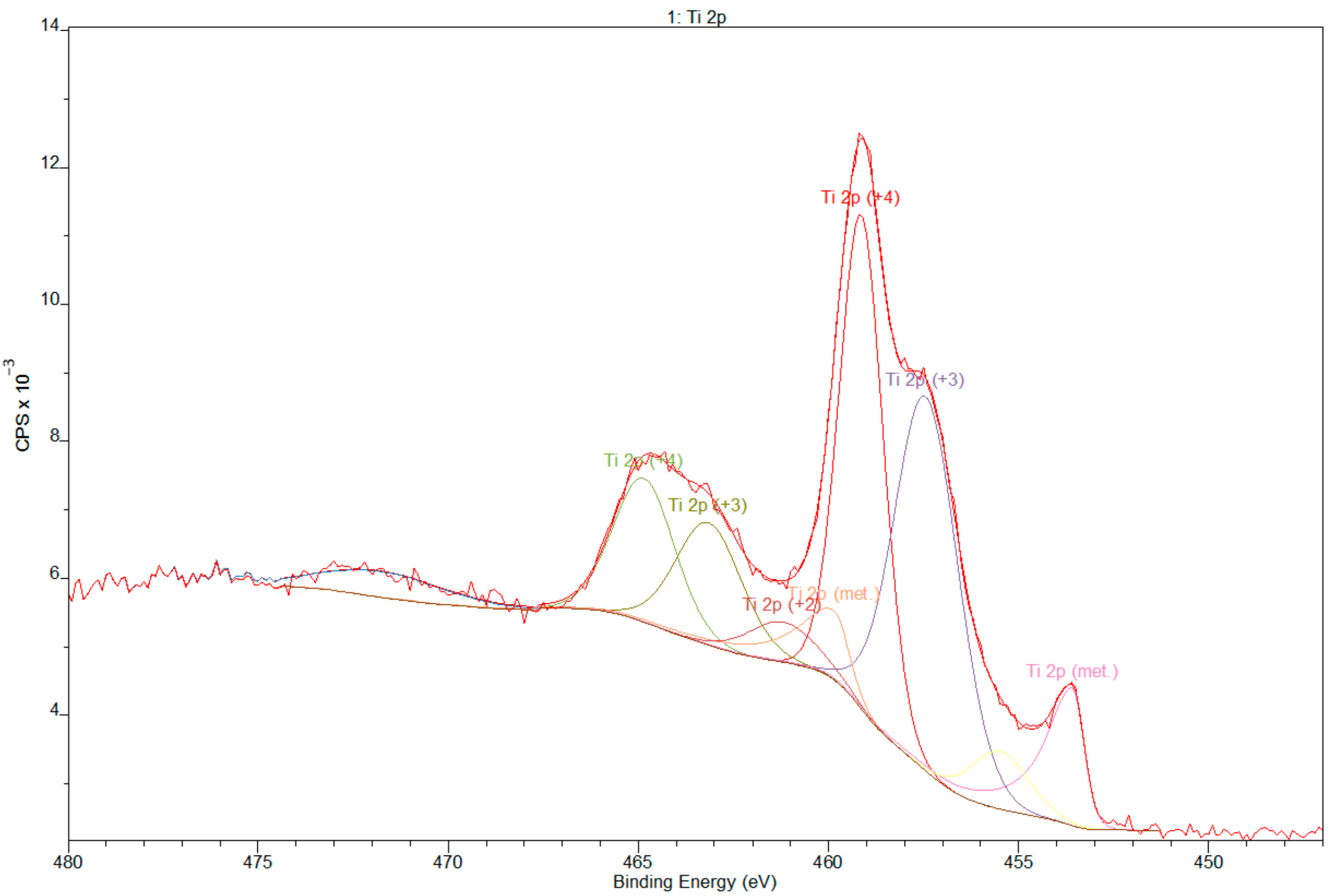
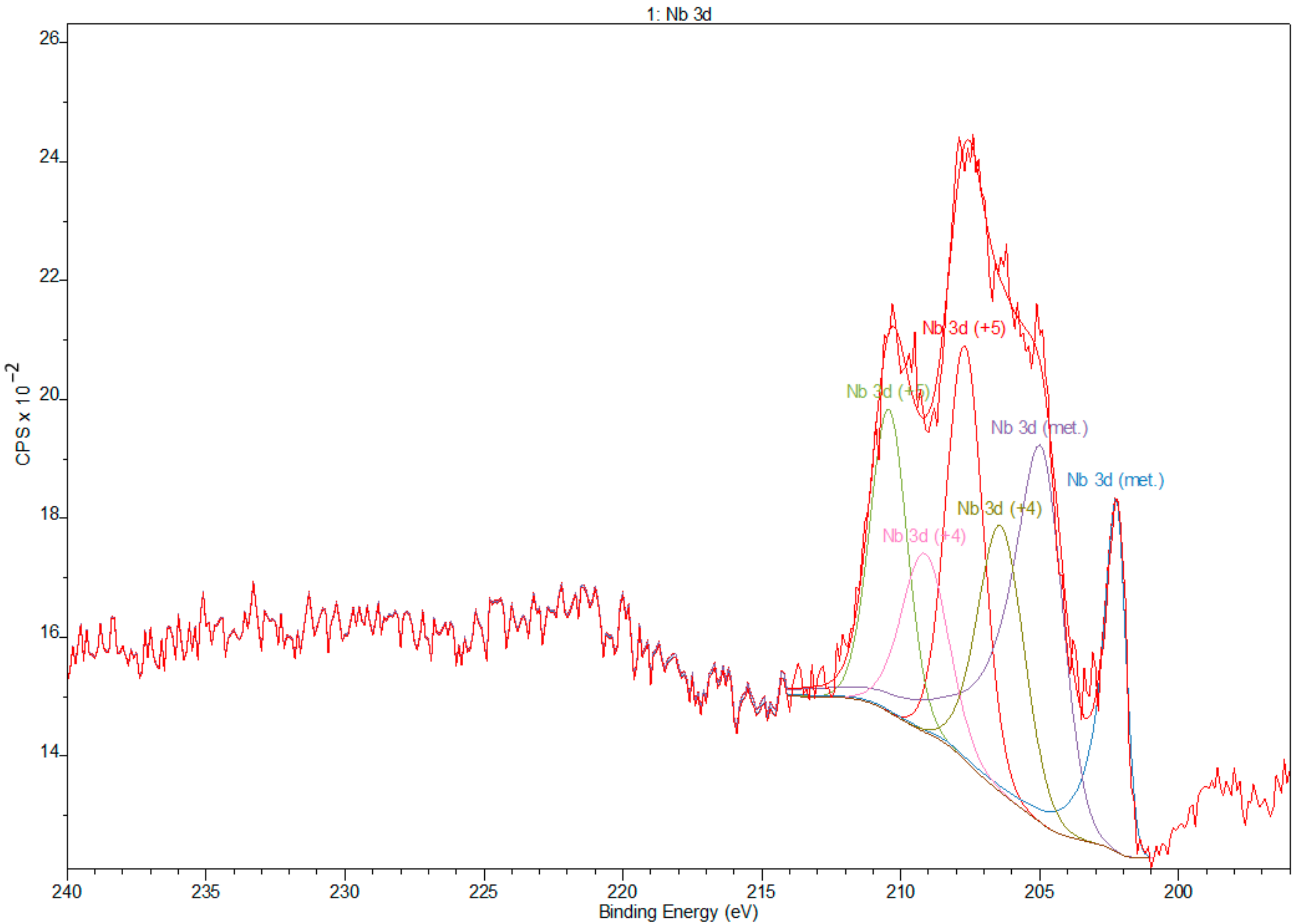
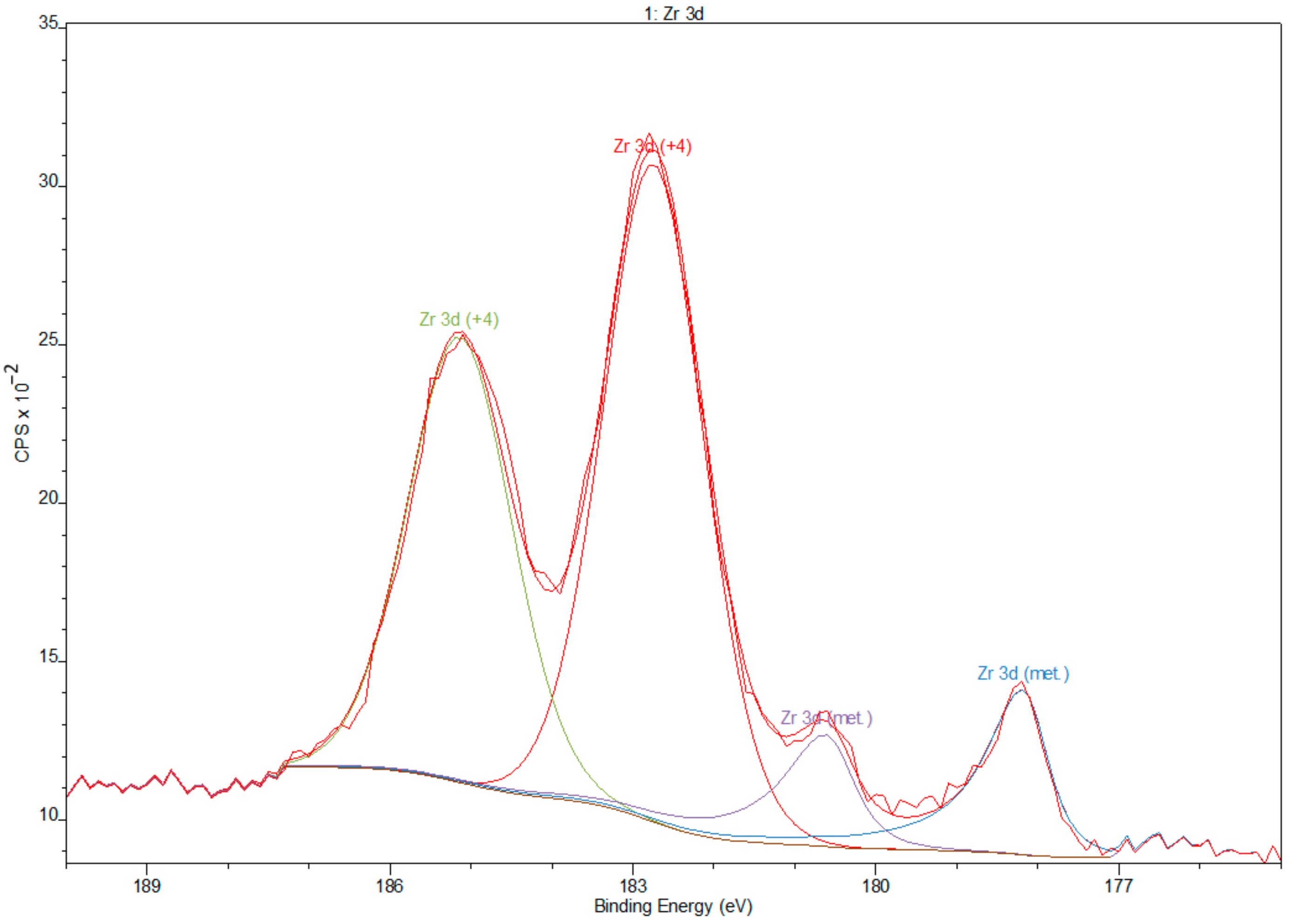
3.2. X-ray Photoelectron Spectroscopy
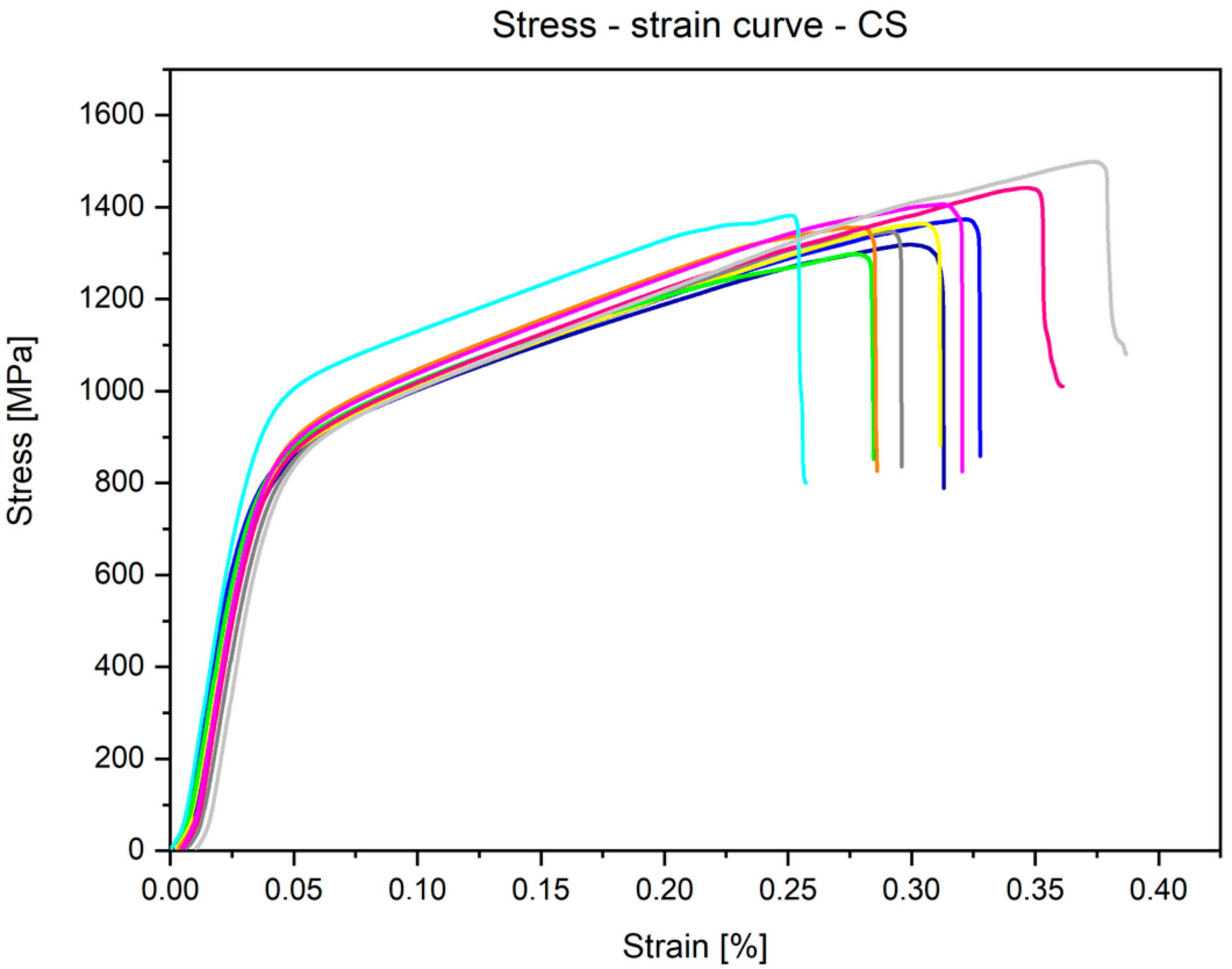
