Hydrogen Gas Phase and Electrochemical Hydriding of LaNi5−xMx (M = Sn, Co, Al) Alloys
Abstract
:1. Introduction
2. Materials and Methods
3. Results and Discussion
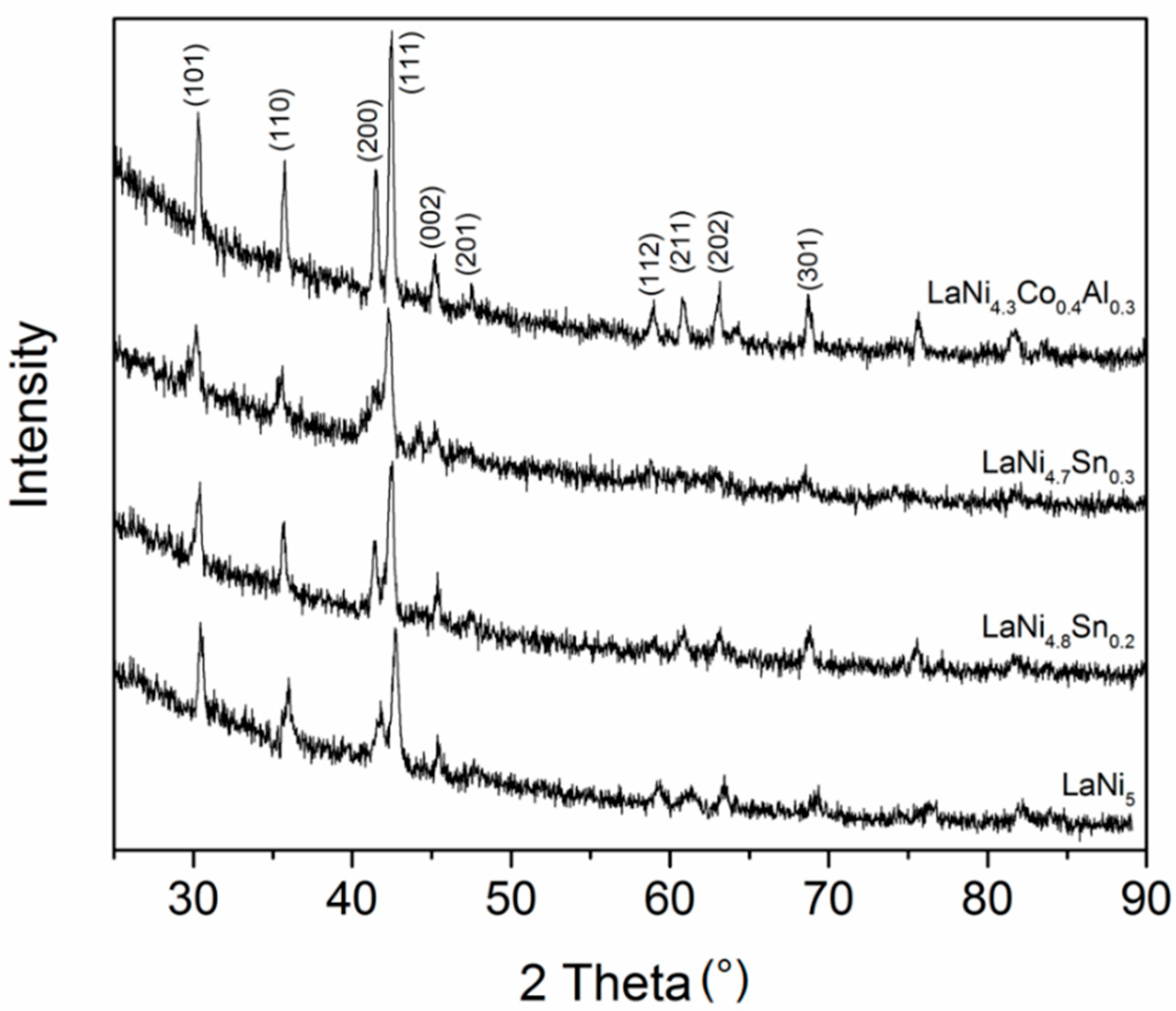
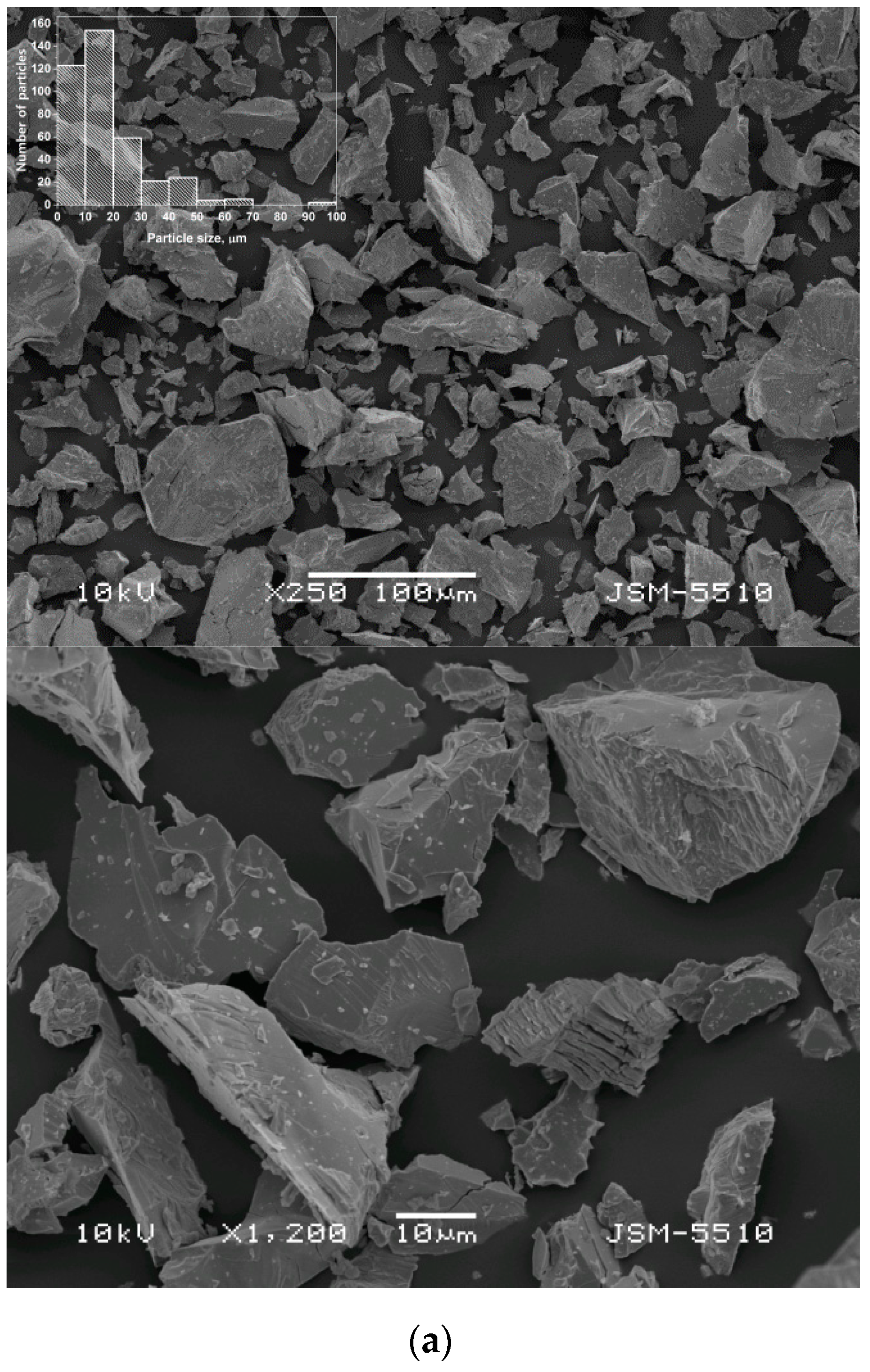
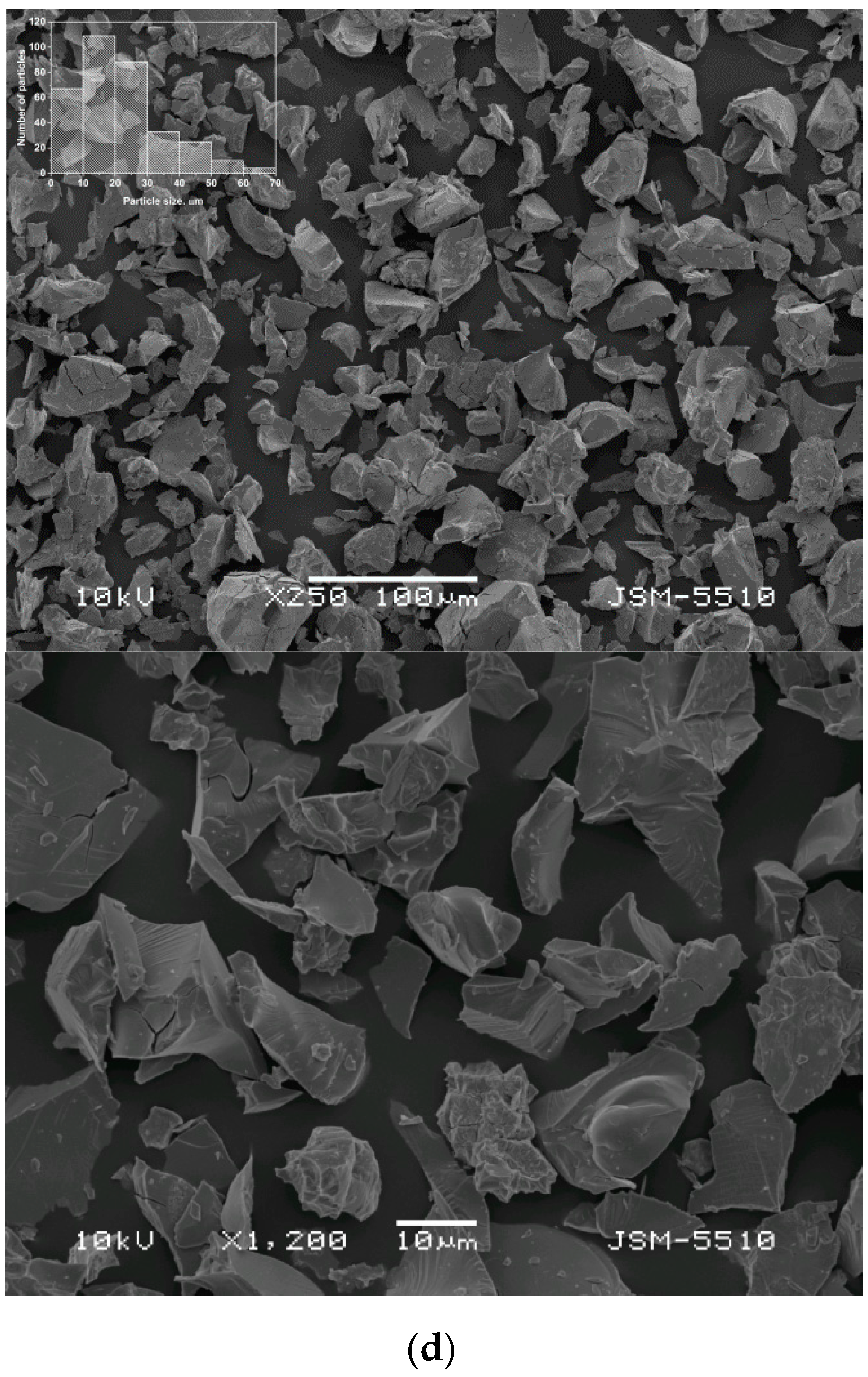
3.1. Alloys Morphology and Microstructure
3.2. Hydrogen Gas Phase Absorption
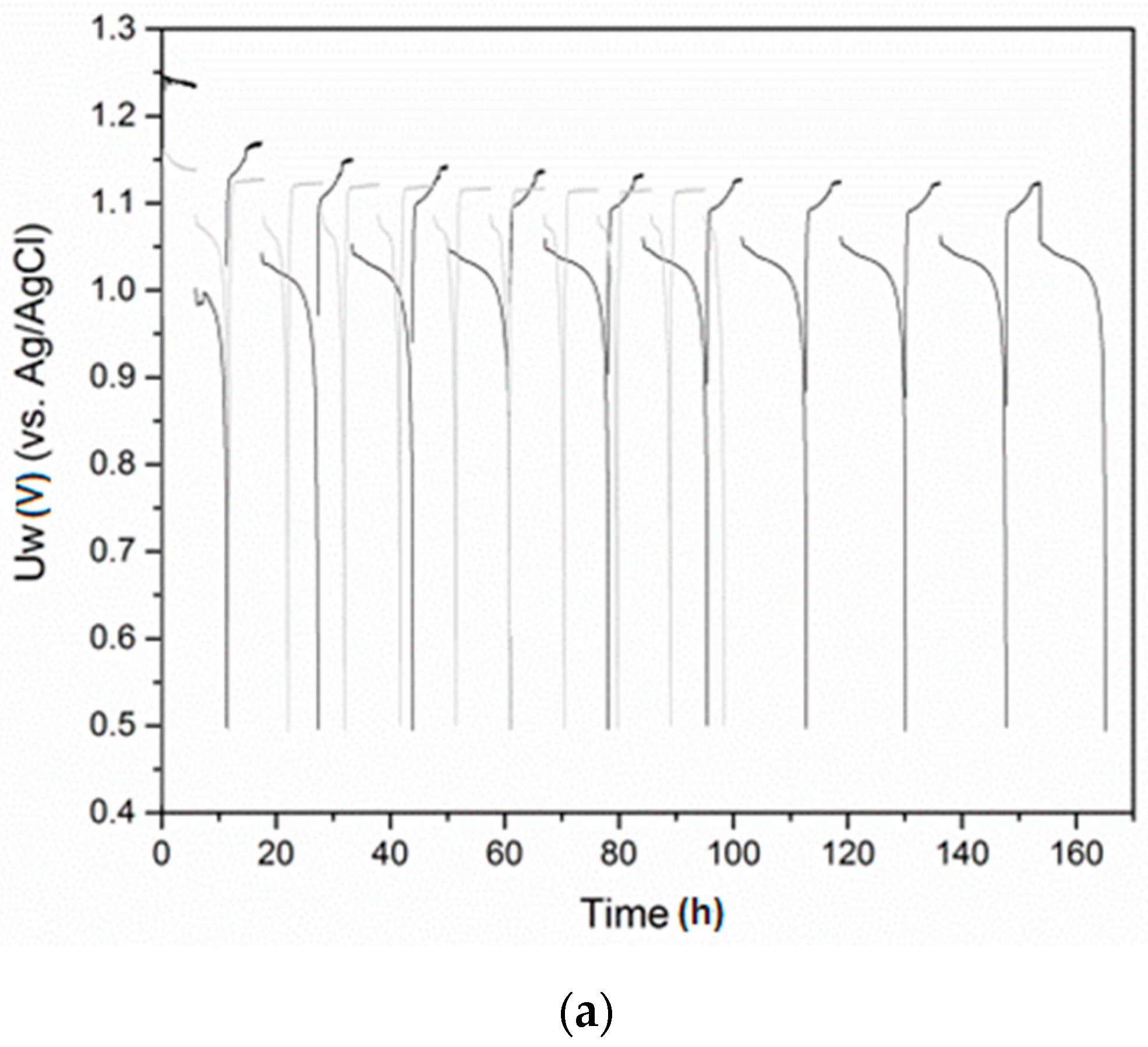
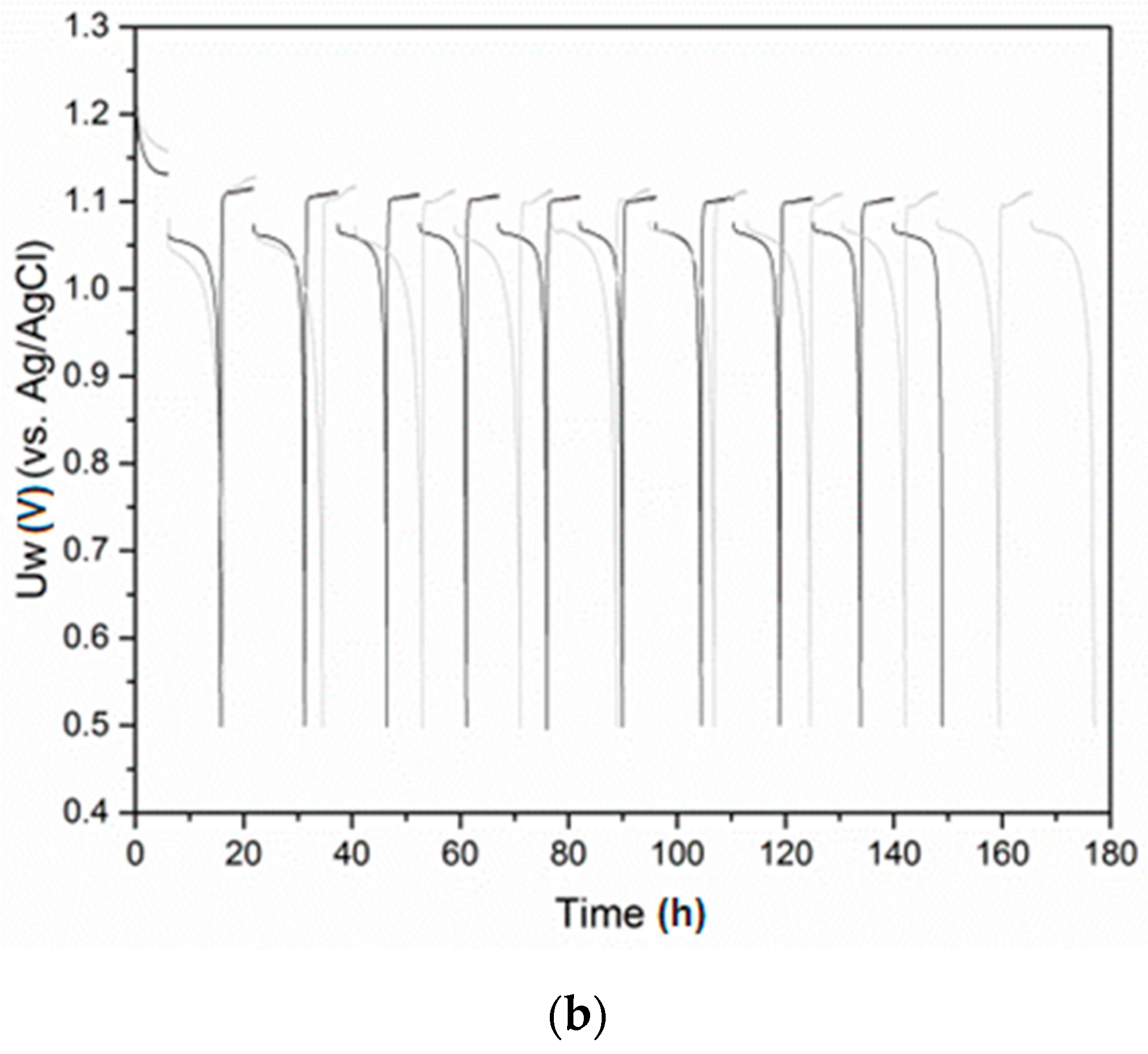
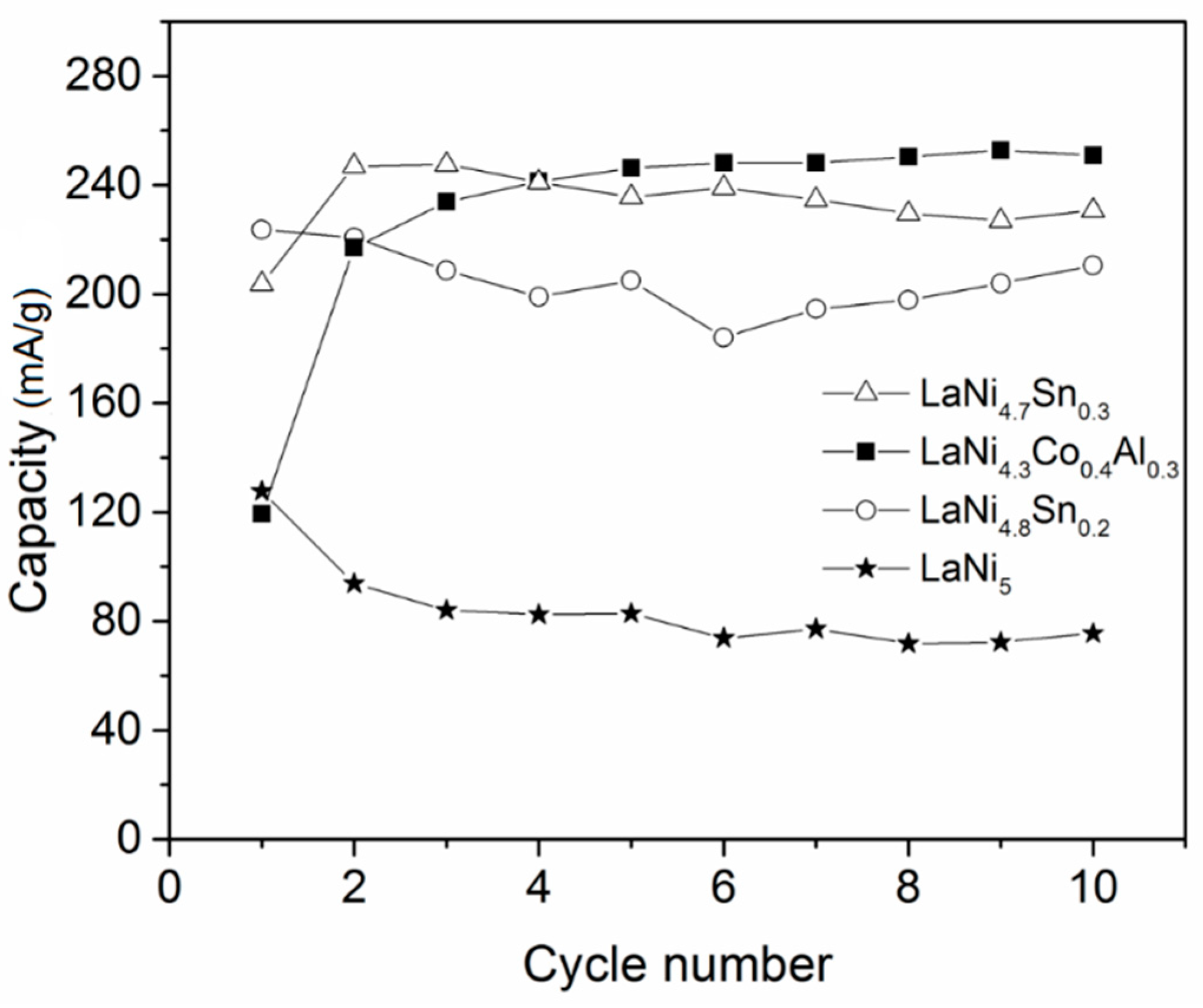
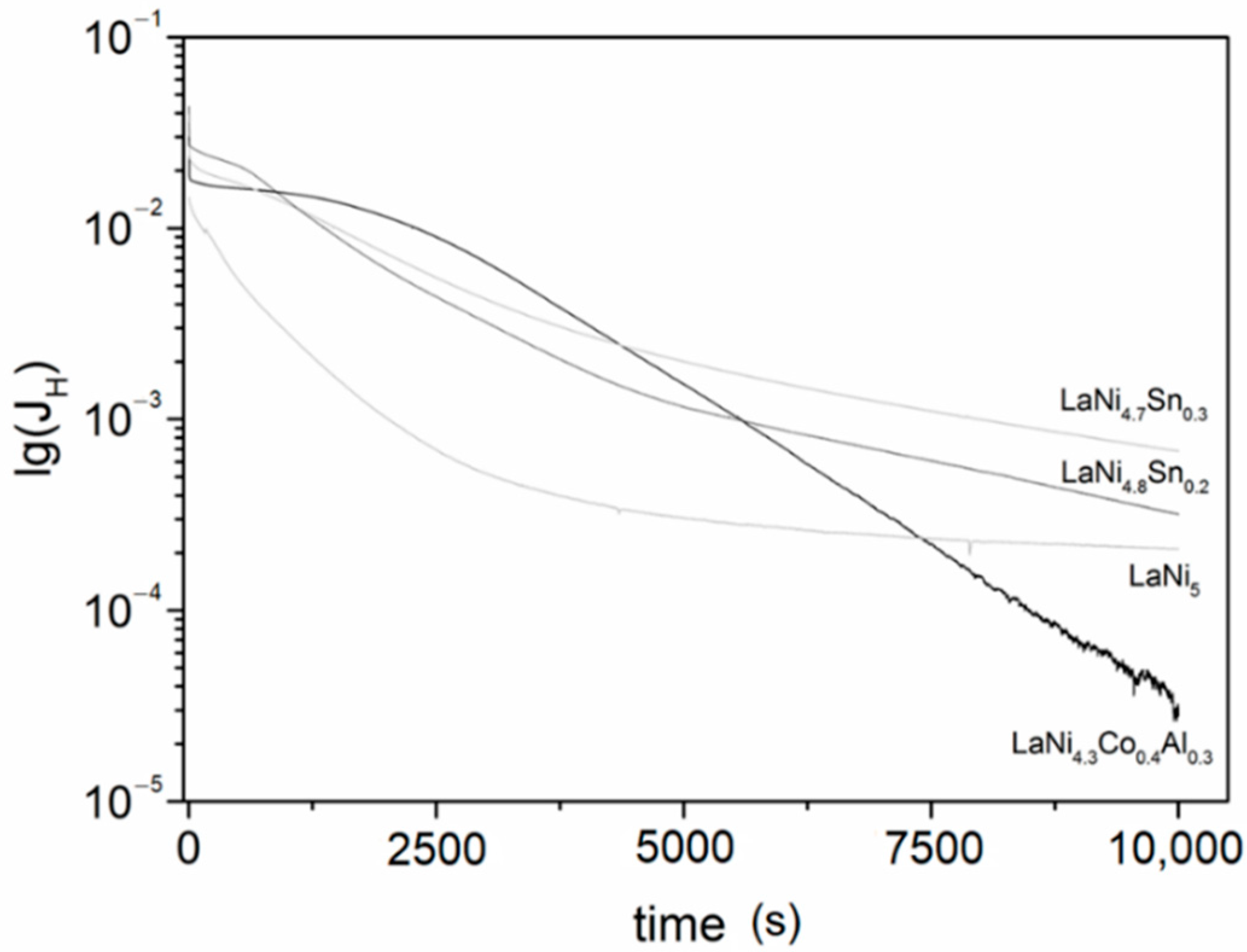
3.3. Electrochemical Hydriding
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- El Kharbachi, A.; Zavorotynska, O.; Latroche, M.; Cuevas, F.; Yartys, V.; Fichtner, M. Exploits, advances and challenges benefiting beyond Li-ion battery. J. Alloys Compd. 2020, 817, 153261. [Google Scholar] [CrossRef]
- Notten, P.H.L.; Latroche, M. Nickel-Metal Hydride: Metal Hydrides. In Encyclopedia of Electrochemical Power Sources; Garche, J., Chris, K., Dyer, C.K., Moseley, P.T., Ogumi, Z., Rand, D.A.J., Scrosati, B., Eds.; Elsevier Science: Amsterdam, The Netherlands, 2009; pp. 502–521. [Google Scholar]
