Straw-Based Activated Carbon: Optimization of the Preparation Procedure and Performance of Volatile Organic Compounds Adsorption
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of SACs
2.3. SACs Characterization
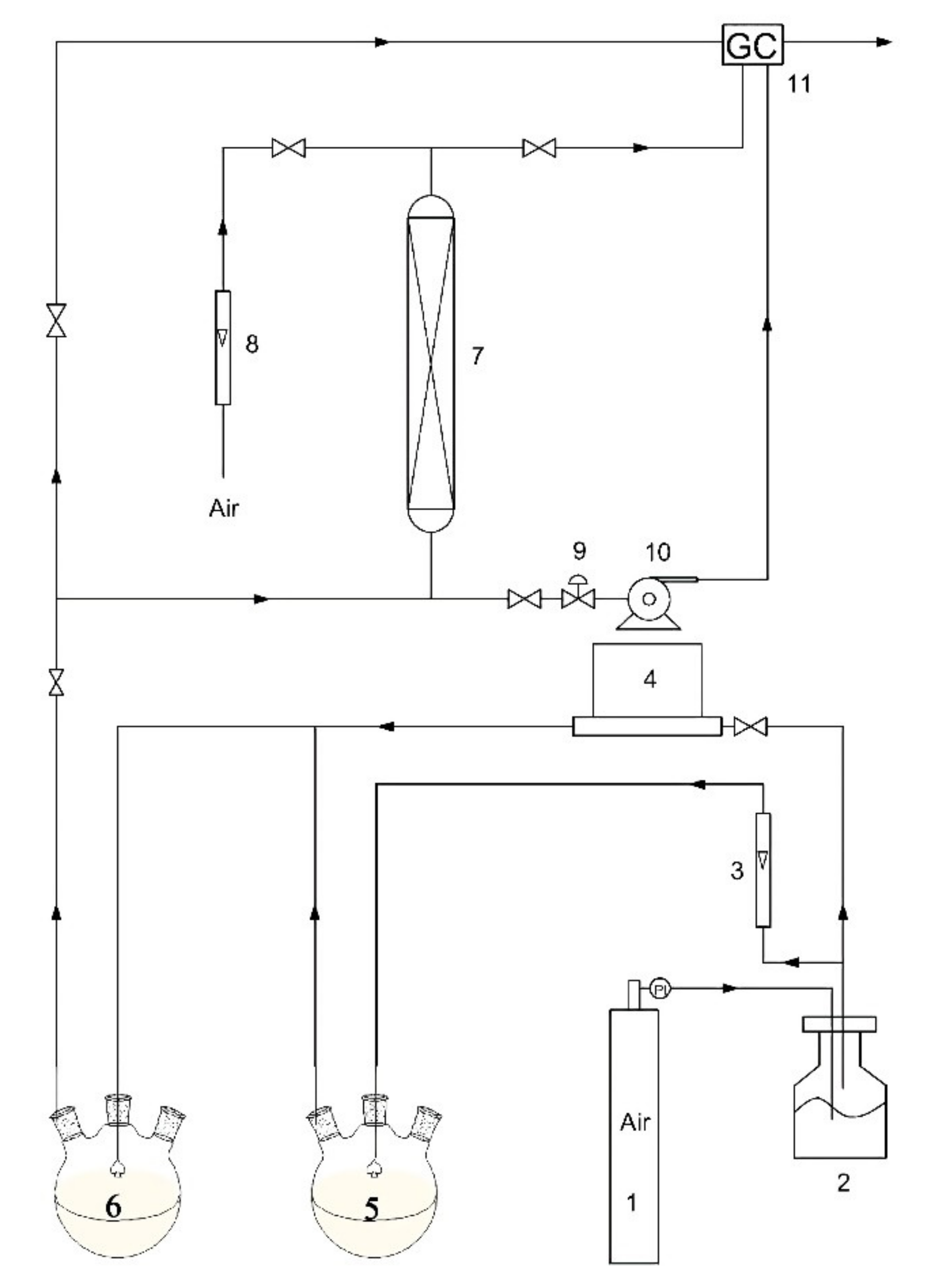
2.4. Adsorption and Regeneration Evaluation
2.5. Optimization of the Preparation Conditions for MSAC via RSM
3. Results
3.1. Pyrolysis Behavior of Different Straws
3.2. Characterization of SACs
3.3. Adsorption Capacity Study
3.4. RSM Optimization of MSAC Preparation
3.4.1. Experimental Results of RSM
- (1)
- For toluene adsorption capacity, Y1, the significant terms are the linear terms of A and C, and quadratic terms of A2 and C2. The effects of A and C are positive, while that of A2 and C2 are negative. A and A2 have the greatest impact on Y1. B has little influence on Y1. This means that the carbonization temperature, A, is the most important factor and is optimal for toluene adsorption capacity, Y1.
