Effect of Processing Conditions on the Flash Onset Temperature in Hydroxyapatite
Abstract
:1. Introduction
2. Materials and Methods
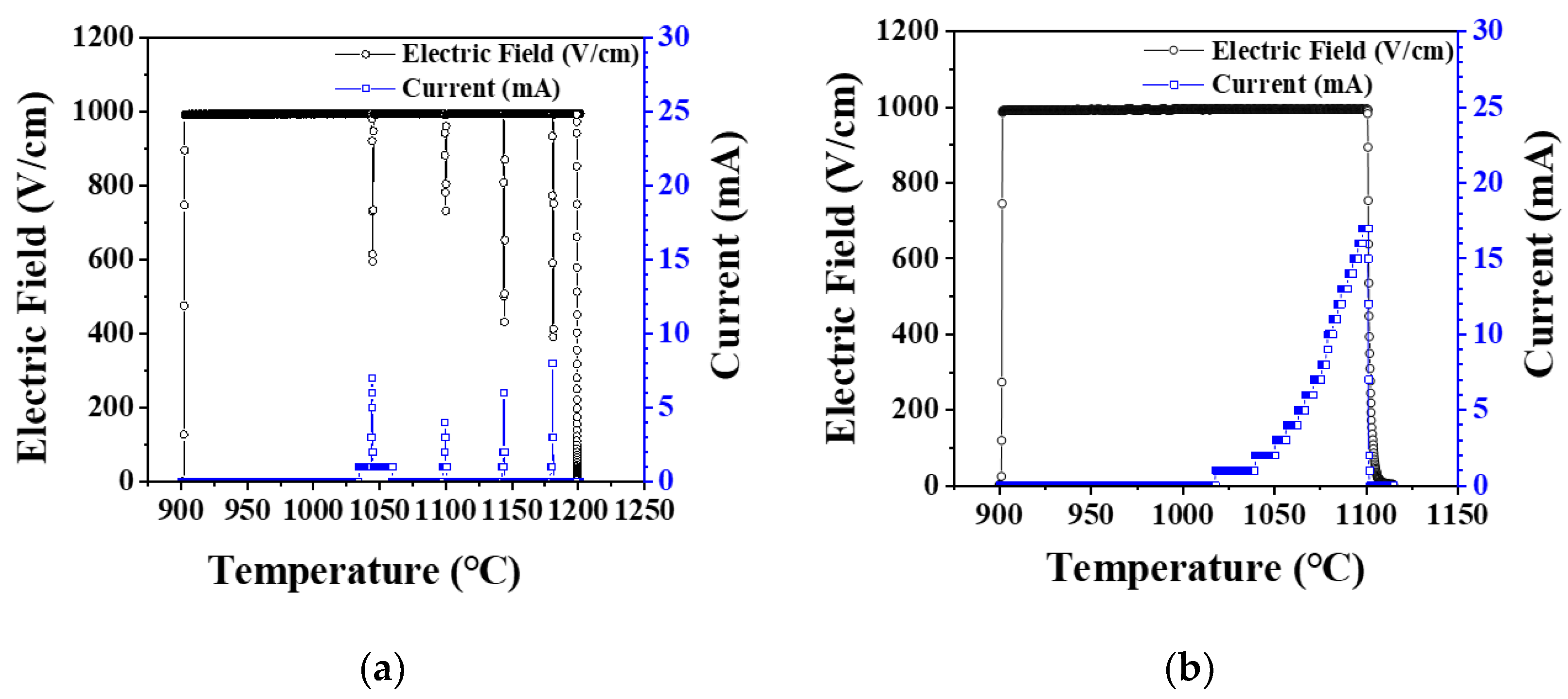
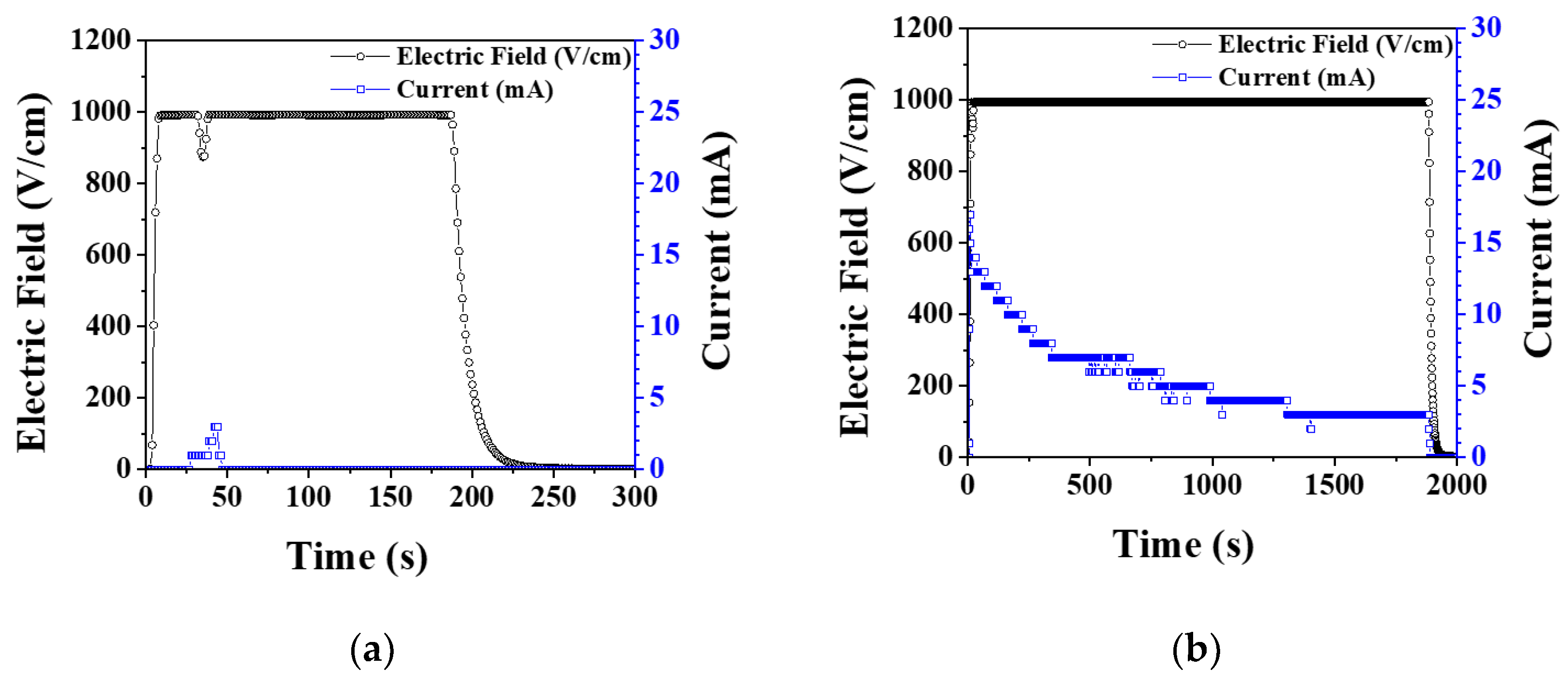
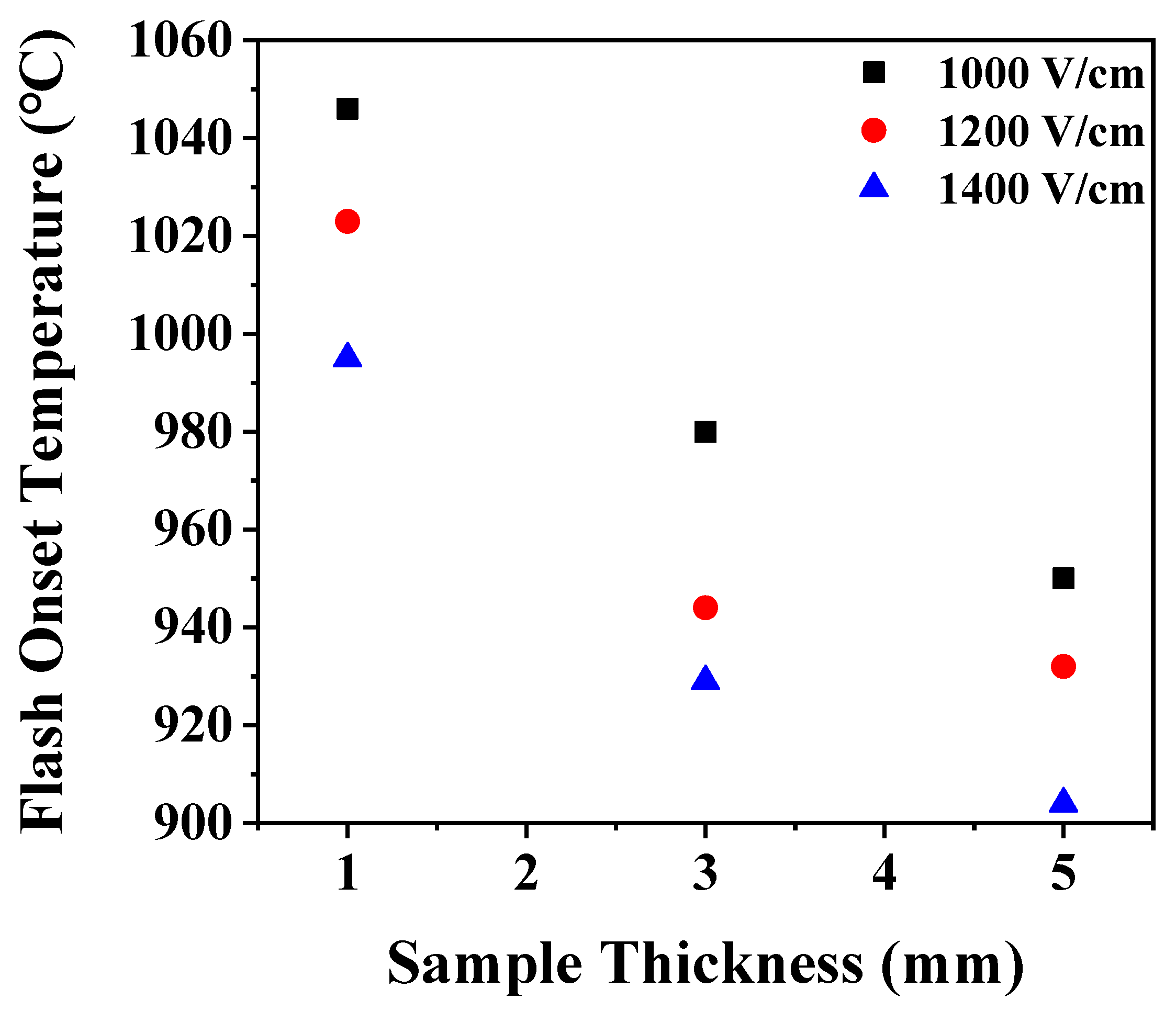
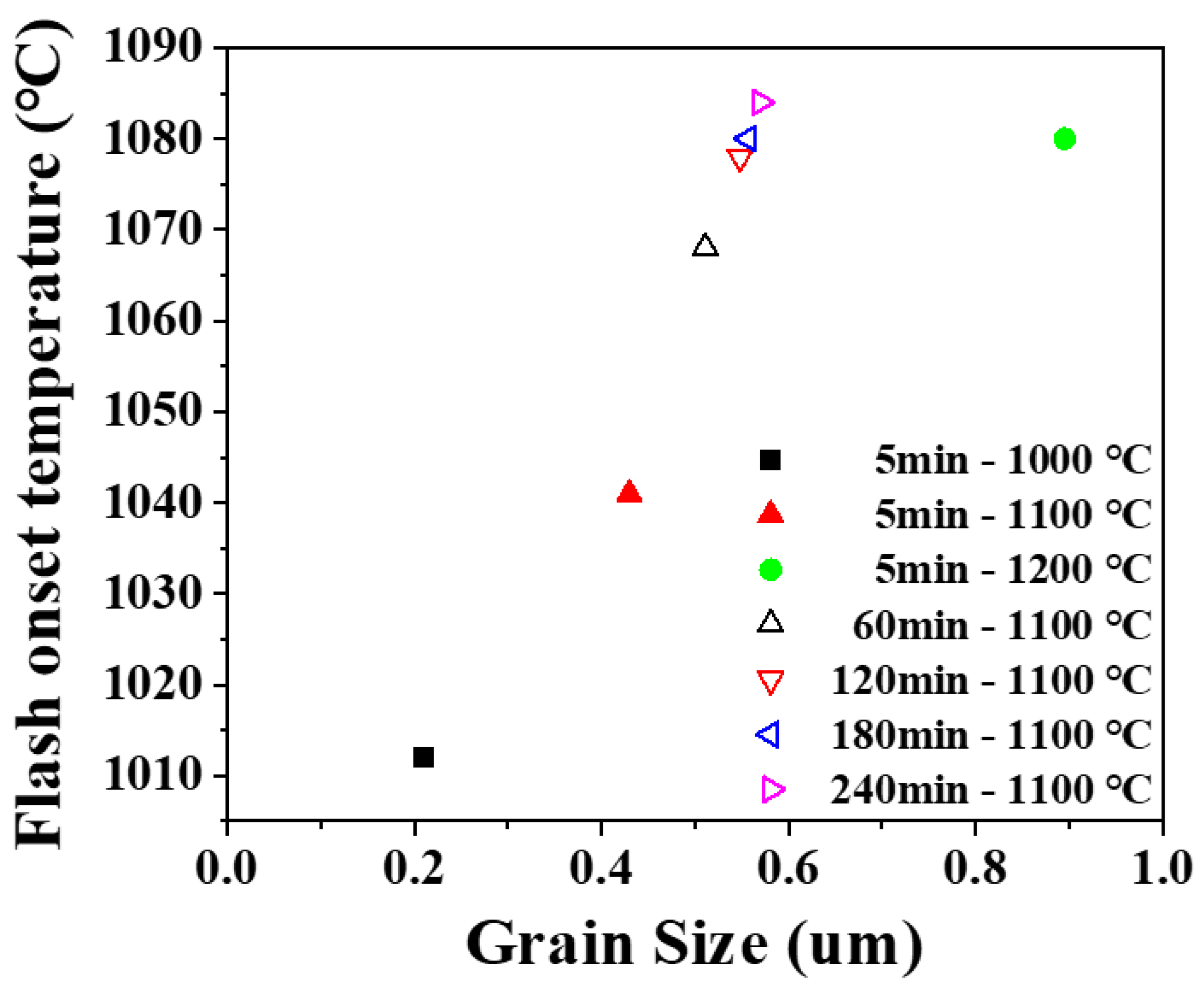
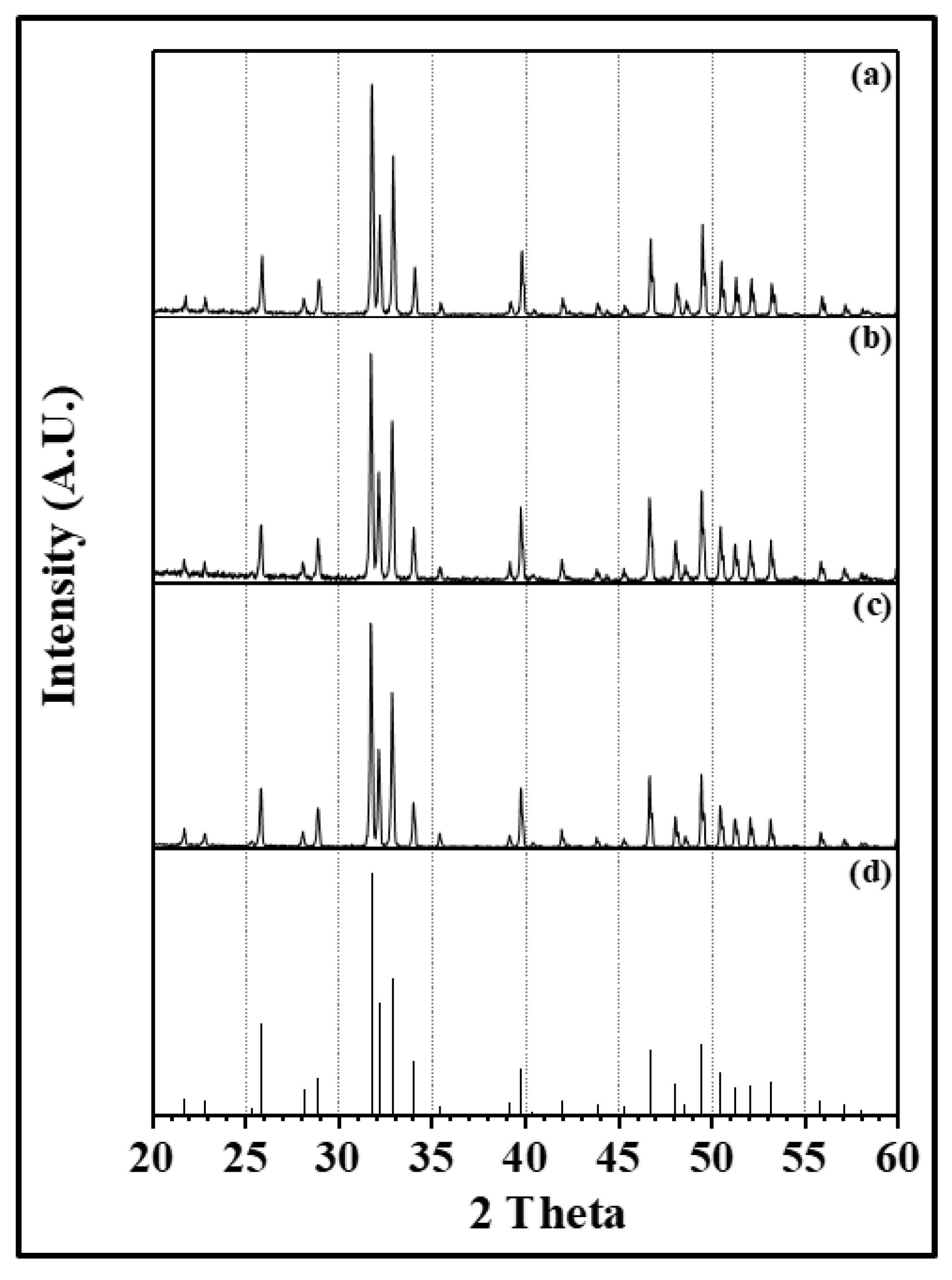
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Olevsky, E.A.; Dudina, D.V. Microwave Sintering. In Field-Assisted Sintering: Science and Applications; Springer International Publishing: Berlin, Germany, 2018; pp. 1–24. ISBN 978-3-319-76031-5. [Google Scholar]
- Yu, M.; Grasso, S.; Mckinnon, R.; Saunders, T.; Reece, M.J. Review of flash sintering: Materials, mechanisms and modelling. Adv. Appl. Ceram. 2017, 116, 24–60. [Google Scholar] [CrossRef] [Green Version]
- Dancer, C.E.J. Flash sintering of ceramic materials. Mater. Res. Express 2016, 3, 102001. [Google Scholar] [CrossRef] [Green Version]
- Cologna, M.; Rashkova, B.; Raj, R. Flash Sintering of Nanograin Zirconia in <5 s at 850 °C. J. Am. Ceram. Soc. 2010, 93, 3559. [Google Scholar] [CrossRef]
- Francis, J.S.; Raj, R. Flash-Sinterforging of Nanograin Zirconia: Field Assisted Sintering and Superplasticity. J. Am. Ceram. Soc. 2012, 95, 138–146. [Google Scholar] [CrossRef]