- Sakintuna, B.; Lamari-Darkrim, F.; Hirscher, M. Metal hydride materials for solid hydrogen storage: A review. Int. J. Hydrogen Energy 2007, 32, 1121–1140. [Google Scholar] [CrossRef]
- Kang, J.; Yan, F.; Zhang, P.; Du, C. Comparison of comprehensive properties of Ni-MH (nickel-metal hydride) and Li-ion (lithium-ion) batteries in terms of energy efficiency. Energy 2014, 70, 618–625. [Google Scholar] [CrossRef]
- Ouyang, L.; Huang, J.; Wang, H.; Liu, J.; Zhu, M. Progress of hydrogen storage alloys for Ni-MH rechargeable power batteries in electric vehicles: A review. Mater. Chem. Phys. 2017, 200, 164–178. [Google Scholar] [CrossRef]
- Ming, Q.; Shuhui, L.; Peilin, Q.; Zhiqiang, L.; Jin, G. The influence of Co content on LaNi3.2−xMn03Cox (x = 0.2~0.8) alloy hydrogen storage and electrochemical properties. Phys. Procedia 2011, 22, 577–583. [Google Scholar] [CrossRef] [Green Version]
- Briki, C.; Belkhiria, S.; Dhaou, M.H.; De Rango, P.; Jemni, A. Experimental study of the influences substitution from Ni by Co, Al and Mn on the hydrogen storage properties of LaNi3.6Mn0.3Al0.4Co0.7 alloy. Int. J. Hydrogen Energy 2017, 15, 10081–10088. [Google Scholar] [CrossRef]