- (2)
- For the adsorption capacity of ethyl acetate, Y2, the most significant terms are the linear terms of A and C and quadratic terms of A2 and C2, which are similar to Y1. Notably, C2 has a much greater effect on Y2 than on Y1, which means the impregnation ratio, C, needs more attention for ethyl acetate adsorption during the optimization. All the interaction terms have little influence on Y2.
- (3)
- For the yield of SAC, Y3, the carbonization temperature, A, is the only significant term that has a negative impact on Y3. This means the yield of SAC decreases with increasing carbonization temperature.
3.4.2. RSM Analysis
3.4.3. Parameter Optimization and Verification
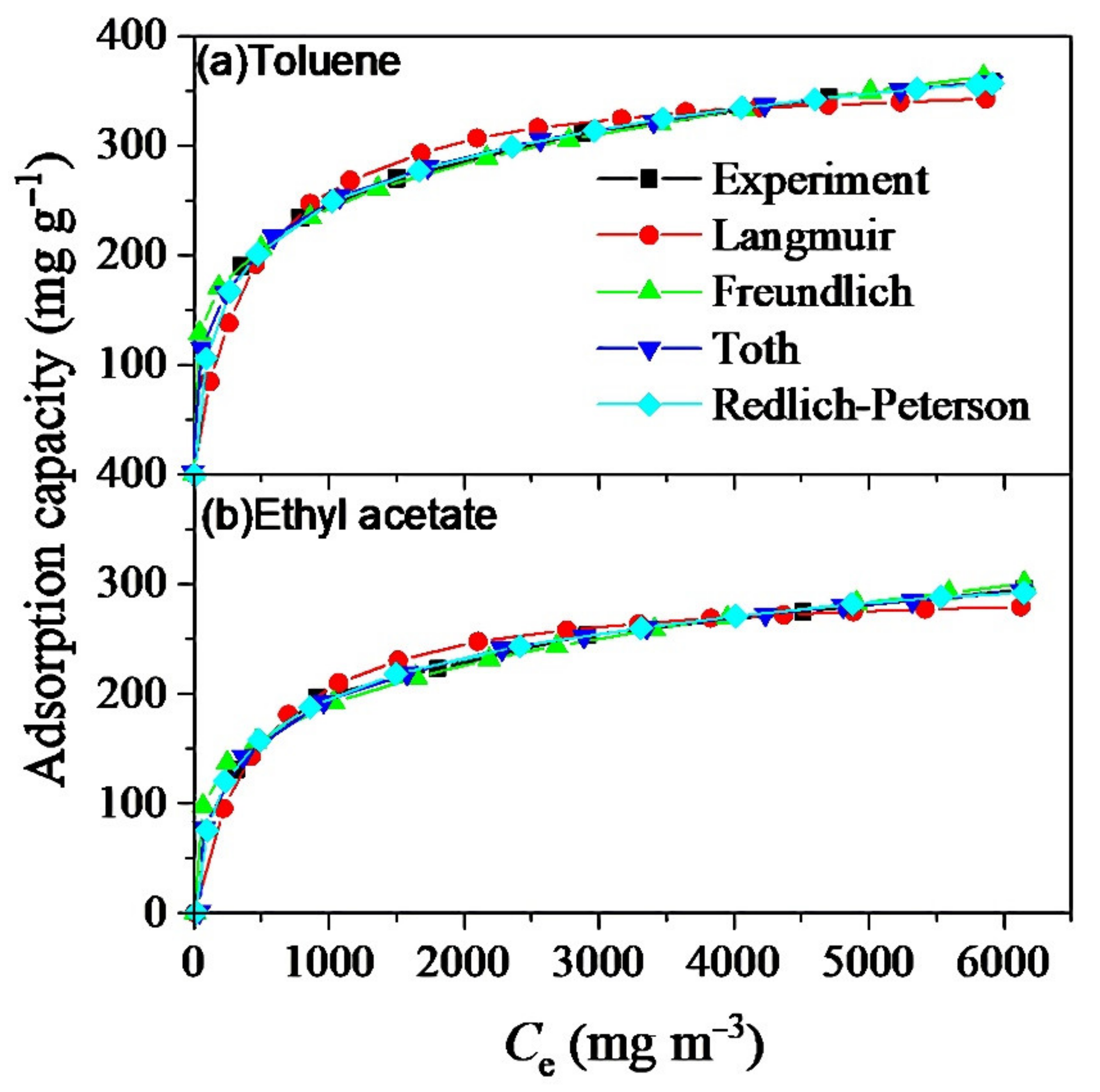
3.5. Adsorption Isotherms of Toluene and Ethyl Acetate
3.5.1. Adsorption Isotherms of Toluene and Ethyl Acetate on MSAC
3.5.2. Regeneration of MSAC
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Nham, N.T.; Tahtamouni, T.M.A.; Nguyen, T.D.; Huong, P.T.; Jitae, K.; Viet, N.M.; Noi, N.V.; Phuong, N.M.; Ahn, N.T.H. Synthesis of iron modified rice straw biochar toward arsenic from groundwater. Mater. Res. Express 2019, 6, 115528. [Google Scholar] [CrossRef]
- Zhang, S.C.; Abdalla, M.A.S.; Luo, Z.J.; Xia, S.B. The wheat straw biochar research on the adsorption/desorption behaviour of mercury in wastewater. Desalination Water Treat. 2018, 112, 147–160. [Google Scholar] [CrossRef] [Green Version]
- Mao, H.; Zhou, D.; Hashisho, Z.; Wang, S.; Chen, H.; Wang, H. Prediction of VOCs Adsorptive Isotherms in Wheat Straw Activated Carbon Based on Dubinin-Radushkevich Model. Bull. Sci. Technol. 2014, 30, 229–231. [Google Scholar]
- Grand View Research. Activated Carbon Market Size, Share & Trends Analysis Report By Product (Powdered, Granular), By Application (Liquid, Gas), By End Use (Water Treatment, Air Purification), By Region, And Segment Forecasts, 2019–2025; Grand View Research: San Francisco, CA, USA, 2019; 158p, ISBN 978-1-68038-073-6. [Google Scholar]
- Maheshwari, U.; Gupta, S. Performance evaluation of activated neem bark for the removal of Zn (II) and Cu (II) along with other metal ions from aqueous solution and synthetic pulp & paper industry effluent using fixed-bed reactor. Process Saf. Environ. Prot. 2016, 102, 547–557. [Google Scholar]
- Ren, Z.G.; Chen, F.; Wang, B.; Song, Z.X.; Zhou, Z.Y.; Ren, D. Magnetic biochar from alkali-activated rice straw for removal of rhodamine B from aqueous solution. Environ. Eng. Res. 2020, 25, 536–544. [Google Scholar] [CrossRef]