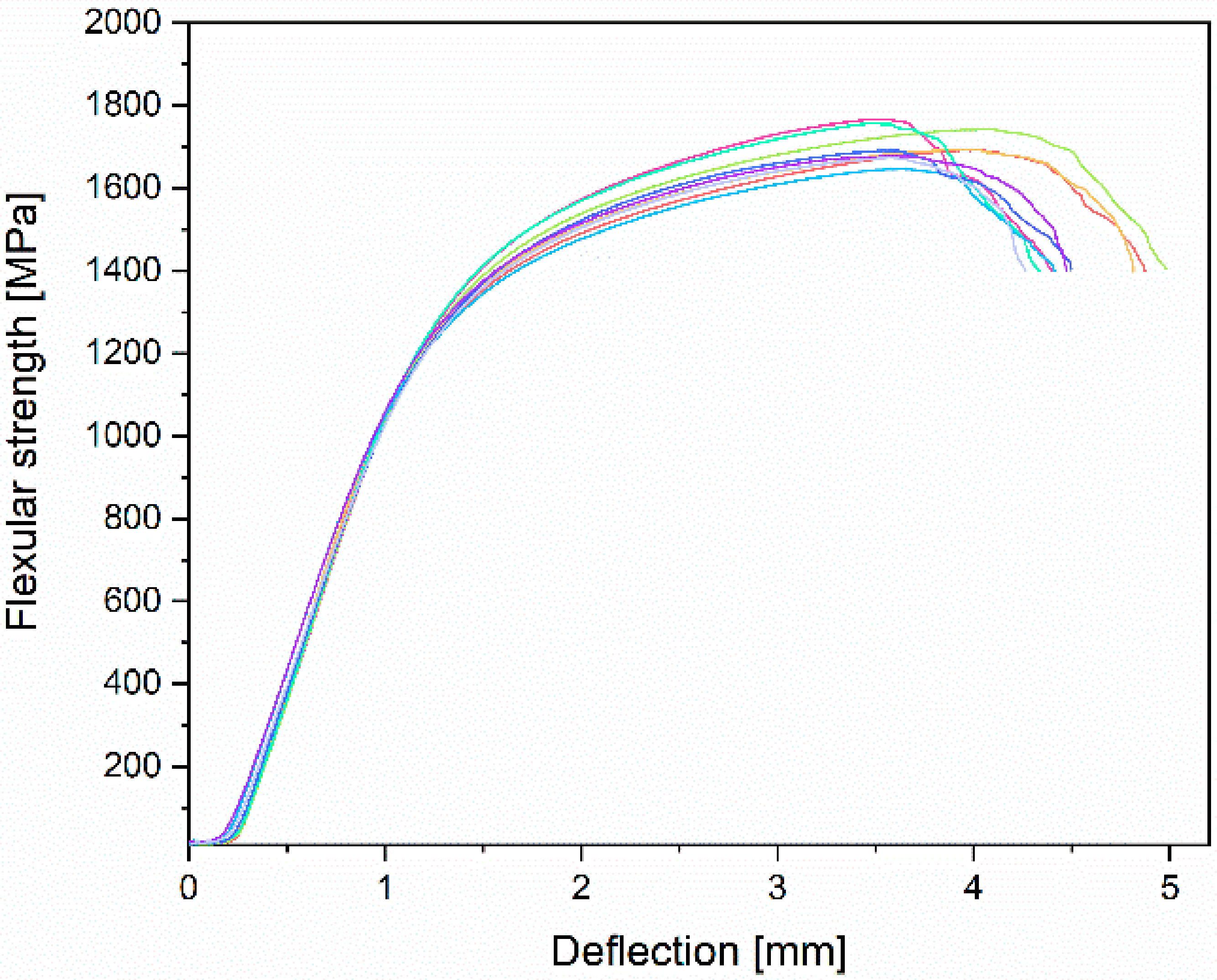
3.3. Mechanical Properties Analyses
3.4. Electrochemical Properties Analyses
3.5. Biological Properties Analyses
4. Discussion
5. Conclusions
- The microstructure at room temperature was confirmed by SEM and XRD investigations and was found to contain α, α’, and β phases, implying that some of the β phase transformed to α’.