- Downs, J.A.; Sglavo, V.M. Electric Field Assisted Sintering of Cubic Zirconia at 390 °C. J. Am. Ceram. Soc. 2013, 96, 1342–1344. [Google Scholar] [CrossRef]
- Qin, W.; Majidi, H.; Yun, J.; Benthem, K.V. Electrode effects on microstructure formation during FLASH sintering of yt-trium-stabilized zirconia. J. Am. Ceram. Soc. 2016, 99, 2253–2259. [Google Scholar] [CrossRef]
- Yoshida, H.; Morita, K.; Kim, B.-N.; Sakka, Y.; Yamamoto, T. Reduction in sintering temperature for flash-sintering of yttria by nickel cation-doping. Acta Mater. 2016, 106, 344–352. [Google Scholar] [CrossRef]
- Cologna, M.; Francis, J.S.C.; Raj, R. Field assisted and flash sintering of alumina and its relationship to conductivity and MgO-doping. J. Eur. Ceram. Soc. 2011, 31, 2827–2837. [Google Scholar] [CrossRef]
- Biesuz, M.; Luchi, P.; Quaranta, A.; Sglavo, V.M. Theoretical and phenomenological analogies between flash sintering and dielectric breakdown in α-alumina. J. Appl. Phys. 2016, 120, 145107. [Google Scholar] [CrossRef]
- Francis, J.S.C.; Cologna, M.; Montinaro, D.; Raj, R. Flash Sintering of Anode-Electrolyte Multilayers for SOFC Applications. J. Am. Ceram. Soc. 2013, 96, 1352–1354. [Google Scholar] [CrossRef]