- Furukawa, N. Development and commercialization of nickel-metal hydride secondary batteries. J. Power Sources 1994, 51, 45–59. [Google Scholar] [CrossRef]
- Smith, G.; Goudy, A.J. Thermodynamics, kinetics and modeling studies of the LaNi5−xCox hydride system. J. Alloys Compd. 2001, 316, 93–98. [Google Scholar] [CrossRef]
- Zhu, Z.; Zhu, S.; Lu, H.; Wu, J.; Yan, K.; Cheng, H.; Liu, J. Stability of LaNi5−xCox alloys cycled in hydrogen—Part 1 evolution in gaseous hydrogen storage performance. Int. J. Hydrogen Energy 2019, 44, 15159–15172. [Google Scholar] [CrossRef]
- Mi, W.; Liu, Z.; Kimura, T.; Kamegawa, A.; Wang, H. Crystal structure and hydrogen storage properties of (La,Ce)Ni5−xMx (M = Al, Fe, or Co) alloys. Int. J. Miner. Metall. Mater. 2019, 26, 108–113. [Google Scholar] [CrossRef]
- Deng, H.; Zhuang, Y.; Liu, J.; Guo, J.; Lü, J.; Yan, J. Electrochemical performance of LaNi5−xSnx alloys. J. Alloys Compd. 2004, 376, 211–214. [Google Scholar] [CrossRef]