- Mechanical properties obtained by the static tensile test are similar to the data found in the literature and conform to the requirements of ASTM F1713. For the annealed conditions: E = 84.1 ± 1.8 GPa, UTS = 733.9 ± 7.5 MPa, YS = 555.6 ± 17.3 MPa, and A = 19.6 ± 1.6%.
- XPS results confirmed the dominant presence of TiO2 on the examined surface and showed the presence of hydroxyl groups on the surface. Moreover, other oxides are present—Nb2O5 and Zr2O. The occurrence of oxides increases the biocompatibility of materials and hydroxide groups allow for covalent attachment of various molecules.
- As shown by our Tafel-plot extrapolations, the Ti-13Nb-13Zr in the Ringer’s solution spontaneously forms a passive layer, due to long passivation area on potentiodynamic polarisation curves.
- The toxic effect was assessed by a cytotoxicity MTT test, which proved that the Ti-13Nb-13Zr alloy did not present cytotoxic effect in the in vitro examination both for MC3T3 and NHDF cells.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Bertol, L.S.; Júnior, W.K.; da Silva, F.P.; Aumund-Kopp, C. Medical design: Direct metal laser sintering of Ti-6Al-4V. Mater. Des. 2010, 31, 3982–3988. [Google Scholar] [CrossRef]
- Spetzger, U.; Frasca, M.; König, S.A. Surgical planning, manufacturing and implantation of an individualized cervical fusion titanium cage using patient-specific data. Eur. Spine J. 2016, 25, 2239–2246. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; Wang, Y.; Wu, S.; Lin, H.; Yang, Y.; Fan, S.; Gu, C.; Wang, J.; Song, C. Customized a Ti6Al4V bone plate for complex pelvic fracture by selective laser melting. Materials 2017, 10, 1. [Google Scholar] [CrossRef] [Green Version]
- Fan, H.; Fu, J.; Li, X.; Pei, Y.; Li, X.; Pei, G.; Guo, Z. Implantation of customized 3-D printed titanium prosthesis in limb salvage surgery: A case series and review of the literature. World J. Surg. Oncol. 2015, 13, 1–10. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Patel, N.; Gohil, P. A review on biomaterials: Scope, applications & human anatomy significance. Int. J. Emerg. Technol. Adv. Eng. 2012, 2, 91–101. [Google Scholar]
- Bai, L.; Gong, C.; Chen, X.; Sun, Y.; Zhang, J.; Cai, L.; Zhu, S.; Xie, S.Q. Additive manufacturing of customized metallic orthopedic implants: Materials, structures, and surface modifications. Metals 2019, 9, 1004. [Google Scholar] [CrossRef] [Green Version]
- Park, Y.J.; Song, Y.H.; An, J.H.; Song, H.J.; Anusavice, K.J. Cytocompatibility of pure metals and experimental binary titanium alloys for implant materials. J. Dent. 2013, 41, 1251–1258. [Google Scholar] [CrossRef]
- Li, Y.; Wong, C.; Xiong, J.; Hodgson, P.; Wen, C. Cytotoxicity of titanium and titanium alloying elements. J. Dent. Res. 2010, 89, 493–497. [Google Scholar] [CrossRef]
- Kolli, R.; Devaraj, A. A Review of Metastable Beta Titanium Alloys. Metals 2018, 8, 506. [Google Scholar] [CrossRef] [Green Version]
- Liu, Q.; Meng, Q.; Guo, S.; Zhao, X. α′ Type Ti–Nb–Zr alloys with ultra-low Young’s modulus and high strength. Prog. Nat. Sci. Mater. Int. 2013, 23, 562–565. [Google Scholar] [CrossRef] [Green Version]
- Mohammed, M.T. Beta Titanium Alloys: The Lowest Elastic Modulus for Biomedical Applications: A Review Welding View project Powder Metallurgy Titanium Alloys View project Arshad Noor Siddiquee Jamia Millia Islamia. Int. J. Chem. Nucl. Metall. Mater. Eng. 2014, 8, 822–827. [Google Scholar]