- Khalil, A.; Sergeevich, N.; Borisova, V. Removal of ammonium from fish farms by biochar obtained from rice straw: Isotherm and kinetic studies for ammonium adsorption. Adsorpt. Sci. Technol. 2018, 36, 1294–1309. [Google Scholar] [CrossRef] [Green Version]
- Balarak, D.; Bazrafshan, E.; Mahdavi, Y.; Lalhmunsiama; Lee, S.M. Kinetic, isotherms and thermodynamic studies in the removal of 2-chlorophenol from aqueous solution using modified rice straw. Desalination Water Treat. 2017, 63, 203–211. [Google Scholar] [CrossRef]
- Naeem, M.A.; Imran, M.; Amjad, M.; Abbas, G.; Tahir, M.; Murtaza, B.; Zakir, A.; Shahid, M.; Bulgariu, L.; Ahmad, I. Batch and Column Scale Removal of Cadmium from Water Using Raw and Acid Activated Wheat Straw Biochar. Water 2019, 11, 1438. [Google Scholar] [CrossRef] [Green Version]
- Vassileva, P.S.; Radoykova, T.H.; Detcheva, A.K.; Avramova, I.A.; Aleksieva, K.I.; Nenkova, S.K.; Valchev, I.V.; Mehandjiev, D.R. Adsorption of Ag+ ions on hydrolyzed lignocellulosic materials based on willow, paulownia, wheat straw and maize stalks. Int. J. Environ. Sci. Technol. 2016, 13, 1319–1328. [Google Scholar] [CrossRef] [Green Version]
- Park, J.H.; Ok, Y.S.; Kim, S.H.; Cho, J.S.; Heo, J.S.; Delaune, R.D.; Seo, D.C. Competitive adsorption of heavy metals onto sesame straw biochar in aqueous solutions. Chemosphere 2016, 142, 77–83. [Google Scholar] [CrossRef] [PubMed]
- Ma, H.F.; Yang, J.J.; Gao, X.; Liu, Z.B.; Liu, X.X.; Xu, Z.G. Removal of chromium (VI) from water by porous carbon derived from corn straw: Influencing factors, regeneration and mechanism. J. Hazard. Mater. 2019, 369, 550–560. [Google Scholar] [CrossRef] [PubMed]
- Zhao, N.; Yin, Z.; Liu, F.; Zhang, M.Y.; Lv, Y.Z.; Hao, Z.P.; Pan, G.; Zhang, J. Environmentally persistent free radicals mediated removal of Cr(VI) from highly saline water by corn straw biochars. Bioresour. Technol. 2018, 260, 294–301. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jiang, W.; Xing, X.J.; Li, S.; Zhang, X.W.; Wang, W.Q. Synthesis, characterization and machine learning based performance prediction of straw activated carbon. J. Clean. Prod. 2019, 212, 1210–1223. [Google Scholar] [CrossRef]
- Yang, F.; Sun, L.; Zhang, W.; Zhang, Y. One-pot synthesis of porous carbon foam derived from corn straw: Atrazine adsorption equilibrium and kinetics. Environ. Sci. Nano 2017, 4, 625–635. [Google Scholar] [CrossRef]
- Li, G.T.; Zhu, W.Y.; Zhang, C.Y.; Zhang, S.; Liu, L.L.; Zhu, L.F.; Zhao, W.G. Effect of a magnetic field on the adsorptive removal of methylene blue onto wheat straw biochar. Bioresour. Technol. 2016, 206, 16–22. [Google Scholar] [CrossRef] [Green Version]