- Zhang, J.; Wang, Z.; Jiang, T.; Xie, L.; Sui, C.; Ren, R.; Qiao, J.; Sun, K. Densification of 8 mol% yttria-stabilized zirconia at low temperature by flash sintering technique for solid oxide fuel cells. Ceram. Int. 2017, 43, 14037–14043. [Google Scholar] [CrossRef]
- Shi, R.; Pu, Y.; Wang, W.; Shi, Y.; Li, J.; Guo, X.; Yang, M. Flash sintering of barium titanate. Ceram. Int. 2019, 45, 7085–7089. [Google Scholar] [CrossRef]
- Yun, J.; Qin, W.; Van Benthem, K.; Thron, A.M.; Kim, S.; Han, Y.-H. Nanovoids in dense hydroxyapatite ceramics after electric field assisted sintering. Adv. Appl. Ceram. 2018, 117, 376–382. [Google Scholar] [CrossRef]
- Zhang, Y.; Luo, J. Promoting the flash sintering of ZnO in reduced atmospheres to achieve nearly full densities at furnace temperatures of <120 °C. Scr. Mater. 2015, 106, 29. [Google Scholar] [CrossRef]
- Biesuz, M.; Pinter, L.; Saunders, T.; Reece, M.; Binner, J.; Sglavo, V.M.; Grasso, S. Investigation of Electrochemical, Optical and Thermal Effects during Flash Sintering of 8YSZ. Materials 2018, 11, 1214. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Francis, J.S.; Cologna, M.; Raj, R. Particle size effects in flash sintering. J. Eur. Ceram. Soc. 2012, 32, 3129–3136. [Google Scholar] [CrossRef]
- Avila, V.; Raj, R. Flash sintering of ceramic films: The influence of surface to volume ratio. J. Am. Ceram. Soc. 2019, 102, 3063–3069. [Google Scholar] [CrossRef]