- Niinomi, M. Mechanical biocompatibilities of titanium alloys for biomedical applications. J. Mech. Behav. Biomed. Mater. 2008, 1, 30–42. [Google Scholar] [CrossRef] [PubMed]
- Niinomi, M.; Liu, Y.; Nakai, M.; Liu, H.; Li, H. Biomedical titanium alloys with Young’s moduli close to that of cortical bone. Regen. Biomater. 2016, 3, 173–185. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Geetha, M.; Singh, A.K.; Asokamani, R.; Gogia, A.K. Ti based biomaterials, the ultimate choice for orthopaedic implants—A review. Prog. Mater. Sci. 2009, 54, 397–425. [Google Scholar] [CrossRef]
- Donato, T.A.G.; de Almeida, L.H.; Nogueira, R.A.; Niemeyer, T.C.; Grandini, C.R.; Caram, R.; Schneider, S.G.; Santos, A.R. Cytotoxicity study of some Ti alloys used as biomaterial. Mater. Sci. Eng. C 2009, 29, 1365–1369. [Google Scholar] [CrossRef]
- Schneider, S.G.; Nunes, C.A.; Rogero, S.O. Mechanical properties and cytotoxic evaluation of the Ti-3Nb-13Zr alloy. Biomecanica 2000, 8, 84–87. [Google Scholar] [CrossRef]
- Mishra, A.K.; Davidson, J.A.; Poggie, R.A.; Kovacs, P.; Ted, J. Mechanical and tribological properties and biocompatibility of diffusion hardened Ti-13Nb-13Zr A new titanium alloy for surgical implants. ASTM Int. 1996, 96–113. [Google Scholar]
- Black, J. Biological Performance of Materials—Fundamentals of Biocompability, 4th ed.; Taylor & Francis Group, LCC: Abingdon, UK, 2006; ISBN 9781420057843. [Google Scholar]
- Abdel-Hady Gepreel, M.; Niinomi, M. Biocompatibility of Ti-alloys for long-term implantation. J. Mech. Behav. Biomed. Mater. 2013, 20, 407–415. [Google Scholar] [CrossRef]
- Ozaki, T.; Matsumoto, H.; Watanabe, S.; Hanada, S. Beta Ti Alloys with Low Young’s Modulus. Mater. Trans. 2005, 45, 2776–2779. [Google Scholar] [CrossRef] [Green Version]
- Chen, Q.; Thouas, G.A. Metallic implant biomaterials. Mater. Sci. Eng. R Rep. 2015, 87, 1–57. [Google Scholar] [CrossRef]
- Saji, V.S.; Choe, H.C. Electrochemical corrosion behaviour of nanotubular Ti-13Nb-13Zr alloy in Ringer’s solution. Corros. Sci. 2009, 51, 1658–1663. [Google Scholar] [CrossRef]
- Cvijović-Alagić, I.; Cvijović, Z.; Mitrović, S.; Panić, V.; Rakin, M. Wear and corrosion behaviour of Ti-13Nb-13Zr and Ti-6Al-4V alloys in simulated physiological solution. Corros. Sci. 2011, 53, 796–808. [Google Scholar] [CrossRef]
- Geetha, M.; Kamachi Mudali, U.; Gogia, A.K.; Asokamani, R.; Raj, B. Influence of microstructure and alloying elements on corrosion behavior of Ti-13Nb-13Zr alloy. Corros. Sci. 2004, 46, 877–892. [Google Scholar] [CrossRef]
- Taekyung, L.; Eshaan, M.; Santhosh, R.; Geetha, M.; Kumar, S.A.; Soo, L.C. Tribological and corrosion behaviors of warm- and hot-rolled Ti-13Nb-13Zr alloys in simulated body fluid conditions. Int. J. Nanomed. 2015, 10, 207–212. [Google Scholar]
- Niemeyer, T.C.; Grandini, C.R.; Pinto, L.M.C.; Angelo, A.C.D.; Schneider, S.G. Corrosion behavior of Ti-13Nb-13Zr alloy used as a biomaterial. J. Alloy Compd. 2009, 476, 172–175. [Google Scholar] [CrossRef]
- Robin, A.; Carvalho, O.A.S.; Schneider, S.G.; Schneider, S.G. Corrosion behavior of Ti-xNb-13Zr alloys in Ringer’s solution. Mater. Corros. 2008, 59, 929–933. [Google Scholar] [CrossRef]
- Dinu, M.; Franchi, S.; Pruna, V.; Cotrut, C.M.; Secchi, V.; Santi, M.; Titorencu, I.; Battocchio, C.; Iucci, G.; Vladescu, A. Ti-Nb-Zr System and Its Surface Biofunctionalization for Biomedical Applications; Elsevier Inc.: Amsterdam, The Netherlands, 2018; ISBN 9780128124567. [Google Scholar]
