Microstructure and Properties of Mg-Al-Ca-Mn Alloy with High Ca/Al Ratio Fabricated by Hot Extrusion
Abstract
:1. Introduction
2. Experimental Procedure
3. Results and Discussion
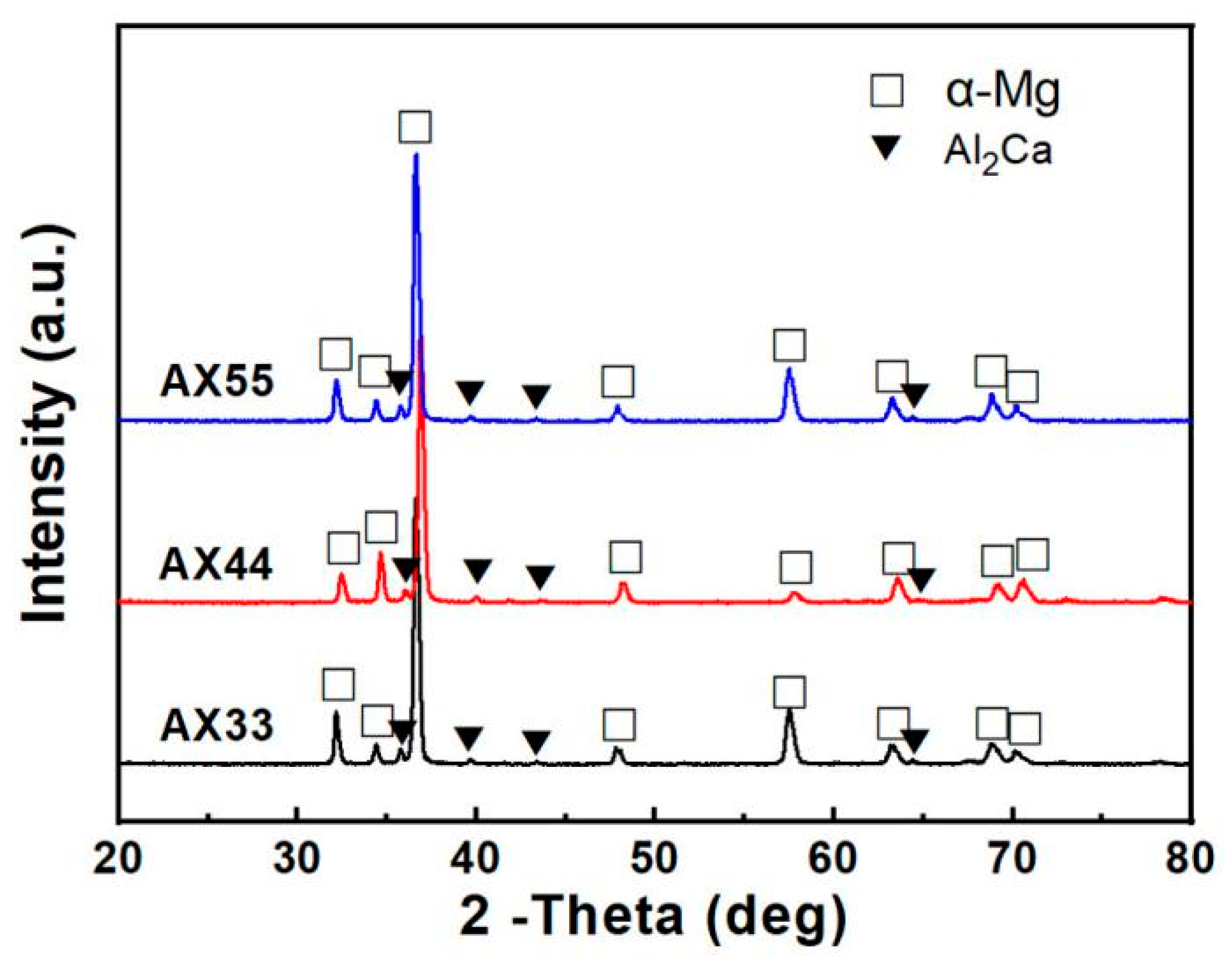
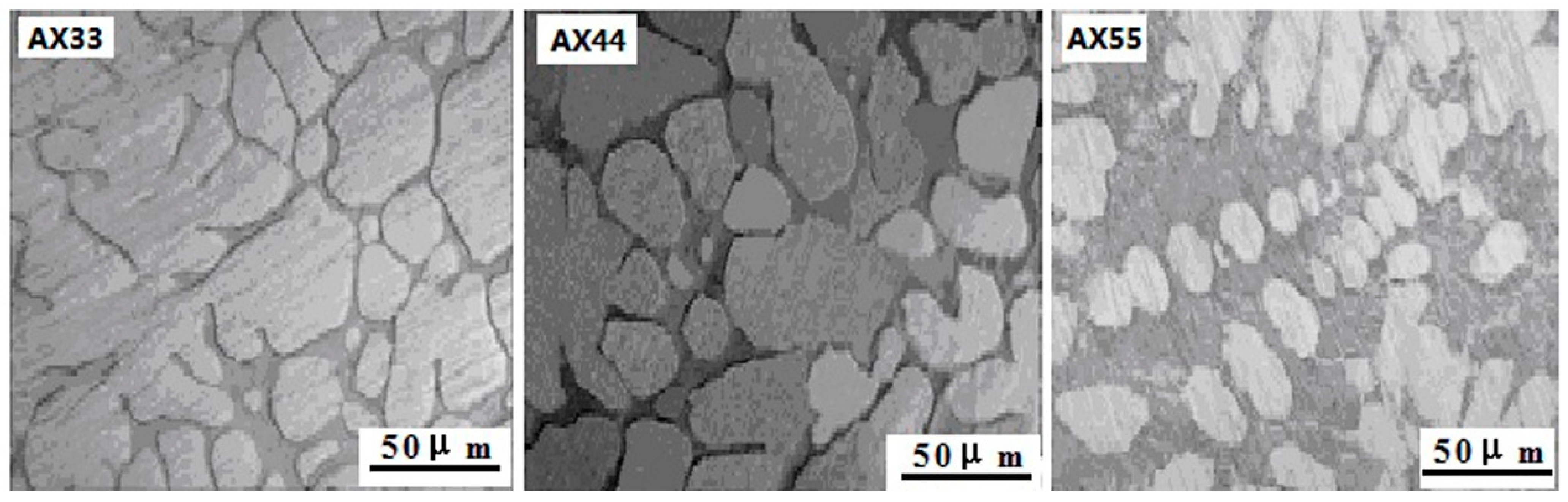
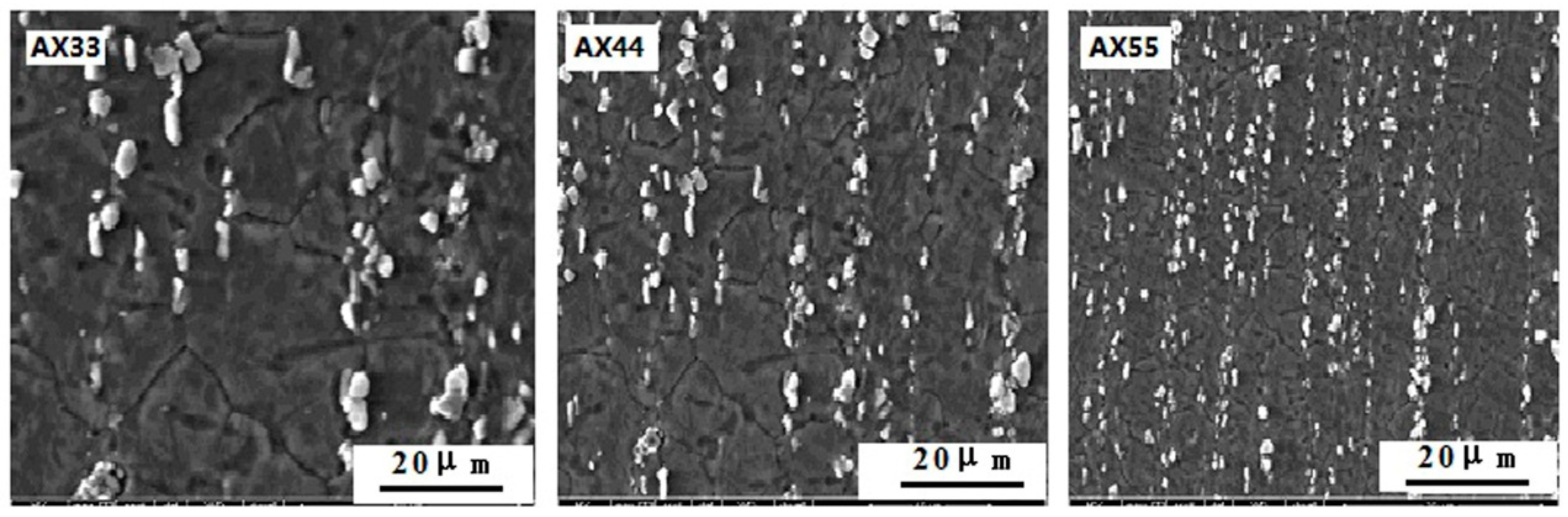
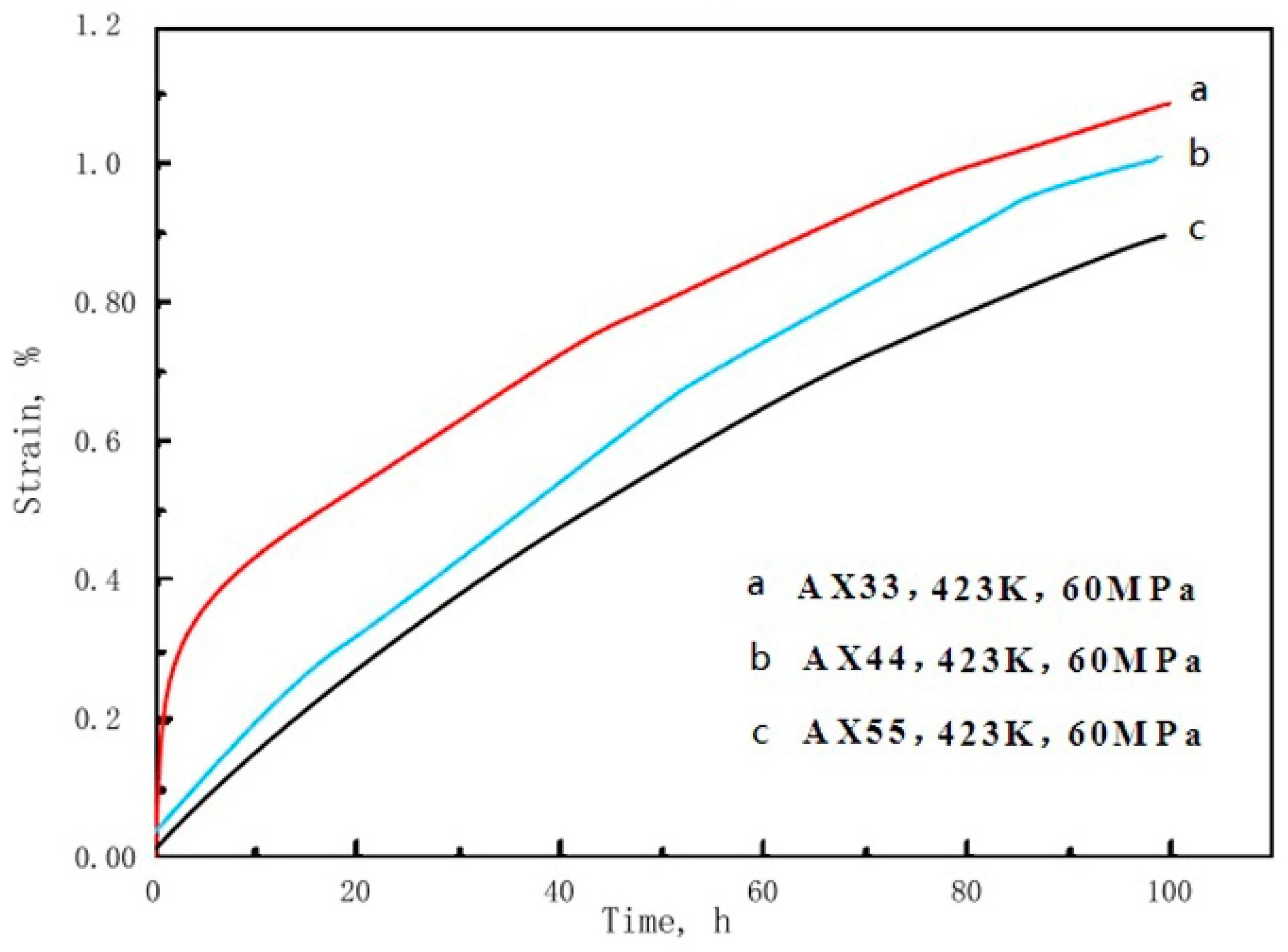
3.1. Results
3.2. Discussion
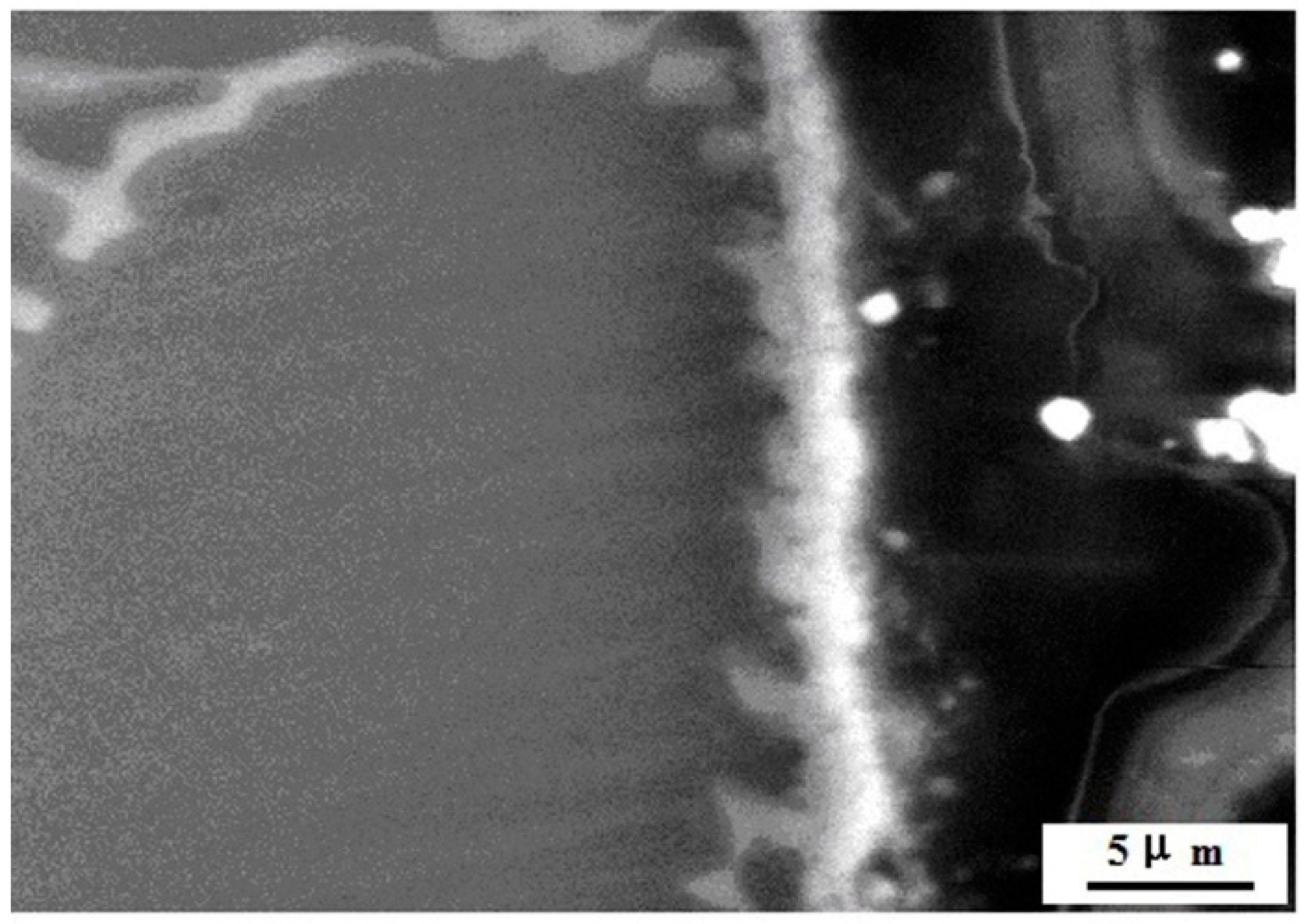
3.2.1. Microstructure Characterization
3.2.2. Tensile Strength
3.2.3. Creep and Superplasticity
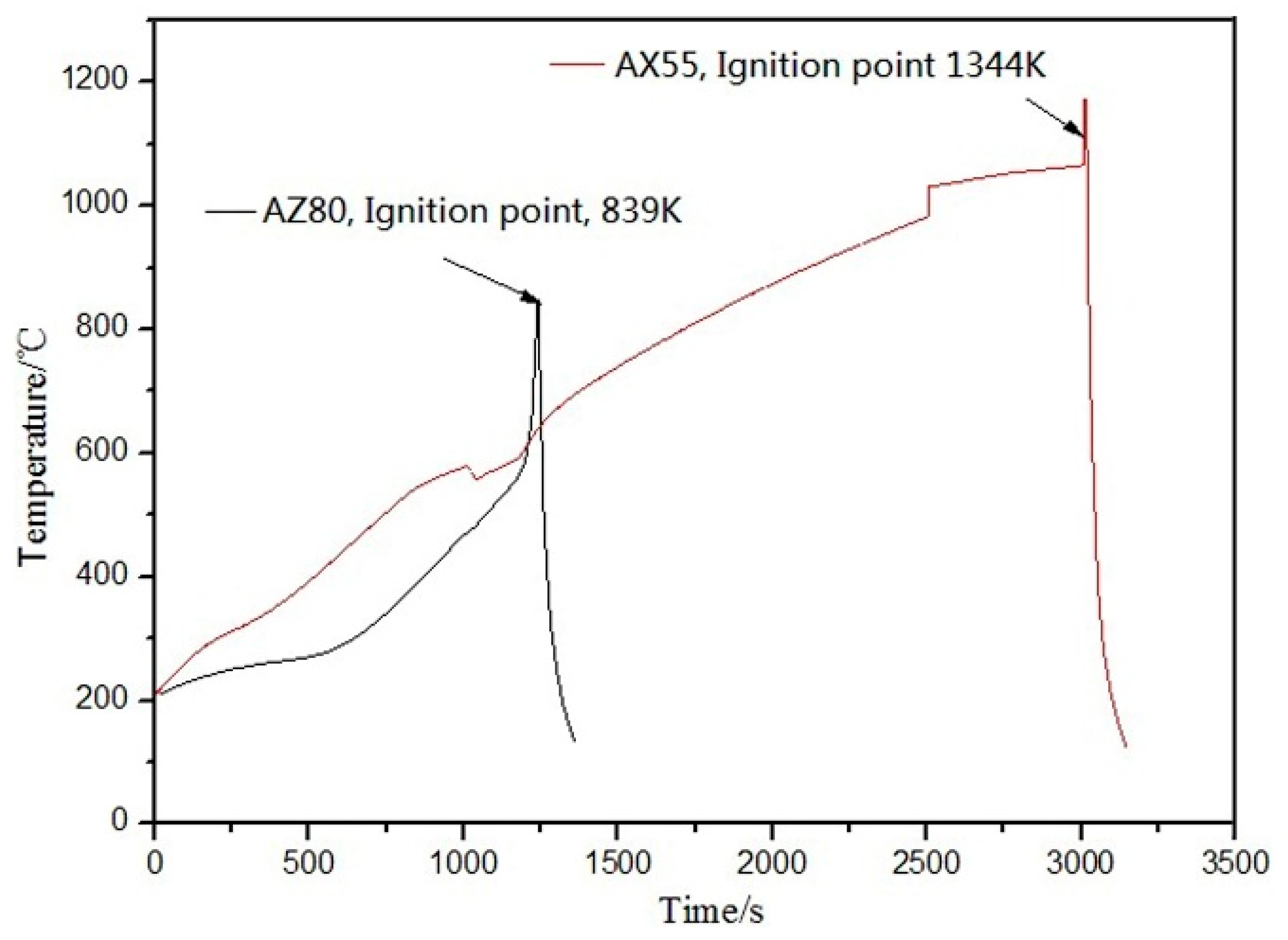
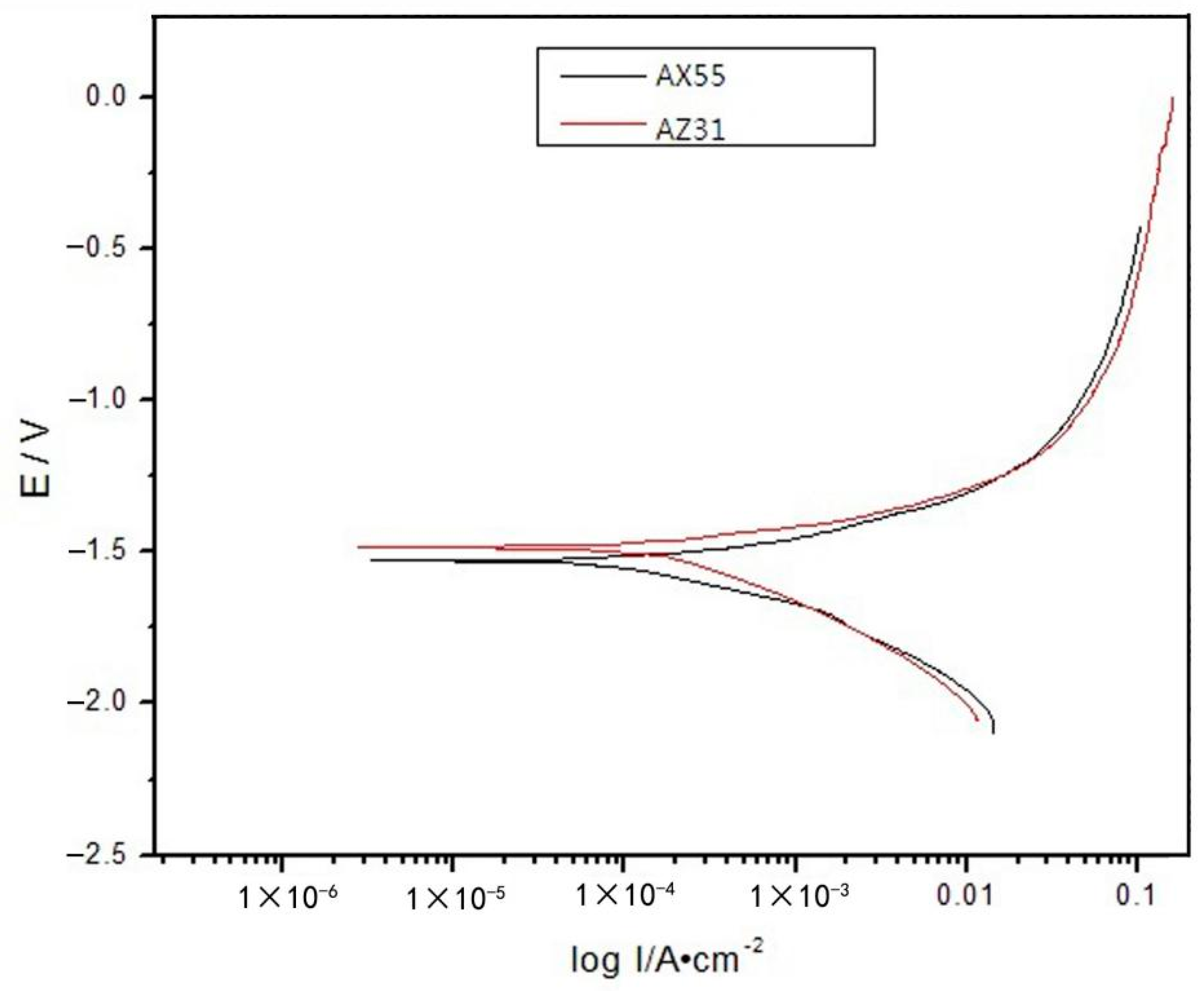
3.2.4. Flame Retardation and Corrosion Resistance
4. Conclusions
Author Contributions
Funding
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zheng, C.; Wu, X.; Zheng, X.; Jin, H.; Liu, Y. Mechanical properties and wear behavior of a dissolvable magnesium alloy used for multistage fracturing. Wear 2021, 466, 203559. [Google Scholar] [CrossRef]
