Thermodynamic Properties, Viscosity, and Structure of CaO–SiO2–MgO–Al2O3–TiO2–Based Slag
Abstract
:1. Introduction
2. Materials and Methods
2.1. Thermodynamic Calculations
2.2. Experimental Materials
2.3. Viscosity Measurement
3. Results and Discussion
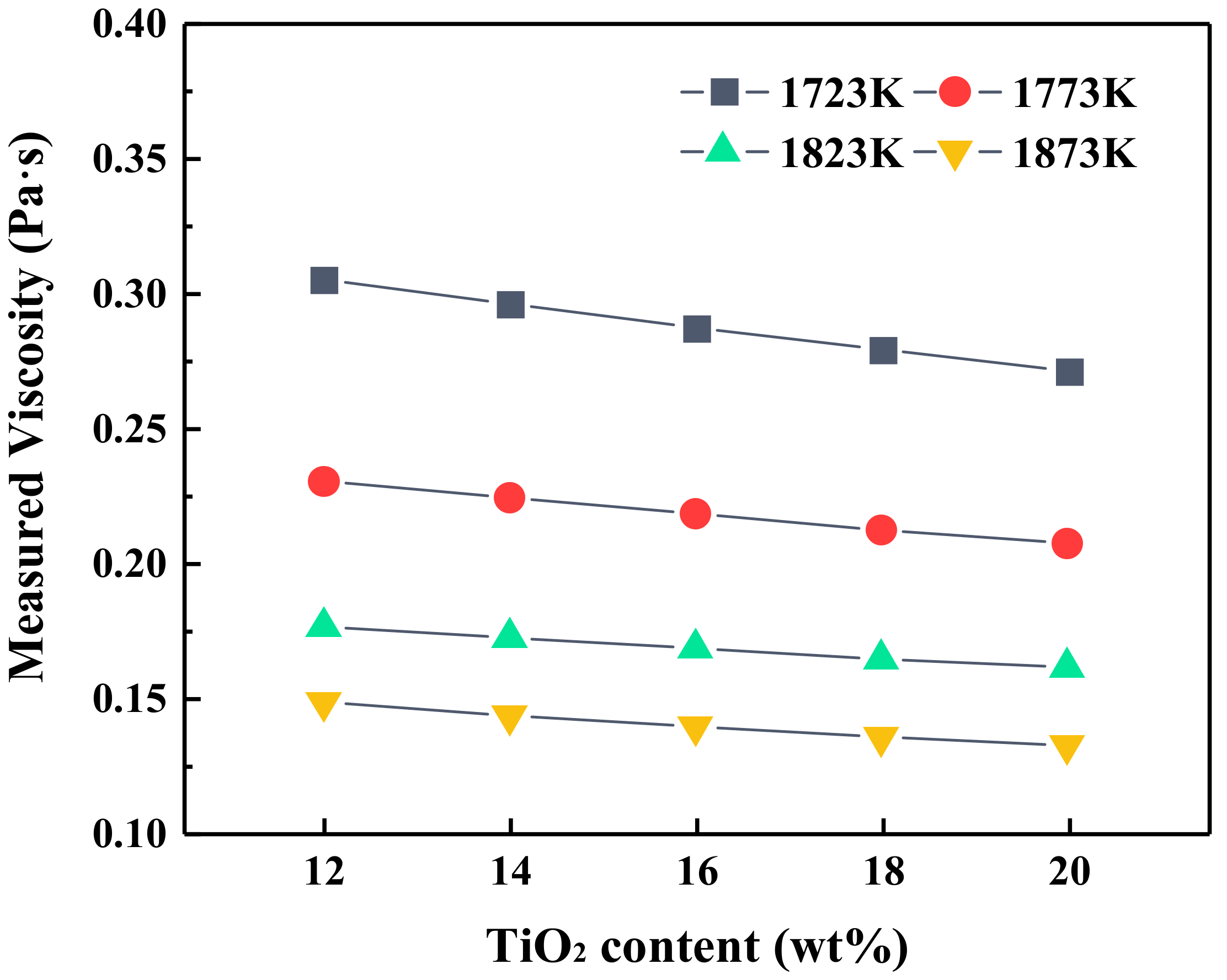
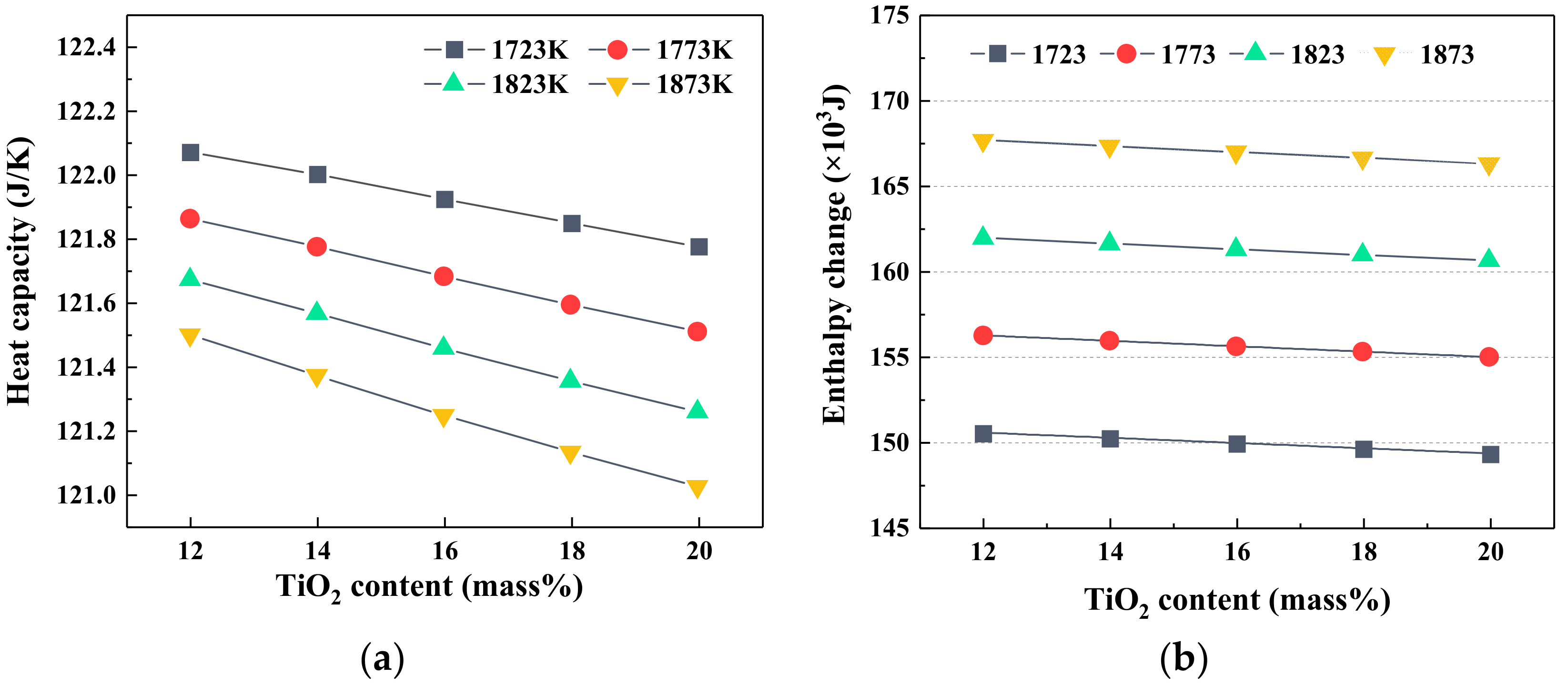
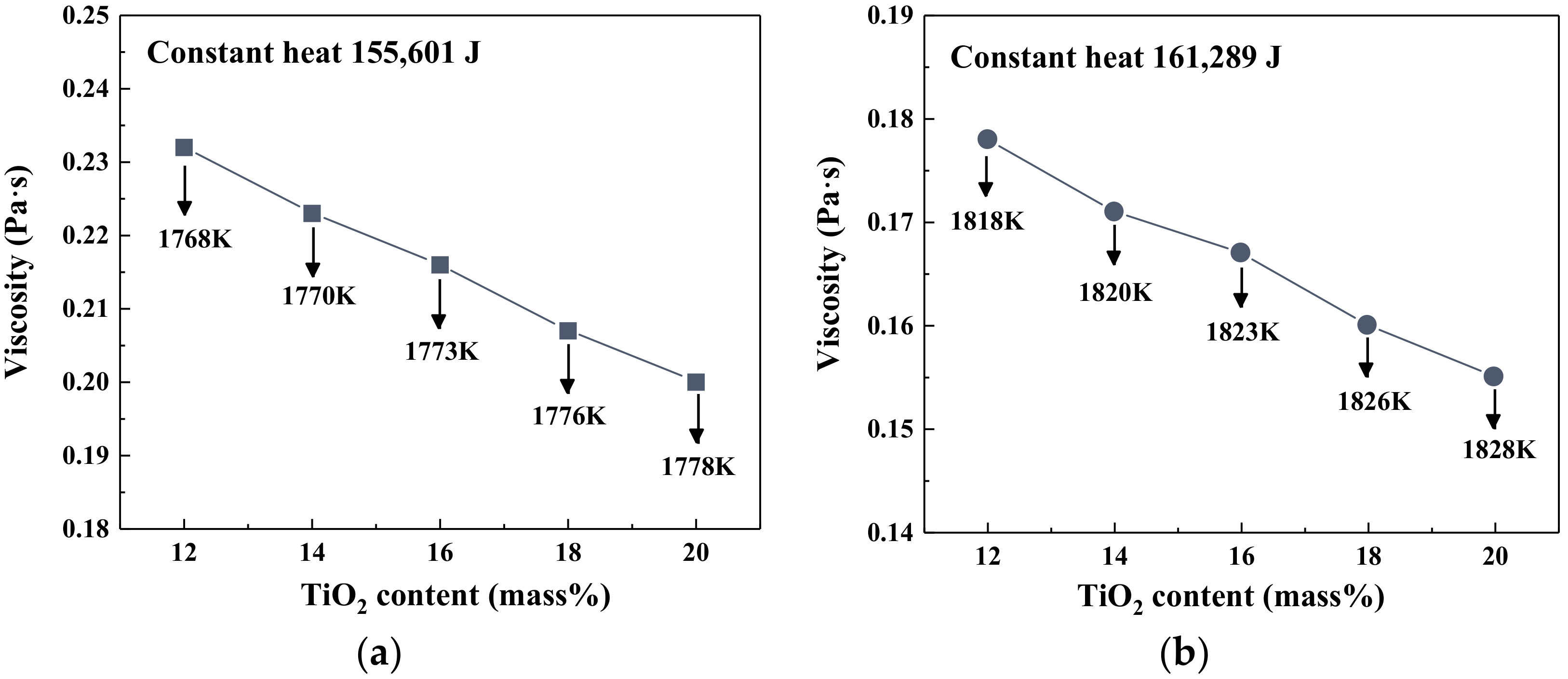
3.1. Effect of TiO2 on Viscosity, Heat Capacity and Enthalpy Change of Slag