- Yang, Q.L.; Wu, P.X.; Liu, J.; Rehman, S.; Ahmed, Z.; Ruan, B.; Zhu, N.W. Batch interaction of emerging tetracycline contaminant with novel phosphoric acid activated corn straw porous carbon: Adsorption rate and nature of mechanism. Environ. Res. 2020, 181, 108899. [Google Scholar] [CrossRef] [PubMed]
- Yin, Y.Y.; Guo, X.Y.; Peng, D. Iron and manganese oxides modified maize straw to remove tylosin from aqueous solutions. Chemosphere 2018, 205, 156–165. [Google Scholar] [CrossRef]
- Guo, X.T.; Yin, Y.Y.; Yang, C.; Dang, Z. Maize straw decorated with sulfide for tylosin removal from the water. Ecotoxicol. Environ. Saf. 2018, 152, 16–23. [Google Scholar] [CrossRef] [PubMed]
- Atkinson, R. Atmospheric chemistry of VOCs and NOx. Atmos. Environ. 2000, 34, 2063–2101. [Google Scholar] [CrossRef]
- Zhang, X.; Gao, B.; Creamer, A.E.; Cao, C.; Li, Y. Adsorption of VOCs onto engineered carbon materials: A review. J. Hazard. Mater. 2017, 338, 102–123. [Google Scholar] [CrossRef]
- Pui, W.K.; Yusoff, R.; Aroua, M.K. A review on activated carbon adsorption for volatile organic compounds (VOCs). Rev. Chem. Eng. 2019, 35, 649–668. [Google Scholar] [CrossRef]
- Mohan, N.; Kannan, G.; Upendra, S.; Subha, R.; Kumar, N. Breakthrough of toluene vapours in granular activated carbon filled packed bed reactor. J. Hazard. Mater. 2009, 168, 777–781. [Google Scholar] [CrossRef] [PubMed]
- Karimnezhad, L.; Haghighi, M.; Fatehifar, E. Adsorption of benzene and toluene from waste gas using activated carbon activated by ZnCl 2. Front. Environ. Sci. Eng. 2014, 8, 835–844. [Google Scholar] [CrossRef]
- Bedane, A.H.; Guo, T.x.; Eić, M.; Xiao, H. Adsorption of volatile organic compounds on peanut shell activated carbon. Can. J. Chem. Eng. 2019, 97, 238–246. [Google Scholar] [CrossRef] [Green Version]
- Gao, L.; Li, C.; Zhang, J.; Du, X.; Li, S.; Zeng, J.; Yi, Y.; Zeng, G. Simultaneous removal of NO and Hg0 from simulated flue gas over CoOx-CeO2 loaded biomass activated carbon derived from maize straw at low temperatures. Chem. Eng. J. 2018, 342, 339–349. [Google Scholar] [CrossRef]
- Schaefer, S.; Muniz, G.; Izquierdo, M.T.; Mathieu, S.; Ballinas-Casarrubias, M.L.; Gonzalez-Sanchez, G.; Celzard, A.; Fierro, V. Rice straw-based activated carbons doped with SiC for enhanced hydrogen adsorption. Int. J. Hydrog. Energy 2017, 42, 11534–11540. [Google Scholar] [CrossRef] [Green Version]
- Huang, Y.F.; Chiueh, P.T.; Shih, C.H.; Lo, S.L.; Sun, L.P.; Zhong, Y.; Qiu, C.S. Microwave pyrolysis of rice straw to produce biochar as an adsorbent for CO2 capture. Energy 2015, 84, 75–82. [Google Scholar] [CrossRef]