- Muralithran, G.; Ramesh, S. The effects of sintering temperature on the properties of hydroxyapatite. Ceram. Int. 2000, 26, 221–230. [Google Scholar] [CrossRef]
- Champion, E. Sintering of calcium phosphate bioceramics. Acta Biomater. 2013, 9, 5855–5875. [Google Scholar] [CrossRef] [PubMed]
- Carrodeguas, R.G.; Aza, S.D. α-Tricalcium phosphate:synthesis, properties and biomedical applications. Acta Biomater. 2011, 7, 3536–3546. [Google Scholar] [CrossRef] [PubMed]
- Guo, X.; Xiao, P.; Liu, J.; Shen, Z. Fabrication of Nanostructured Hydroxyapatite via Hydrothermal Synthesis and Spark Plasma Sintering. J. Am. Ceram. Soc. 2005, 88, 1026–1029. [Google Scholar] [CrossRef]
- Eriksson, M.; Liu, Y.; Hu, J.; Gao, L.; Nygren, M.; Shen, Z. Transparent hydroxyapatite ceramics with nanograin structure prepared by high pressure spark plasma sintering at the minimized sintering temperature. J. Eur. Ceram. Soc. 2011, 31, 1533–1540. [Google Scholar] [CrossRef]
- Liu, Y.; Shen, Z. Dehydroxylation of hydroxyapatite in dense bulk ceramics sintered by spark plasma sintering. J. Eur. Ceram. Soc. 2012, 32, 2691–2696. [Google Scholar] [CrossRef]