- Iosub, V.; Latroche, M.; Joubert, J.-M.; Percheron-Guégan, A. Optimisation of MmNi5−xSnx (Mm=La, Ce, Nd and Pr, 0.27 < x < 0.5) compositions as hydrogen storage materials. J. Alloys Compd. 2006, 31, 101–108. [Google Scholar]
- Borzone, E.M.; Blanco, M.V.; Baruj, A.; Meyer, G.O. Stability of LaNi5−xSnx cycled in hydrogen. Int. J. Hydrogen Energy 2014, 39, 8791–8796. [Google Scholar] [CrossRef]
- Luo, S.; Luo, W.; Clewley, J.D.; Flanagan, T.B.; Wade, L.A. Thermodynamic studies of the LaNi5−xSnx–H system from x = 0 to 0.5. J. Alloys Compd. 1995, 231, 467–472. [Google Scholar] [CrossRef]
- Lin, Q.; Zhao, S.; Zhu, D.; Chen, N. Structure and properties of the MlNi system with tin substitution. Solid State Ionics 2000, 136–137, 663–666. [Google Scholar] [CrossRef]
- Bowman, R.C., Jr.; Payzant, E.A.; Wilson, P.R.; Pearson, D.P.; Ledovskikh, A.; Danilov, D.; Notten, P.H.L.; An, K.; Skorpenske, H.D.; Wood, D.L. Characterization and analyses of degradation and recovery of LaNi4.78Sn0.22 hydrides following thermal aging. J. Alloys Compd. 2013, 580, S207–S210. [Google Scholar] [CrossRef]