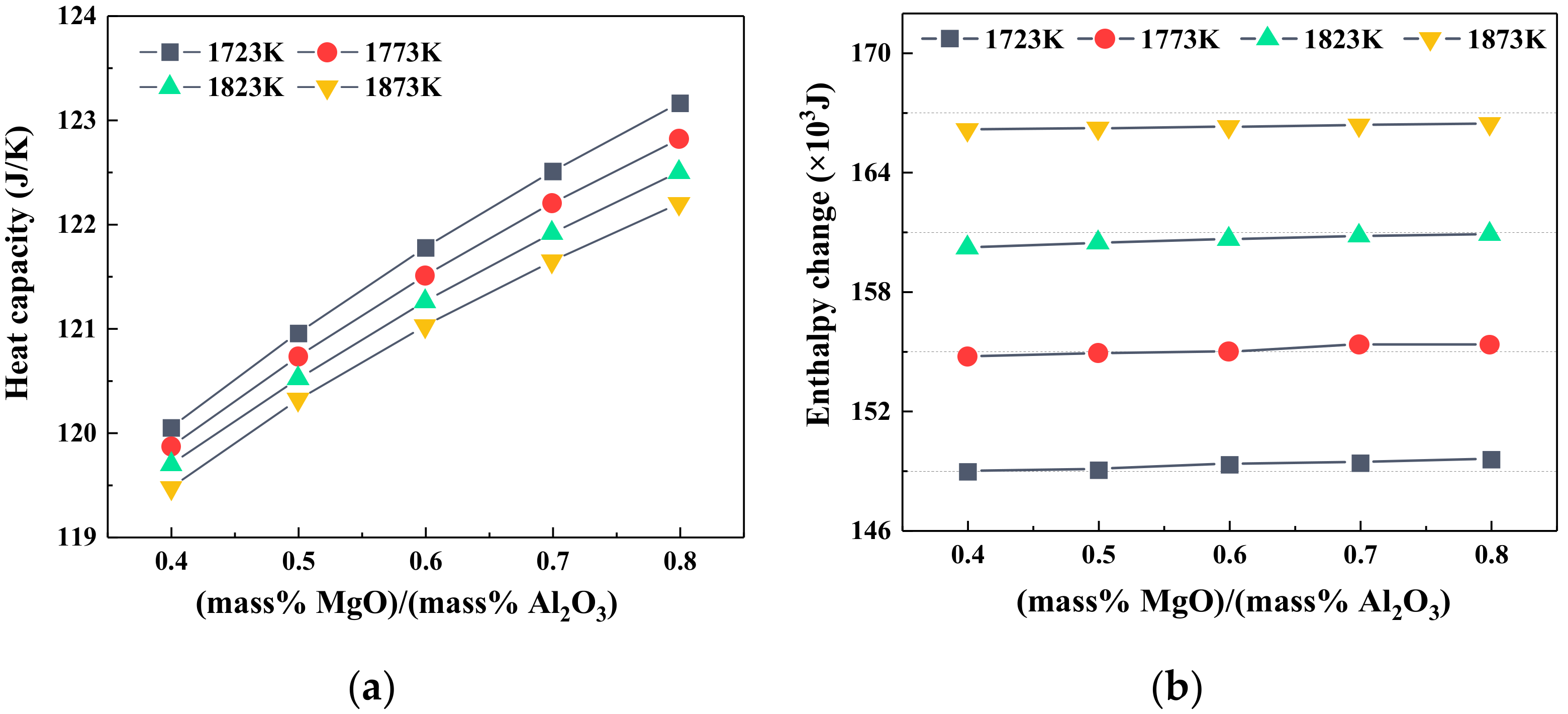
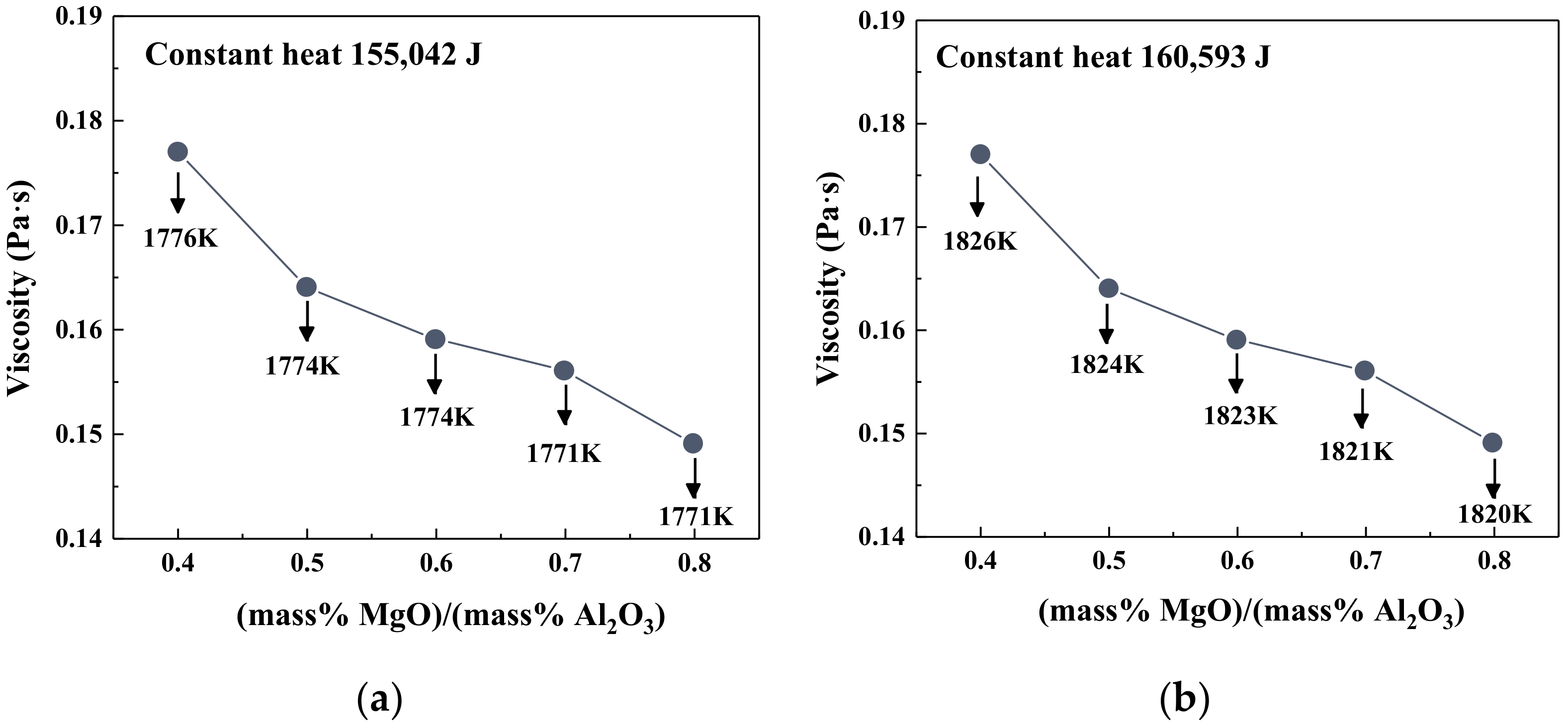
3.2. Effect of the MgO/Al2O3 Ratio on Viscosity, Heat Capacity, and Enthalpy Change of Slag
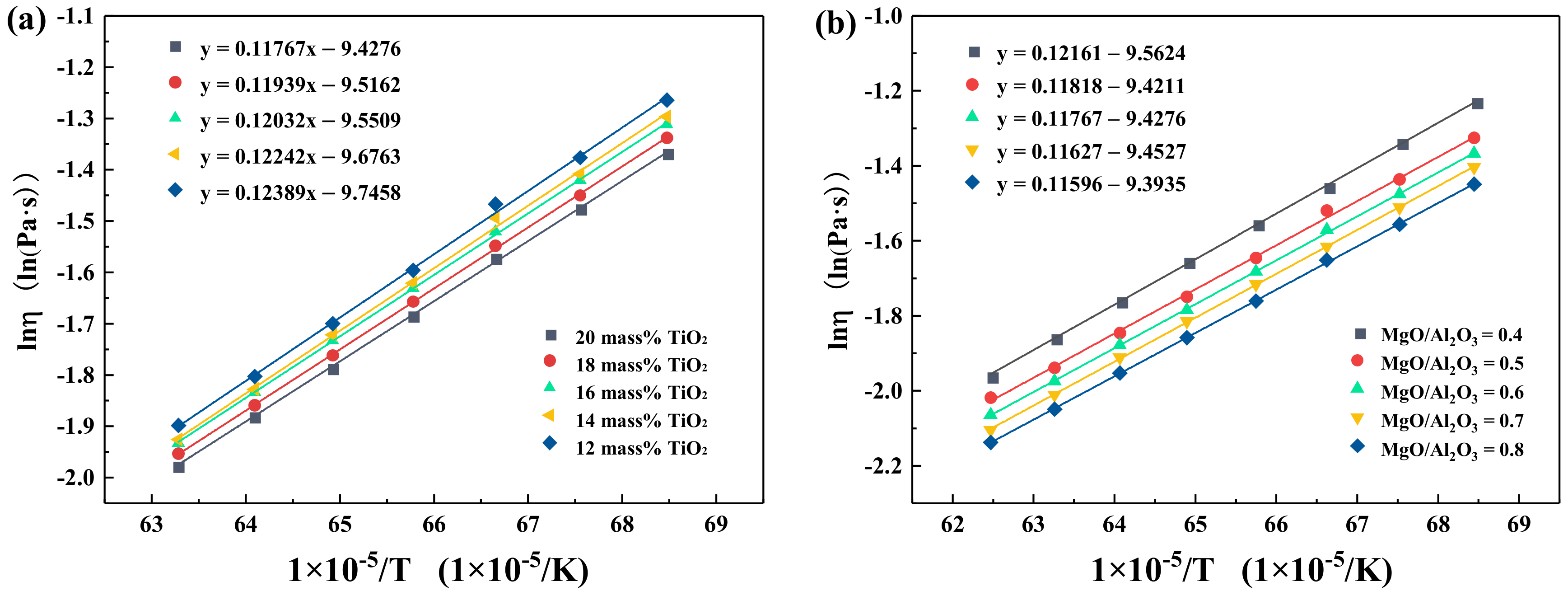