- Chen, G.; Fu, Y.; Cui, Y.; Gao, J.; Guo, X.; Gao, H.; Wu, S.; Lu, J.; Li, Q.; Shi, S. Effect of surface mechanical attrition treatment on corrosion fatigue behavior of AZ31B magnesium alloy. Int. J. Fatigue 2019, 127, 461–469. [Google Scholar] [CrossRef]
- You, S.; Huang, Y.; Kainer, K.U.; Hort, N. Recent research and developments on wrought magnesium alloys. J. Magnes. Alloys 2017, 5, 239–253. [Google Scholar] [CrossRef]
- Sułkowski, B.; Janoska, M.; Boczkal, G.; Chulist, R.; Mroczkowski, M.; Pałka, P. The effect of severe plastic deformation on the Mg properties after CEC deformation. J. Magnes. Alloys 2020, 8, 761–768. [Google Scholar] [CrossRef]
- Sadeghi, A.; Mortezapour, H.; Samei, J.; Pekguleryuz, M.; Wilkinson, D. Anisotropy of mechanical properties and crystallographic texture in hot rolled AZ31+XSr sheets. J. Magnes. Alloys 2019, 7, 466–473. [Google Scholar] [CrossRef]
- Tahreen, N.; Chen, D.; Nouri, M.; Li, D. Effects of aluminum content and strain rate on strain hardening behavior of cast magnesium alloys during compression. Mater. Sci. Eng. A 2013, 594, 235–245. [Google Scholar] [CrossRef]
- Wang, Q.D.; Chen, W.Z.; Zeng, X.Q.; Lu, Y.Z.; Ding, W.J.; Zhu, Y.P.; Xu, X.P.; Mabuchi, M. Effects of Ca Addition on the Microstructure and Mechanical Properties of AZ91 Magnesium Alloy. J. Mater. Sci. 2001, 36, 3035–3040. [Google Scholar]
- Sakamoto, M.; Akiyama, S.; Ogi, K. Suppression of ignition and burning of molten Mg alloys by Ca bearing stable oxide film. J. Mater. Sci. Lett. 1997, 16, 1048–1050. [Google Scholar] [CrossRef]