- Shen, Y.; Zhang, N. Facile synthesis of porous carbons from silica-rich rice husk char for volatile organic compounds (VOCs) sorption. Bioresour. Technol. 2019, 282, 294–300. [Google Scholar] [CrossRef]
- Díaz-Muñoz, L.L.; Bonilla-Petriciolet, A.; Reynel-Ávila, H.E.; Mendoza-Castillo, D.I. Sorption of heavy metal ions from aqueous solution using acid-treated avocado kernel seeds and its FTIR spectroscopy characterization. J. Mol. Liq. 2016, 215, 555–564. [Google Scholar] [CrossRef]
- Vivo-Vilches, J.F.; Bailón-García, E.; Pérez-Cadenas, A.F.; Carrasco-Marín, F.; Maldonado-Hódar, F.J. Tailoring the surface chemistry and porosity of activated carbons: Evidence of reorganization and mobility of oxygenated surface groups. Carbon 2014, 68, 520–530. [Google Scholar] [CrossRef]
- Zhu, J.; Li, Y.; Xu, L.; Liu, Z. Removal of toluene from waste gas by adsorption-desorption process using corncob-based activated carbons as adsorbents. Ecotoxicol. Environ. Saf. 2018, 165, 115–125. [Google Scholar] [CrossRef]
- Bezerra, M.A.; Santelli, R.E.; Oliveira, E.P.; Villar, L.S.; Escaleira, L.A. Response surface methodology (RSM) as a tool for optimization in analytical chemistry. Talanta 2008, 76, 965–977. [Google Scholar] [CrossRef] [PubMed]
- Ahmadpour, A.; Do, D.D. The preparation of active carbons from coal by chemical and physical activation. Carbon 1996, 34, 471–479. [Google Scholar] [CrossRef]
- Pezoti, O.; Cazetta, A.L.; Souza, I.; Bedin, K.C.; Martins, A.C.; Silva, T.L.; Almeida, V.C. Adsorption studies of methylene blue onto ZnCl2-activated carbon produced from buriti shells (Mauritia flexuosa L.). J. Ind. Eng. Chem. 2014, 20, 4401–4407. [Google Scholar] [CrossRef]
- Prauchner, M.J.; Sapag, K.; Rodríguez-Reinoso, F. Tailoring biomass-based activated carbon for CH4 storage by combining chemical activation with H3PO4 or ZnCl2 and physical activation with CO2. Carbon 2016, 110, 138–147. [Google Scholar] [CrossRef]
- Blacher, S.; Sahouli, B.; Heinrichs, B.; Lodewyckx, P.; Pirard, R.; Pirard, J.P. Micropore size distributions of activated carbons. Langmuir 2000, 16, 6754–6756. [Google Scholar] [CrossRef]
- Scherdel, C.; Reichenauer, G.; Wiener, M. Relationship between pore volumes and surface areas derived from the evaluation of N 2-sorption data by DR, BET and t-plot. Microporous Mesoporous Mater. 2010, 132, 572–575. [Google Scholar] [CrossRef]
- Song, T.; Liao, J.; Xiao, J.; Shen, L.H. Effect of micropore and mesopore structure on CO2 adsorption by activated carbons from biomass. New Carbon Mater. 2015, 30, 156–166. [Google Scholar] [CrossRef]