3.3. Effect of TiO2 and MgO/Al2O3 Ratio on the Activation Energy for Viscous Flow
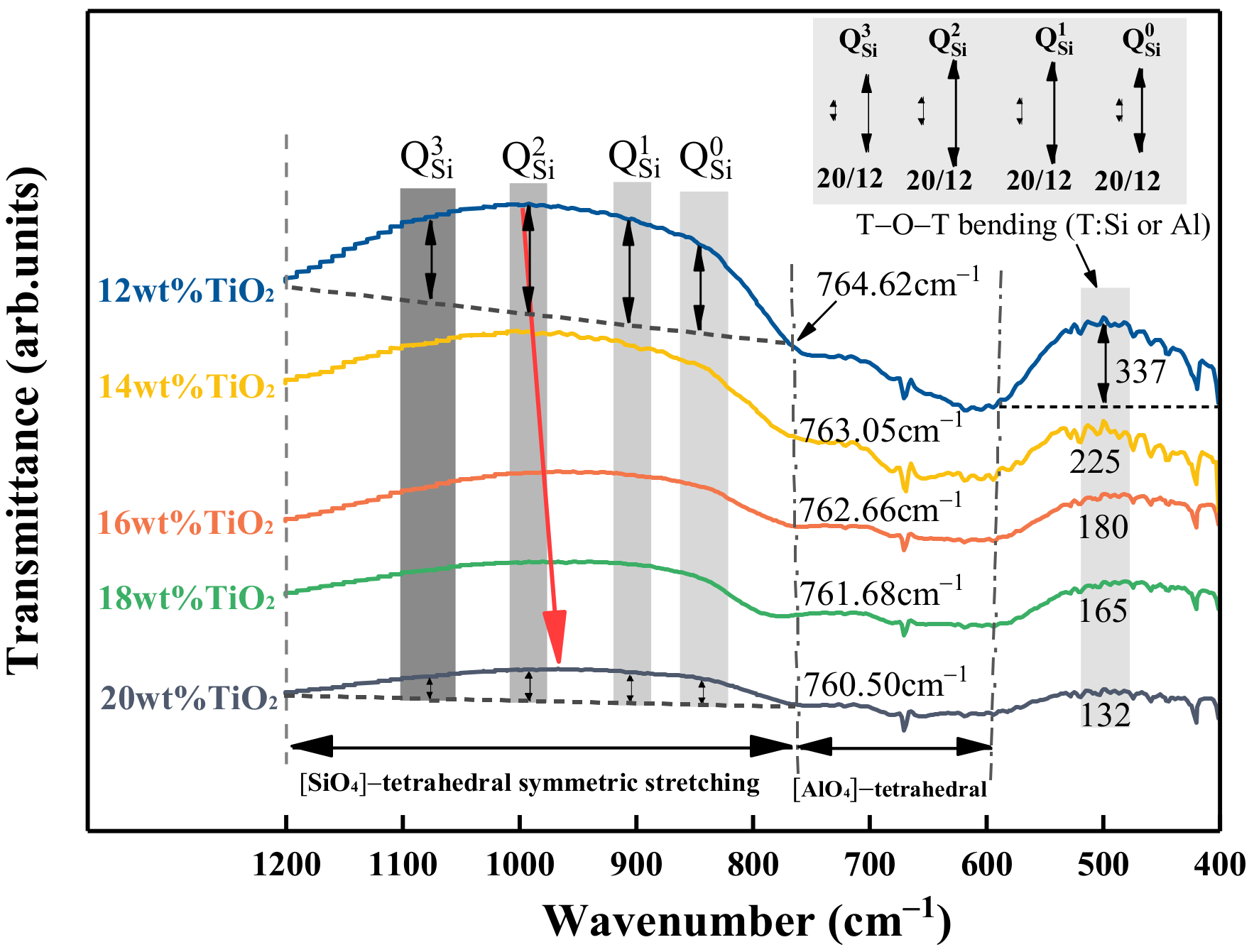
3.4. Effect of TiO2 on the Structure Using FTIR Spectroscopy
4. Conclusions
- At the fixed heat quantity supply, the increase in TiO2 content led to an increase in slag temperature but a decrease in the viscosity. As the MgO/Al2O3 ratio increased, the temperature of slag had the opposite variation trend, but the viscosity of slag still decreased.