- Bajpai, I.; Han, Y.-H.; Yun, J.; Francis, J.; Kim, S.; Raj, R. Preliminary investigation of hydroxyapatite microstructures prepared by flash sintering. Adv. Appl. Ceram. 2016, 115, 276–281. [Google Scholar] [CrossRef]
- Hwang, C.; Yun, J. Flash sintering of hydroxyapatite ceramics. J. Asian Ceram. Soc. 2021, 9, 304–311. [Google Scholar] [CrossRef]
- Takahashi, T.; Tanase, S.; Yamamoto, O. Electrical conductivity of some hydroxyapatites. Electrochim. Acta 1978, 23, 369–373. [Google Scholar] [CrossRef]
- Gittings, J.P.; Bowen, C.R.; Dent, A.C.E.; Turner, I.G.; Baxter, F.R.; Chaudhuri, J.B. Electrical characterization of hy-droxyapatite-based bioceramics. Acta Biomater. 2009, 5, 743–754. [Google Scholar] [CrossRef]
- Rajeswari, K.; Suresh, M.B.; Chakravarty, D.; Das, D.; Johnson, R. Effect of nano-grain size on the ionic conductivity of spark plasma sintered 8YSZ electrolyte. Int. J. Hydrogen Energy 2012, 37, 511–517. [Google Scholar] [CrossRef]
- Ruys, A.J.; Wei, M.; Sorrell, C.C.; Dickson, M.R.; Brandwood, A.; Milthorpe, B.K. Sintering effects on the strength of hy-droxyapatite. Biomaterials 1995, 16, 409–415. [Google Scholar] [CrossRef]
- Yamashita, K.; Kitagaki, K.; Umegaki, T. Thermal Instability and Proton Conductivity of Ceramic Hydroxyapatite at High Temperatures. J. Am. Ceram. Soc. 1995, 78, 1191–1197. [Google Scholar] [CrossRef]