- Li, X.; Liu, S.; Liu, Y.; Du, Y. Thermodynamic Re-assessment of the Lanthanum-Tin System. J. Phase Equilib. Diffus. 2019, 40, 653–667. [Google Scholar] [CrossRef]
- Chen, J.; Dou, S.X.; Bradhurst, D.H.; Liu, H.K. Studies on the diffusion coefficient of hydrogen through metal hydride electrodes. Int. J. Hydrogen Energ. 1998, 23, 177–182. [Google Scholar] [CrossRef]
- Yuan, X.; Ma, Z.-F.; Nuli, Y.; Xu, N. Study on hydrogen diffusion behavior in AB5-type hydrogen storage alloys with galvanostatic intermittent titration technique (GITT). J. Alloys Compd. 2004, 385, 90–95. [Google Scholar] [CrossRef]
- Giza, K. Electrochemical studies of LaNi4.3Co0.4Al0.3 hydrogen storage alloy. Intermetallics 2013, 34, 128–131. [Google Scholar] [CrossRef]
- Laurencelle, F.; Dehouche, Z.; Goyette, J. Hydrogen sorption cycling performance of LaNi4.8Sn0.2. J. Alloys Compd. 2006, 424, 266–271. [Google Scholar] [CrossRef]
- Bowman, R.C., Jr.; Luo, C.H.; Ahn, C.C.; Witham, C.K.; Fultz, B. The effect of tin on the degradation of LaNi5−ySny metal hydrides during thermal cycling. J. Alloys Compd. 1995, 217, 185–192. [Google Scholar] [CrossRef]