- Köseoğlu, E.; Akmil-Başar, C. Preparation, structural evaluation and adsorptive properties of activated carbon from agricultural waste biomass. Adv. Powder Technol. 2015, 26, 811–818. [Google Scholar] [CrossRef]
- Sayğılı, H.; Güzel, F. High surface area mesoporous activated carbon from tomato processing solid waste by zinc chloride activation: Process optimization, characterization and dyes adsorption. J. Clean. Prod. 2016, 113, 995–1004. [Google Scholar] [CrossRef]
- Luo, R.; Kong, L.; Tian, S.; He, C.; Huang, H.; Xiong, Y. Preparation and characterization of a hierarchical porous char from sewage sludge with superior adsorption capacity for toluene by a new two-step pore-fabricating process. Bioresour. Technol. 2013, 146, 457–462. [Google Scholar]
- Brunauer, S.; Deming, L.S.; Deming, W.E.; Teller, E. On a Theory of the van der Waals Adsorption of Gases. J. Am. Chem. Soc. 1940, 62, 1723–1732. [Google Scholar] [CrossRef]
- Gupta, V.K.; Agarwal, S.; Asif, M.; Fakhri, A.; Sadeghi, N. Application of response surface methodology to optimize the adsorption performance of a magnetic graphene oxide nanocomposite adsorbent for removal of methadone from the environment. J. Colloid Interface Sci. 2017, 497, 193–200. [Google Scholar] [CrossRef] [PubMed]
- Nahm, S.W.; Wang, G.S.; Park, Y.K.; Sang, C.K. Thermal and chemical regeneration of spent activated carbon and its adsorption property for toluene. Chem. Eng. J. 2012, 210, 500–509. [Google Scholar] [CrossRef]
- Sui, H.; Liu, H.; An, P.; He, L.; Li, X.; Cong, S. Application of silica gel in removing high concentrations toluene vapor by adsorption and desorption process. J. Taiwan Inst. Chem. Eng. 2017, 74, 218–224. [Google Scholar] [CrossRef]




| Sample | Specific Surface Area (m2 g−1) | Pore Volume (cm3 g−1) | Saturation Adsorption Capacity (mg/g) | |||||
|---|---|---|---|---|---|---|---|---|
| SBET | Smic | Sext | Vt | Vmic | Vmes | Toluene | Ethyl Acetate | |
| CMSAC | 1474 | 1336 | 137.5 | 0.765 | 0.551 | 0.215 | 369.5 ± 7.2 | 217.4 ± 10.2 |
| PMSAC | 1733 | 1632 | 100.4 | 0.815 | 0.690 | 0.125 | 419.5 ± 3.8 | 297.5 ± 3.9 |
| MSAC | 1515 | 1413 | 102.0 | 0.731 | 0.581 | 0.150 | 376.1 ± 11.7 | 260.7 ± 8.4 |
| CSAC | 1390 | 1302 | 88.04 | 0.622 | 0.541 | 0.122 | 364.6 ± 9.0 | 250.0 ± 13.5 |
| PSAC | 1378 | 1265 | 112.5 | 0.708 | 0.518 | 0.190 | 374.2 ± 13.8 | 252.8 ± 6.7 |
| No. | Variables | Experimental Value | ||||
|---|---|---|---|---|---|---|
| A | B | C | Y1 (mg/g) | Y2 (mg/g) | Y3 (%) | |