- White, A.A.; Kinloch, I.A.; Windle, A.H.; Best, S.M. Optimization of the sintering atmosphere for high-density hydrox-yapatite carbon nanotube composites. J. R. Soc. Interface 2010, 7, 529–539. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ou, S.-F.; Chiou, S.-Y.; Ou, K.-L. Phase transformation on hydroxyapatite decomposition. Ceram. Int. 2013, 39, 3809–3816. [Google Scholar] [CrossRef]
- Yamashita, K.; Owada, H.; Nakagawa, H.; Umegaki, T.; Kanazawa, T. Trivalent-Cation-Substituted Calcium Oxyhydrox-yapatite. J. Am. Ceram. 1986, 69, 590–594. [Google Scholar] [CrossRef]
- Mistler, R.E.; Coble, R.L. Grain-boundary diffusion and boundary widths in metals and ceramics. J. Appl. Phys. 1974, 45, 1507–1509. [Google Scholar] [CrossRef]
- Kingery, W.D. Plausible Concepts Necessary and Sufficient for Interpretation of Ceramic Grain-Boundary Phenomena: II, Solute Segregation, Grain-Boundary Diffusion, and General Discussion. J. Am. Ceram. Soc. 1974, 57, 74–83. [Google Scholar] [CrossRef]
- Jaseliunaite, J.; Galdikas, A. Kinetic Modeling of Grain Boundary Diffusion: The Influence of Grain Size and Surface Pro-cesses. Materials 2020, 13, 1051. [Google Scholar] [CrossRef] [Green Version]
- Johnson, P.D.; Prener, J.S.; Kingsley, J.D. Apatite: Origin of Blue Color. Sci. 1963, 141, 1179–1180. [Google Scholar] [CrossRef]