- The apparent activation energy decreased from 123.89 kJ/mol to 117.67 kJ/mol with the increment of TiO2 content, and it also decreased from 121.61 kJ/mol to 115.96 kJ/mol with higher MgO/Al2O3 ratios.
- The vibration bands of [SiO4]-tetrahedron, [AlO4]-tetrahedron, and T–O–T became less pronounced. TiO2 played a role in weakening the stability of the molten slag structure and depolymerizing the complex network structural units into simpler structures, which eventually decreased the viscosity.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Jiao, K.; Zhang, J.; Chen, C.; Liu, Z.; Jiang, X. Operation characteristic of super-large blast furnace slag in China. ISIJ Int. 2017, 57, 983–998. [Google Scholar]
- Husslage, W.M.; Bakker, T.; Steeghs, A.G.S.; Reuter, M.A.; Heerema, R.H. Flow of molten slag and iron at 1500 °C to 1600 °C through packed coke beds. Metall. Mater. Trans. B 2005, 36, 765–776. [Google Scholar] [CrossRef]
- Gao, K.; Jiao, K.; Zhang, J.; Dianyu, E. Thermodynamic properties and viscosities of high-titanium slags. ISIJ Int. 2020, 60, 1902–1908. [Google Scholar] [CrossRef]
- Xing, X.; Pang, Z.; Zheng, J.; Du, Y.; Ren, S.; Ju, J. Effect of MgO and K2O on high-Al silicon–manganese alloy slag viscosity and structure. Minerals 2020, 10, 810. [Google Scholar] [CrossRef]
- Hyunsik, P.; Park, J.Y.; Hyun, K.; Sohn, I. Effect of TiO2 on the viscosity and slag structure in blast furnace type slags. Steel Res. Int. 2012, 83, 150–156. [Google Scholar]
- Sohn, I.; Wang, W.; Hiroyuki, M.; Fumitaka, T.; Dong, J. Influence of TiO2 on the viscous behavior of calcium silicate melts containing 17 mass% Al2O3 and 10 mass% MgO. ISIJ Int. 2012, 52, 158–160. [Google Scholar] [CrossRef] [Green Version]
- Liao, J.L.; Li, J.; Wang, X.D.; Zhang, Z.T. Influence of TiO2 and basicity on viscosity of Ti bearing slag. Ironmak. Steelmak. 2012, 39, 133–139. [Google Scholar] [CrossRef]
- Zhang, G.; Chou, K.; Zhang, J. Influence of TiO2 on viscosity of aluminosilicate melts. Ironmak. Steelmak. 2014, 41, 47–50. [Google Scholar] [CrossRef]
- Yan, Z.; Lv, X.; He, W.; Xu, J. Effect of TiO2 on the liquid zone and apparent viscosity of SiO2–CaO–8 wt.%MgO–14 wt.%Al2O3 system. ISIJ Int. 2017, 57, 31–36. [Google Scholar] [CrossRef] [Green Version]
- Zhang, X.; Jiang, T.; Xue, X.; Hu, B. Influence of MgO/Al2O3 ratio on the viscosity of blast furnace slag with high Al2O3 content. Steel Res. Int. 2016, 87, 87–93. [Google Scholar] [CrossRef]
- Kou, M.; Wu, S.; Ma, X.; Wang, L.; Chen, M.; Cai, Q.; Zhao, B. Phase equilibrium studies of CaO-SiO2-MgO-Al2O3 system with binary basicity of 1.5 related to blast furnace slag. Metall. Mater. Trans. B 2016, 47, 1093–1102. [Google Scholar] [CrossRef]
- Suzuki, M.; Jak, E. Quasi-chemical viscosity model for fully liquid slag in the Al2O3–CaO–MgO–SiO2 system–Part I: Revision of the model. Metall. Mater. Trans. B 2013, 44, 1435–1450. [Google Scholar] [CrossRef] [Green Version]
- Suzuki, M.; Jak, E. Quasi-chemical viscosity model for fully liquid slag in the Al2O3–CaO–MgO–SiO2 system–Part II: Evaluation of slag viscosities. Metall. Mater. Trans. B 2013, 44, 1451–1465. [Google Scholar] [CrossRef] [Green Version]