- Ratnakumar, B.V.; Witham, C.; Bowman, R.C., Jr.; Hightower, A.; Fultz, B. Electrochemical Studies on LaNi5−xSnx Metal Hydride Alloys. J. Electrochem. Soc. 1996, 143, 2578–2584. [Google Scholar]
- Sato, M.; Stange, M.; Yartys, V. Desorption behaviour of hydrogen in the LaNi4.7Sn0.3-H system. J. Alloys Compd. 2005, 396, 197–201. [Google Scholar] [CrossRef]
- Kwon, H.; Kim, J.; Yoo, J.-H.; Cho, S.-W. Control of hydrogen storage properties of (La,Ce,Nd,Pr)(Ni,Co,Mn,Al)5 alloys with microstructural parameters. J. Alloys Compd. 2013, 570, 114–118. [Google Scholar] [CrossRef]
- Kisi, E.H.; Buckley, C.E.; Gray, E.M. The hydrogen activation of LaNi5. J. Alloys Compd. 1992, 185, 369–384. [Google Scholar] [CrossRef]
- An, X.H.; Pan, Y.B.; Luo, Q.; Zhang, X.; Zhang, J.Y.; Li, Q. Application of a new kinetic model for the hydriding kinetics of LaNi5−xAlx (0 ≤ x ≤ 1.0) alloys. J. Alloys Compd. 2010, 506, 63–69. [Google Scholar] [CrossRef]
- Zhang, W.; Cimato, J.; Goudy, A.J. The hydriding and dehydriding kinetics of some LaNi5−xAlx alloys. J. Alloys Compd. 1993, 201, 175–179. [Google Scholar] [CrossRef]