- Kingsley, J.D.; Prener, J.S.; Segall, B. Spectroscopy of MnO43− in Calcium Halophosphates. Phys. Rev. 1965, 137, A189–A202. [Google Scholar] [CrossRef]
- Bystrov, V.; Piccirillo, C.; Tobaldi, D.M.; Castro, P.; Coutinho, J.; Kopyl, S.; Pullar, R. Oxygen vacancies, the optical band gap (Eg) and photocatalysis of hydroxyapatite: Comparing modelling with measured data. Appl. Catal. B Environ. 2016, 196, 100–107. [Google Scholar] [CrossRef]
- Yubao, L.; Klein, C.P.A.T.; Xingdong, Z.; de Groot, K. Relationship between the colour change of hydroxyapatite and the trace element manganese. Biomaterials 1993, 14, 969–972. [Google Scholar]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hwang, C.; Yun, J. Effect of Processing Conditions on the Flash Onset Temperature in Hydroxyapatite. Materials 2021, 14, 5229. https://doi.org/10.3390/ma14185229
Hwang C, Yun J. Effect of Processing Conditions on the Flash Onset Temperature in Hydroxyapatite. Materials. 2021; 14(18):5229. https://doi.org/10.3390/ma14185229
Chicago/Turabian StyleHwang, Changhun, and Jondo Yun. 2021. "Effect of Processing Conditions on the Flash Onset Temperature in Hydroxyapatite" Materials 14, no. 18: 5229. https://doi.org/10.3390/ma14185229