- Thomsen, P.; Johansson, C.B. A selection of oral implant materials based on experimental studies. In Bio Implant Interface Improving Biomaterials Tissue Reactions; CRC PRESS: Boca Raton, FL, USA, 2003; pp. 183–203. [Google Scholar] [CrossRef]
- ASTM E8/E8M-16a. Standard Test Methods for Tension Testing of Metallic Materials; ASTM International: West Conshohocken, PA, USA, 2016. [Google Scholar]
- ASTM E9-09. Standard Test Methods of Compression Testing of Metallic Materials at Room Temperature; ASTM International: West Conshohocken, PA, USA, 2009. [Google Scholar]
- ASTM E290-14. Standard Test Methods for Bend Testing of Material for Ductility; ASTM International: West Conshohocken, PA, USA, 2014. [Google Scholar]
- EN ISO 10993-5: 2012. Biological Evaluation of Medical Devices. Tests for In Vitro Cytotoxicity; ISO: Geneva, Switzerland, 2012. [Google Scholar]
- EN ISO 10993-5: 2009. Biological Evaluation of Medical Devices. Tests for In Vitro Cytotoxicity; ISO: Geneva, Switzerland, 2009. [Google Scholar]
- ASTM F1713-08 (2013). Standard Specification for Wrought Titanium-13Niobium-13Zirconium Alloy for Surgical Implant Applications (UNS R58130); ASTM International: West Conshohocken, PA, USA, 2013. [Google Scholar]
- National Institute of Standards and Technology. NIST X-ray Photoelectron Spectroscopy Database; National Institute of Standards and Technology: Gaithersburg, MD, USA, 2000. [Google Scholar]
- Biesinger, M.C.; Lau, L.W.M.; Gerson, A.R.; Smart, R.S.C. Resolving surface chemical states in XPS analysis of first row transition metals, oxides and hydroxides: Sc, Ti, V., Cu and Zn. Appl. Surf. Sci. 2010, 257, 887–898. [Google Scholar] [CrossRef]
- Kasemo, B.; Lausmaa, J. Aspects of surface physics on titanium implants. Swed. Dent. J. Suppl. 1985, 28, 19–36. [Google Scholar]
- Lausmaa, J. Surface spectroscopic characterization of titanium implant materials. J. Electron Spectros. Relat. Phenom. 1996, 81, 343–361. [Google Scholar] [CrossRef]
- Diebold, U.; Madey, T.E. TiO2 by XPS. Surf. Sci. Spectra 1996, 4, 227–231. [Google Scholar] [CrossRef]
- Buabthong, P.; Stasiewicz, N.B.; Mitrovic, S.; Lewis, N.S. Vanadium, niobium and tantalum by XPS. Surf. Sci. Spectra 2017, 24, 024001. [Google Scholar] [CrossRef]
- Barreca, D.; Battiston, G.A.; Gerbasi, R.; Tondello, E.; Zanella, P. Zirconium Dioxide Thin Films Characterized by XPS. Surf. Sci. Spectra 2000, 7, 303–309. [Google Scholar] [CrossRef]
- Davidson, J.A.; Kovacs, P. Biocompatible Low Modulus Titanium Alloy for Medical Implants. U.S. Patent No. 5,169,597, 8 December 1992. [Google Scholar]
- Ozaltin, K.; Panigrahi, A.; Chrominski, W.; Bulutsuz, A.G.; Kulczyk, M.; Zehetbauer, M.J.; Lewandowska, M. Microstructure and Texture Evolutions of Biomedical Ti-13Nb-13Zr Alloy Processed by Hydrostatic Extrusion. Metall. Mater. Trans. A Phys. Metall. Mater. Sci. 2017, 48, 5747–5755. [Google Scholar] [CrossRef] [Green Version]
- Khorasani, A.M.; Goldberg, M.; Doeven, E.H.; Littlefair, G. Titanium in Biomedical Applications—Properties and Fabrication: A Review. J. Biomater. Tissue Eng. 2015, 5, 593–619. [Google Scholar] [CrossRef]
- Laheurte, P.; Prima, F.; Eberhardt, A.; Gloriant, T.; Wary, M.; Patoor, E. Mechanical properties of low modulus β titanium alloys designed from the electronic approach. J. Mech. Behav. Biomed. Mater. 2010, 3, 565–573. [Google Scholar] [CrossRef]