- Zheng, G.; Popov, B.N.; White, R.E. Determination of transport and electrochemical kinetic parameters of bare and copper-coated LaNi4.27Sn0.24 electrodes in alkaline solution. J. Electrochem. Soc. 1996, 143, 834–839. [Google Scholar] [CrossRef]
- Kim, H.-S.; Nishizawa, M.; Uchida, I. Single particle electrochemistry for hydrogen storage alloys, MmNi3.55Co0.75Mn0.4Al0.3. Electrochim. Acta 1999, 45, 483–488. [Google Scholar] [CrossRef]
- Cao, J.; Xie, H.; Wen, Z.; Cao, G.; Ji, L.; Fan, Y.; Liu, B. Effects of Mo0.46Fe0.54 alloy on microstructure and electrochemical hydrogen storage performances of La0.75Ce0.25Ni4.2Mn0.9−xCu0.3(Mo0.46Fe0.54)x (x = 0−0.20) alloys. Int. J. Electrochem. Sci. 2017, 12, 5854–5866. [Google Scholar] [CrossRef]
- Zhang, B.; Liu, H.; Jing, X.; Ning, Y.; Han, X. Investigations on kinetics properties of hydrogen adsorbing/desorbing reactions for metal hydride electrodes. Int. J. Hydrogen Energ. 2018, 43, 22427–22437. [Google Scholar] [CrossRef]




| Alloy | Lattice Parameters, Å | Cell Volume, Å3 | Atomic Radius Factor (According to [18]) | |
|---|---|---|---|---|
| a | c | |||
| LaNi5 | 4.982 | 4.008 | 86.14 | 870 |