- Yim, C.D.; Kim, Y.M.; You, B.S. Effect of Ca Addition on the Corrosion Resistance of Gravity Cast AZ31 Magnesium Alloy. Mater. Trans. 2007, 48, 1023–1028. [Google Scholar]
- Qin, G.W.; Ren, Y.; Huang, W.; Li, S.; Pei, W. Grain refining mechanism of Al-containing Mg alloys with the addition of Mn-Al alloys. J. Alloys Compd. 2010, 507, 410–413. [Google Scholar] [CrossRef]
- Watanabe, H.; Yamaguchi, M.; Takigawa, Y.; Higashi, K. Mechanical Properties of Mg-Al-Ca Alloy Processed by Hot Extrusion. Mater. Sci. Eng. A 2007, 454-455, 384–388. [Google Scholar]
- Zhong, Y.; Liu, J.; Witt, R.A.; Sohn, Y.H.; Liu, Z.K. Al2(Mg,Ca) phases in Mg-Al-Ca ternary system: First-principles prediction and experimental identification. Scr. Mater. 2006, 55, 573–576. [Google Scholar] [CrossRef]
- Ninomiya, R.; Ojiro, T.; Kubota, K. Improved heat resistance of Mg-Al alloys by the Ca addition. Acta Metall. Mater. 1995, 43, 669–674. [Google Scholar] [CrossRef]