- Chang, Z.Y.; Jiao, K.X.; Zhang, J.-L.; Ning, X.-J.; Liu, Z.Q. Effect of TiO2 and MnO on viscosity of blast furnace slag and thermodynamic analysis. ISIJ Int. 2018, 58, 2173–2179. [Google Scholar] [CrossRef] [Green Version]
- Kim, J.; Sohn, I. Influence of TiO2/SiO2 and MnO on the viscosity and structure in the TiO2–MnO–SiO2 welding flux system. J. Non-Cryst. Solids 2013, 359, 235–243. [Google Scholar] [CrossRef]
- Zheng, K.; Zhang, Z.; Liu, L.; Wang, X. Investigation of the viscosity and structural properties of CaO–SiO2–TiO2 slags. Metall. Mater. Trans. B 2014, 45, 1389–1397. [Google Scholar] [CrossRef]
- Jiao, K.X.; Zhang, J.-L.; Wang, Z.Y.; Chen, C.-H.; Liu, Y.X. Effect of TiO2 and FeO on the viscosity and structure of blast furnace primary slags. Steel Res. Int. 2017, 88, 1600296. [Google Scholar] [CrossRef]
- Li, P.; Ning, X. Effects of MgO/Al2O3 ratio and basicity on the viscosities of CaO–MgO–SiO2–Al2O3 slags: Experiments and modeling. Metall. Mater. Trans. B 2016, 47, 446–457. [Google Scholar]
- Jiao, K.; Chang, Z.-Y.; Chen, C.; Zhang, J.-L. Thermodynamic properties and viscosities of CaO–SiO2–MgO–Al2O3 slags. Metall. Mater. Trans. B 2019, 50, 1012–1022. [Google Scholar] [CrossRef]
- Zhang, S.; Zhang, X.; Liu, W.; Lv, X.; Bai, C.; Wang, L. Relationship between structure and viscosity of CaO–SiO2–Al2O3–MgO–TiO2 slag. J. Non-Cryst. Solids 2014, 402, 214–222. [Google Scholar] [CrossRef]
- Park, J.H.; Min, D.J.; Song, H.S. FT-IR spectroscopic study on structure of CaO–SiO2 and CaO–SiO2–CaF2 slags. ISIJ Int. 2002, 42, 344–351. [Google Scholar] [CrossRef]
- Hu, K.; Lv, X.; Li, S.; Lv, W.; Song, B.; Han, K. Viscosity of TiO2–FeO–Ti2O3–SiO2–MgO–CaO–Al2O3 for high-titania slag smelting process. Metall. Mater. Trans. B 2018, 49, 1963–1973. [Google Scholar] [CrossRef]
- Qi, J.; Liu, C.; Jiang, M. Role of Li2O on the structure and viscosity in CaO–Al2O3–Li2O–Ce2O3 melts. J. Non-Cryst. Solids 2017, 475, 101–107. [Google Scholar] [CrossRef]
- Mysen, B.; Ryerson, F.; Virgo, D. The Influence of TiO2 on the structure and derivative properties of silicate melts. Am. Miner. 1980, 68, 1150–1165. [Google Scholar]
- Seung, M.; Park, J.; Sohn, I. Surface kinetics of nitrogen dissolution and its correlation to the slag structure in the CaO-SiO2, CaO-Al2O3, and CaO-SiO2-Al2O3 slag system. J. Non-Cryst. Solids 2011, 357, 2868–2875. [Google Scholar]
- Jiang, Y.; Lin, X.; Ideta, K.; Takebe, H.; Miyawaki, J.; Yoon, S.-H.; Mochida, I. Microstructural transformations of two representative slags at high temperatures and effects on the viscosity. J. Ind. Eng. Chem. 2014, 20, 1338–1345. [Google Scholar] [CrossRef]
- Wang, W.; Dai, S.; Zhou, L.-J.; Zhang, J.; Tian, W.; Xu, J. Viscosity and Structure of MgO–SiO2-based Slag Melt with varying B2O3 Content. Ceram. Int. 2020, 46, 3631–3636. [Google Scholar] [CrossRef]
- Sun, Y.; Liao, J.; Zheng, K.; Wang, X.; Zhang, Z. Effect of B2O3 on the structure and viscous behavior of Ti-bearing blast furnace slags. JOM 2014, 66, 2168–2175. [Google Scholar] [CrossRef]