- Terlinde, G.; Fischer, G.; Metallwerke, O.F. Beta Titanium Alloys. In Titanium and Titanium Alloys. Fundamentals and Applications; Leyens, Christoph, Peters, M., Eds.; Wiley-VCH: Weinheim, Germany, 2003; ISBN 3527305343. [Google Scholar]
- Zhang, L.C.; Chen, L.Y. A Review on Biomedical Titanium Alloys: Recent Progress and Prospect. Adv. Eng. Mater. 2019, 21, 1–29. [Google Scholar] [CrossRef] [Green Version]
- Gil, F.J.; La, P.; Rios, J. V Implant—Abutment connections: Influence of the design on the microgap and their fatigue and fracture behavior of dental implants. J. Mater. Sci. Mater. Med. 2014, 1825–1830. [Google Scholar] [CrossRef]
- Lorenzetti, M.; Pellicer, E.; Sort, J.; Baró, M.D.; Kovač, J.; Novak, S.; Kobe, S. Improvement to the corrosion resistance of Ti-based implants using hydrothermally synthesized nanostructured anatase coatings. Materials 2014, 7, 180–194. [Google Scholar] [CrossRef] [Green Version]
- Hammer, P. Are new TiNbZr alloys potential substitutes of the Ti6Al4V alloy for dental applications? Biomed. Mater. 2013, 8. [Google Scholar] [CrossRef]
- Stróż, A.; Łosiewicz, B.; Zubko, M.; Chmiela, B.; Balin, K.; Dercz, G.; Gawlikowski, M.; Goryczka, T. Production, structure and biocompatible properties of oxide nanotubes on Ti13Nb13Zr alloy for medical applications. Mater. Charact. 2017, 132, 363–372. [Google Scholar] [CrossRef]
- Chouirfa, H.; Bouloussa, H.; Migonney, V.; Falentin-Daudré, C. Review of titanium surface modification techniques and coatings for antibacterial applications. Acta Biomater. 2019, 83, 37–54. [Google Scholar] [CrossRef]
- Li, S.; Ni, J.; Liu, X.; Zhang, X.; Yin, S.; Rong, M.; Guo, Z.; Zhou, L. Surface characteristics and biocompatibility of sandblasted and acid-etched titanium surface modified by ultraviolet irradiation: An in vitro study. J. Biomed. Mater. Res. Part B Appl. Biomater. 2012, 100, 1587–1598. [Google Scholar] [CrossRef]
- Barjaktarević, D.R.; Cvijović-Alagić, I.L.; Dimić, I.D.; Đokić, V.R.; Rakin, M.P. Anodization of Ti-based materials for biomedical applications: A review. Metall. Mater. Eng. 2016, 22, 129–144. [Google Scholar] [CrossRef]
- Cremasco, A.; Messias, A.D.; Esposito, A.R.; Duek, E.A.D.R.; Caram, R. Effects of alloying elements on the cytotoxic response of titanium alloys. Mater. Sci. Eng. C 2011, 31, 833–839. [Google Scholar] [CrossRef]


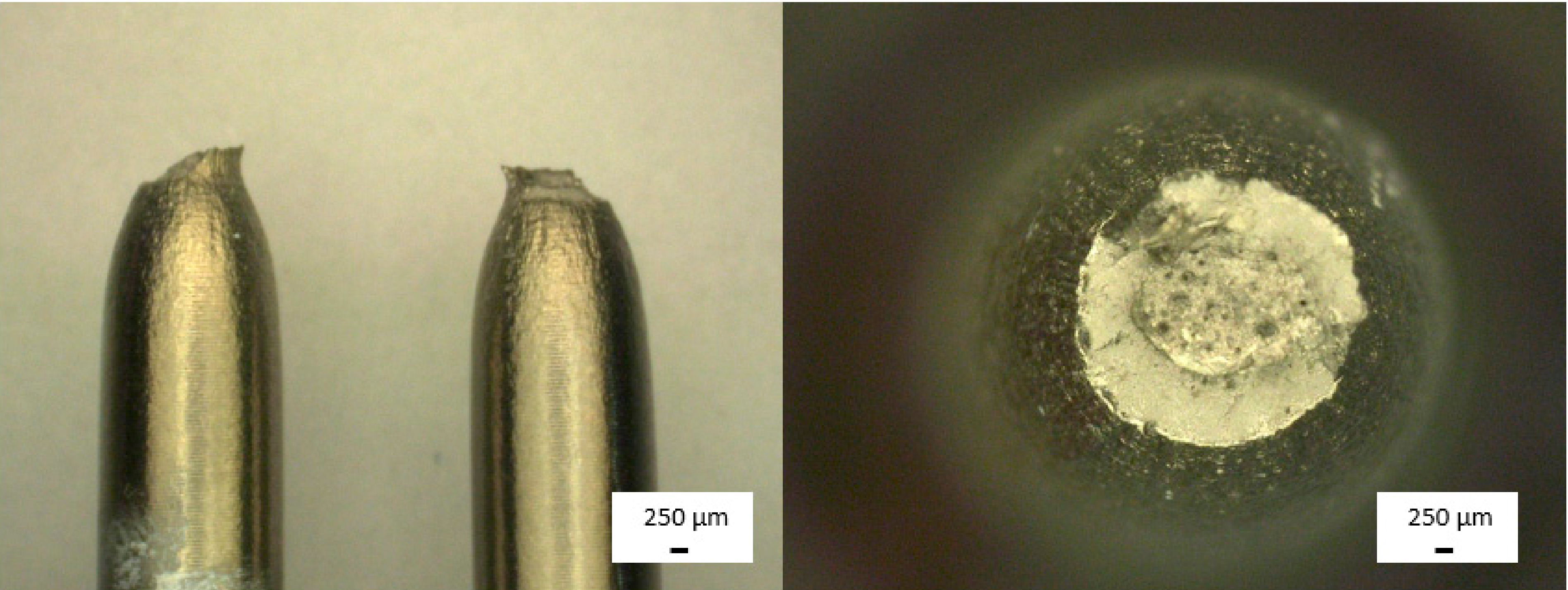

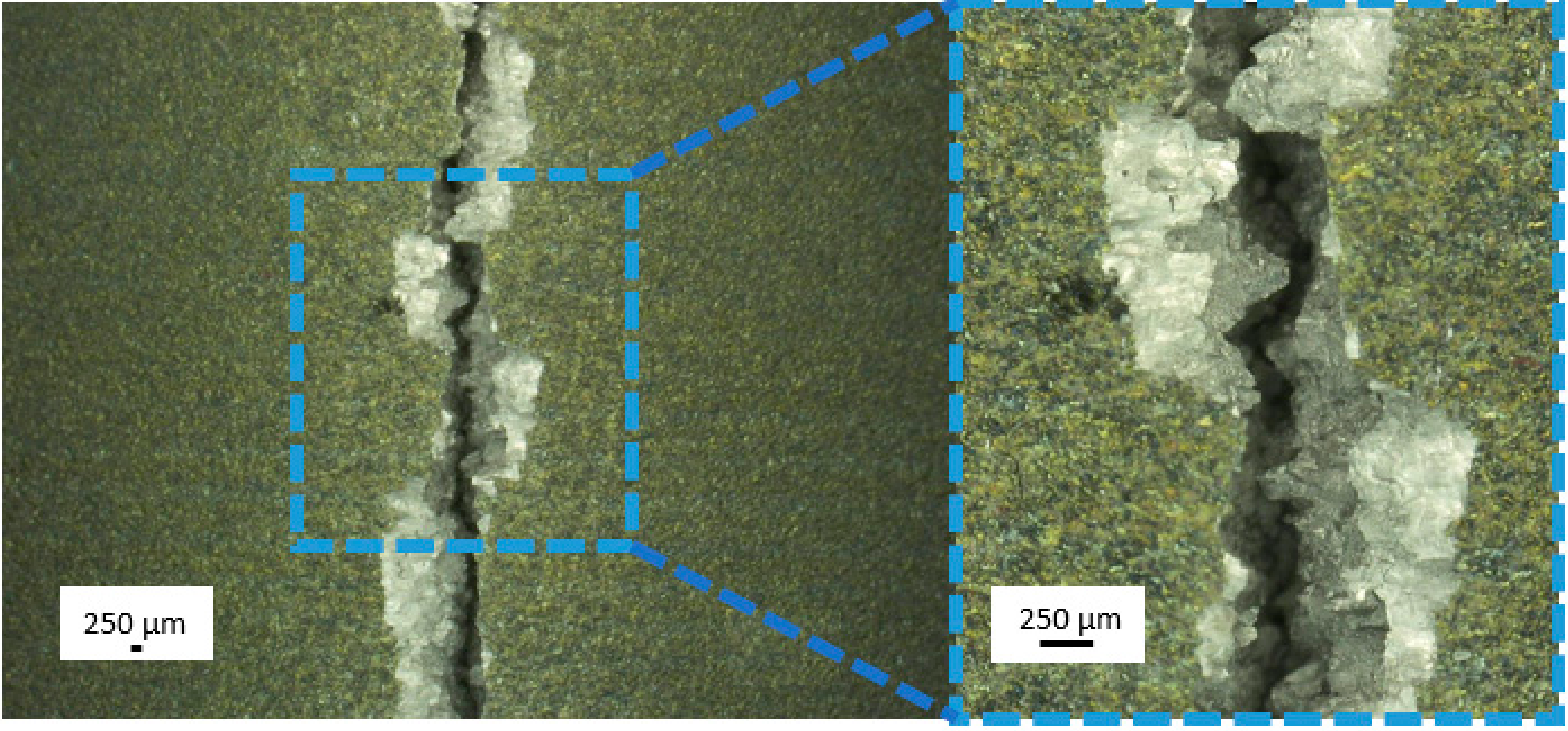

| Type of Test | Measured Parameters | Standard | Dimensions | Traverse Speed | Number of Specimens |
|---|---|---|---|---|---|
| Static tensile test | UTS YS A E | ASTM E8/E8M-16a | G = 20.0 ± 0.1 mm D = 4.0 ± 0.1 mm R = 4 mm Amin = 24 mm | 2 mm/min | 10 |
| Static compression test | CS YSc | ASTM E9-09 | D = 6.5 ± 0.1 mm L = 12.5 ± 0.5 mm L/D = 2 | 1 mm/min | 10 |
| Static bending test | FS | ASTM E290-14 | W = 20.0 ± 0.2 mm T = 3.0 ± 0.1 mm L = 75.0 ± 0.2 mm | 1 mm/min | 10 |
| Elements | Ti | Nb | Zr | O (max) | Fe (max) | H (max) | C (max) | N (max) |
|---|---|---|---|---|---|---|---|---|
| ASTM F1713 | 77.46–74.46 | 12.50–14.00 ± 0.30 | 12.50–14.00 ± 0.40 | 0.15 ± 0.02 | 0.25 ± 0.10 | 0.012 ± 0.0020 | 0.08 ± 0.02 | 0.05 ± 0.02 |
| Producer report | 74.71 | 12.8 | 12.2 | 0.094 | 0.1 | 0.005 | 0.080 | 0.008 |
| Name | Position (eV) | FWHM | Raw Area | %At Conc |
|---|---|---|---|---|
| O 1s | 531.00 | 2.40 | 318459 | 56.66 |
| C 1s | 285.00 | 3.30 | 26929.9 | 16.88 |
| Ti 2p | 459.00 | 3.47 | 256238 | 18.03 |
| Zr 3d | 183.00 | 3.98 | 36626.4 | 3.51 |
| Nb 3d | 207.00 | 5.80 | 41470.6 | 3.35 |