- Suzuki, A.; Saddock, N.; Jones, J.; Pollock, T. Solidification paths and eutectic intermetallic phases in Mg-Al-Ca ternary alloys. Acta Mater. 2005, 53, 2823–2834. [Google Scholar] [CrossRef]
- Zhao, P.; Geng, H.; Wang, Q. Effect of melting technique on the microstructure and mechanical properties of AZ91 commercial magnesium alloys. Mater. Sci. Eng. A 2006, 429, 320–323. [Google Scholar] [CrossRef]
- Ozturk, K.; Zhong, Y.; Liu, Z.-K.; Luo, A.A. Creep resistant Mg-Al-Ca alloys: Computational thermodynamics and experimental investigation. JOM 2003, 55, 40–44. [Google Scholar] [CrossRef]
- Luo, J.; Mei, Z.; Tian, W.; Wang, Z. Diminishing of work hardening in electroformed polycrystalline copper with nano-sized and uf-sized twins. Mater. Sci. Eng. A 2006, 441, 282–290. [Google Scholar] [CrossRef]
- Bahador, A.; Umeda, J.; Yamanoğlu, R.; Ghandvar, H.; Issariyapat, A.; Bakar, T.A.A.; Kondoh, K. Cu-TiB2 composites evaluated by the in-situ tensile test and microstructure characterization. J. Alloys Compd. 2020, 847, 156555. [Google Scholar] [CrossRef]
- Nie, J.F. Effects of precipitate shape and orientation on dispersion strengthening in magnesium alloys. Scr. Mater. 2003, 48, 1009–1015. [Google Scholar] [CrossRef]
- Xu, S.W.; Oh-ishi, K.; Kamado, S.; Takahashi, H.; Homma, T. Effects of different cooling rates during two casting processes on the microstructures and mechanical properties of extruded Mg-Al-Ca-Mn alloy. Mater. Sci. Eng. A 2012, 542, 71–78. [Google Scholar] [CrossRef]
- Xiao, D.; Chen, Z.; Wang, X.; Zhang, M.; Chen, D. Microstructure, mechanical and creep properties of high Ca/Al ratio Mg-Al-Ca alloy. Mater. Sci. Eng. A 2016, 660, 166–171. [Google Scholar] [CrossRef]