| LaNi4.8Sn0.2 | 5.032 | 3.981 | 87.31 | 872 |
| LaNi4.7Sn0.3 | 5.042 | 4.039 | 88.91 | 873 |
| LaNi4.3Co0.4Al0.3 | 5.018 | 4.019 | 87.65 | 867 |
| Alloy | LaNi5 | LaNi4.8Sn0.2 | LaNi4.7Sn0.3 | LaNi4.3Co0.4Al0.3 |
| DH (cm2/s) | 2.8 × 10−11 | 8.8 × 10−11 | 1.1 × 10−10 | 3.3 × 10−10 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Todorova, S.; Abrashev, B.; Rangelova, V.; Mihaylov, L.; Vassileva, E.; Petrov, K.; Spassov, T. Hydrogen Gas Phase and Electrochemical Hydriding of LaNi5−xMx (M = Sn, Co, Al) Alloys. Materials 2021, 14, 14. https://doi.org/10.3390/ma14010014
Todorova S, Abrashev B, Rangelova V, Mihaylov L, Vassileva E, Petrov K, Spassov T. Hydrogen Gas Phase and Electrochemical Hydriding of LaNi5−xMx (M = Sn, Co, Al) Alloys. Materials. 2021; 14(1):14. https://doi.org/10.3390/ma14010014
Chicago/Turabian StyleTodorova, Stanislava, Borislav Abrashev, Vesselina Rangelova, Lyuben Mihaylov, Evelina Vassileva, Konstantin Petrov, and Tony Spassov. 2021. "Hydrogen Gas Phase and Electrochemical Hydriding of LaNi5−xMx (M = Sn, Co, Al) Alloys" Materials 14, no. 1: 14. https://doi.org/10.3390/ma14010014