| N 1s | 401.00 | 1.69 | 2298.6 | 0.73 |
| UTS (MPa) (min) | YS (MPa) (min) | A (%) (min) | E (GPa) | CS (MPa) | YSc (MPa) | FS (MPa) | |
|---|---|---|---|---|---|---|---|
| ASTM F1713 (unannealed) | 550 | 345 | 15 | 64–77 | n.d. | n.d. | n.d. |
| Supplier Certificate (annealed *) | 690 | 446 | 25 | n.d. | n.d. | n.d. | n.d. |
| This research | 733.9 ± 7.5 | 555.6 ± 17.3 | 19.6 ± 1.6 | 84.1 ± 1.8 | 1378.9 ± 55.8 | 757.3 ± 34.9 | 1708.2 ± 43.9 |
| Ecorr | ba | bc | Icorr | Rp | |
|---|---|---|---|---|---|
| mV | mV dec−1 | mV dec−1 | μA·cm−2 | kΩ·cm2 | |
| mean | −366.83 | 208.33 | 191.64 | 0.091 | 523.97 |
| SD | 42.67 | 46.06 | 16.43 | 0.033 | 170.97 |
| Test | Number of Samples | Concentration | Survival Rate (%) | Morphological Changes in Cell Cultures | Evaluation of Morphological Changes in Cultures | Cytotoxicity |
|---|---|---|---|---|---|---|
| Negative test | 5 | Untreated cells | 97.33 | No intra-plasmatic granules, no cell lysis was found. | 0 | none |
| Positive test | 5 | 2 µg/mL | 50.91 | Cell culture almost or completely destroyed. | 4 | strong |
| Ti-13Nb-13Zr | 5 | 100% | 111.67 ± 2.44 | No intra-plasmatic granules, no cell lysis was found. Culture density comparable to negative culture. | 0 | none |
| 5 | 50% | 123.9 ± 4.65 | 0 | none | ||
| 5 | 25% | 125.39 ± 4.37 | 0 | none | ||
| 5 | 12.5% | 128.32 ± 4.22 | 0 | none |
| Test | Number of Samples | Concentration | Survival Rate (%) | Morphological Changes in Cell Cultures | Evaluation of Morphological Changes in Cultures | Cytotoxicity |
|---|---|---|---|---|---|---|
| Negative test | 5 | Untreated cells | 98.17 | No intra-plasmatic granules, No cell lysis was found. | 0 | none |
| Positive test | 5 | 2 µg/mL | 51.67 | Cell culture almost or completely destroyed. | 4 | strong |
| Ti-13Nb-13Zr | 5 | 100% | 111.75 ± 3.04 | No intra-plasmatic granules, no cell lysis was found. Culture density comparable to negative culture. | 0 | none |
| 5 | 50% | 116.5 ± 1.58 | 0 | none | ||
| 5 | 25% | 125.31 ± 4.65 | 0 | none | ||
| 5 | 12.5% | 129.05 ± 9.08 | 0 | none |
| UTS (MPa) | YS (MPa) | A (%) | E (GPa) | |
|---|---|---|---|---|
| This research (annealed) | 733.9 ± 7.5 | 555.6 ± 17.3 | 19.6 ± 1.6 | 84.1 ± 1.8 |
| Hardened [17,43] | 798 ± 17 | 599 ± 24 | 20 ± 3 | 83±2 |
| WQ [17,43] | 703–786 | 433–554 | 21–29 | 64–77 |
| WQ + Aged. [17,43] | 994 ± 42 | 864 ± 43 | 13 ± 3 | 81 ± 4 |
| WQ + DH [17,43] | 1034 ± 5 | 906 ± 10 | 11 ± 1 | 83 ± 2 |
| WQ + 50–75%CW [17,43] | 1000–1055 | 900–1000 | 10–15 | 44–51 |
| ST + WQ [16] | 860 | 640 | 15 | 60 |
| 52% CW [16] | 1280 | 1270 | 12 | 52 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hoppe, V.; Szymczyk-Ziółkowska, P.; Rusińska, M.; Dybała, B.; Poradowski, D.; Janeczek, M. Assessment of Mechanical, Chemical, and Biological Properties of Ti-Nb-Zr Alloy for Medical Applications. Materials 2021, 14, 126. https://doi.org/10.3390/ma14010126
Hoppe V, Szymczyk-Ziółkowska P, Rusińska M, Dybała B, Poradowski D, Janeczek M. Assessment of Mechanical, Chemical, and Biological Properties of Ti-Nb-Zr Alloy for Medical Applications. Materials. 2021; 14(1):126. https://doi.org/10.3390/ma14010126
Chicago/Turabian StyleHoppe, Viktoria, Patrycja Szymczyk-Ziółkowska, Małgorzata Rusińska, Bogdan Dybała, Dominik Poradowski, and Maciej Janeczek. 2021. "Assessment of Mechanical, Chemical, and Biological Properties of Ti-Nb-Zr Alloy for Medical Applications" Materials 14, no. 1: 126. https://doi.org/10.3390/ma14010126