- Wang, H.; Chen, Z.; Liu, L.; Sun, Y.; Wang, X. Roles of P2O5 Addition on the viscosity and structure of CaO–SiO2–Al2O3–Na2O–P2O5 melts. ISIJ Int. 2018, 58, 1644–1649. [Google Scholar] [CrossRef]
- Kim, H.; Matsuura, H.; Tsukihashi, F.; Wang, W.; Min, D.; Sohn, I. Effect of Al2O3 and CaO/SiO2 on the viscosity of calcium-silicate–based slags containing 10 Mass pct MgO. Metall. Mater. Trans. B 2013, 44, 5–12. [Google Scholar] [CrossRef]
- Xu, R.; Zhang, J.; Ma, R.; Jiao, K.; Zhao, Y. Influence of TiO2 on the viscosity of a high alumina slag and on carbon brick corrosion. Steel Res. Int. 2017, 1700353. [Google Scholar] [CrossRef]
- Huang, W.; Zhao, Y.; Yu, S.; Zhang, L.; Ye, Z.; Wang, N.; Chen, M. Viscosity property and structure analysis of FeO–SiO2–V2O3–TiO2–Cr2O3 slags. ISIJ Int. 2016, 56, 594–601. [Google Scholar] [CrossRef] [Green Version]
- Wang, Z.; Shu, Q.; Chou, K. Viscosity of fluoride-free mold fluxes containing B2O3 and TiO2. Steel Res. Int. 2013, 84, 766–776. [Google Scholar] [CrossRef]
- Duan, R.-G.; Liang, K.-M.; Gu, S.-R. The stable energy of glass structure unit. Mater. Trans. JIM. 1998, 39, 1162–1163. [Google Scholar] [CrossRef] [Green Version]



| No. | CaO | SiO2 | Al2O3 | MgO | TiO2 | MgO/Al2O3 | Liquid Temperature (K) |
|---|---|---|---|---|---|---|---|
| 1 | 30.03 | 27.30 | 14.17 | 8.50 | 20 | 0.6 | 1432 |
| 2 | 31.08 | 28.25 | 14.17 | 8.50 | 18 | – | 1430 |
| 3 | 32.13 | 29.20 | 14.17 | 8.50 | 16 | – | 1427 |
| 4 | 33.17 | 30.16 | 14.17 | 8.50 | 14 | – | 1421 |
| 5 | 34.22 | 31.11 | 14.17 | 8.50 | 12 | – | 1413 |
| 6 | 30.03 | 27.30 | 16.19 | 6.48 | 20 | 0.4 | 1440 |
| 7 | 30.03 | 27.30 | 15.11 | 7.56 | 20 | 0.5 | 1436 |
| 8 | 30.03 | 27.30 | 13.34 | 9.33 | 20 | 0.7 | 1428 |
| 9 | 30.03 | 27.30 | 12.59 | 10.08 | 20 | 0.8 | 1424 |
| TiO2 (mass%) | Ea (kJ/mol) | MgO/Al2O3 (mass%/mass%) | Ea (kJ/mol) |
|---|---|---|---|
| 20 | 117.67 | 0.4 | 121.61 |
| 18 | 119.39 | 0.5 | 118.18 |
| 16 | 120.32 | 0.6 | 117.67 |
| 14 | 122.42 | 0.7 | 116.27 |
| 12 | 123.89 | 0.8 | 115.96 |
| Wavenumber (cm−1) | Assignments | References |
|---|---|---|
| 400–600 | T-O-T bond bending vibrations (T denotes Si, Al, or Ti elements, etc.) | [17,18,27,28] |
| 600–760 | The symmetric stretching vibration of [AlO4]-tetrahedron | [14,18,29,30] |
| ~850 | Q0 ([SiO4]4− monomers) | [14,16,29,30] |
| ~900 | Q1 ([Si2O7]6− dimers) | [14,16,29,30] |
| ~950–1000 | Q2 ([Si3O10]8− chains) | [14,16,29,30] |
| ~1050 | Q3 ([Si3O9]6− rings) | [14,16,29,30] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fang, J.; Pang, Z.; Xing, X.; Xu, R. Thermodynamic Properties, Viscosity, and Structure of CaO–SiO2–MgO–Al2O3–TiO2–Based Slag. Materials 2021, 14, 124. https://doi.org/10.3390/ma14010124
Fang J, Pang Z, Xing X, Xu R. Thermodynamic Properties, Viscosity, and Structure of CaO–SiO2–MgO–Al2O3–TiO2–Based Slag. Materials. 2021; 14(1):124. https://doi.org/10.3390/ma14010124
Chicago/Turabian StyleFang, Jinle, Zhuogang Pang, Xiangdong Xing, and Runsheng Xu. 2021. "Thermodynamic Properties, Viscosity, and Structure of CaO–SiO2–MgO–Al2O3–TiO2–Based Slag" Materials 14, no. 1: 124. https://doi.org/10.3390/ma14010124