| 1 | 0 | 0 | 0 | 372.9 | 257.8 | 35.1 |
| 2 | −1 | 1 | 1 | 284.7 | 189.4 | 39.4 |
| 3 | −1 | 1 | −1 | 269.1 | 147.9 | 39.4 |
| 4 | 0 | 0 | 0 | 376.3 | 257.2 | 36.8 |
| 5 | 0 | 0 | 1.68 | 361.5 | 201.8 | 38.9 |
| 6 | −1 | −1 | 1 | 301.1 | 162.5 | 40.4 |
| 7 | 0 | 0 | 0 | 373.7 | 262.9 | 37.3 |
| 8 | 0 | 0 | 0 | 369.6 | 255.4 | 36 |
| 9 | 0 | −1.68 | 0 | 365.9 | 210.6 | 37 |
| 10 | 1 | 1 | −1 | 339.2 | 198.3 | 36.5 |
| 11 | 0 | 1.68 | 0 | 357.7 | 240.9 | 35.9 |
| 12 | −1.68 | 0 | 0 | 203.5 | 120.8 | 44.3 |
| 13 | −1 | −1 | −1 | 247.1 | 148.2 | 40.4 |
| 14 | 1.68 | 0 | 0 | 321.2 | 188.3 | 34.2 |
| 15 | 1 | −1 | 1 | 349.4 | 236.1 | 34.7 |
| 16 | 1 | −1 | −1 | 342.3 | 186.8 | 36.5 |
| 17 | 0 | 0 | −1.68 | 314.9 | 166.4 | 38.6 |
| 18 | 0 | 0 | 0 | 371.6 | 270.1 | 36.5 |
| 19 | 0 | 0 | 0 | 368.9 | 263.7 | 37.1 |
| 20 | 1 | 1 | 1 | 359.3 | 213.6 | 34.7 |
| Adsorption Isotherm | Constants | Materials | |
|---|---|---|---|
| Toluene | Ethyl Acetate | ||
| Langmuir | 373.7 | 304.0 | |
| 0.0022 | 0.0021 | ||
| R2 | 0.9899 | 0.9890 | |
| Freundlich | 53.18 | 39.83 | |
| n | 0.2245 | 0.2295 | |
| R2 | 0.9989 | 0.9971 | |
| Sips | 647.8 | 511.2 | |
| 0.0496 | 0.0352 | ||
| n | 0.3962 | 0.4181 | |
| R2 | 0.9998 | 0.9982 | |
| Toth | f | 872.8 | 660.3 |
| g | 1.512 | 1.898 | |
| d | 0.2241 | 0.2458 | |
| R2 | 0.9997 | 0.9983 | |
| Redlich–Peterson | 2.8835 | 1.913 | |
| 0.0342 | 0.028 | ||
| 0.8293 | 0.8302 | ||
| R2 | 0.9999 | 0.9985 | |
| Flow Rate of Purge Gas (L/min) | Desorption Pressure (kPa) | |||||
|---|---|---|---|---|---|---|
| 11 | 21 | 34 | ||||
| Toluene | Ethyl Acetate | Toluene | Ethyl Acetate | Toluene | Ethyl Acetate | |
| 0.2 | 74 ± 2 | 75 ± 5 | 71 ± 3 | 73 ± 2 | 66 ± 2 | 70 ± 1 |
| 0.6 | 77 ± 3 | 81 ± 3 | 76 ± 4 | 80 ± 2 | 76 ± 2 | 77 ± 1 |
| 1.0 | 78 ± 1 | 82 ± 2 | 78 ± 2 | 82 ± 1 | 78 ± 1 | 81 ± 3 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, Z.; Li, Y.; Zhu, J. Straw-Based Activated Carbon: Optimization of the Preparation Procedure and Performance of Volatile Organic Compounds Adsorption. Materials 2021, 14, 3284. https://doi.org/10.3390/ma14123284
Li Z, Li Y, Zhu J. Straw-Based Activated Carbon: Optimization of the Preparation Procedure and Performance of Volatile Organic Compounds Adsorption. Materials. 2021; 14(12):3284. https://doi.org/10.3390/ma14123284
Chicago/Turabian StyleLi, Zhen, Yonghong Li, and Jiang Zhu. 2021. "Straw-Based Activated Carbon: Optimization of the Preparation Procedure and Performance of Volatile Organic Compounds Adsorption" Materials 14, no. 12: 3284. https://doi.org/10.3390/ma14123284