- Rao, K.P.; Prasad, Y.V.R.K.; Suresh, K.; Hort, N.; Kainer, K.U. Hot Deformation Behavior of Mg-2Sn-2Ca Alloy in As-cast Condition and After Homogenization. Mater. Sci. Eng. A 2012, 552, 444–450. [Google Scholar] [CrossRef]
- Xu, S.W.; Matsumoto, N.; Yamamoto, K.; Kamado, S.; Honma, T.; Kojima, Y. High temperature tensile properties of as-cast Mg-Al-Ca alloys. Mater. Sci. Eng. A 2009, 509, 105–110. [Google Scholar] [CrossRef]
- Kim, W.J.; Lee, K.E.; Park, J.P.; Kim, M.G.; Wang, J.Y.; Yoon, U.S. Microstructure and superplasticity of Mg-Al-Ca electromagnetic casting alloys after hot extrusion. Mater. Sci. Eng. A 2008, 494, 391–396. [Google Scholar] [CrossRef]
- Hsu, S.E.; Edwards, G.R.; Sherby, O.D. Influence of texture on dislocation creep and grain boundary sliding in fine-grained cadmium. Acta Metall. 1983, 31, 763–772. [Google Scholar] [CrossRef]
- Wei, Y.H.; Wang, Q.D.; Zhu, Y.P. Superplasticity and grain boundary sliding in rolled AZ91 magnesium alloy at high strain rates. Mater. Sci. Eng. A 2003, 360, 107–115. [Google Scholar] [CrossRef]
- You, B.S.; Park, W.W.; Chung, I.S. The effect of calcium additions on the oxidation behavior in magnesium alloys. Scr. Mater. 2000, 42, 1089–1094. [Google Scholar] [CrossRef]
- Czerwinski, F. The oxidation behaviour of an AZ91D magnesium alloy at high temperatures. Acta Mater. 2002, 50, 2639–2654. [Google Scholar] [CrossRef]
- Kim, Y.M.; Chang, D.Y.; Kim, H.S.; You, B.S. Key Factor Influencing the Ignition Resistance of Magnesium Alloys at Elevated Temperatures. Scr. Mater. 2011, 65, 958–961. [Google Scholar] [CrossRef]
- Pardo, A.; Merino, M.C.; Coy, A.E.; Arrabal, R.; Viejo, F.; Matykina, E. Corrosion Behaviour of Magnesium/Aluminium Alloys in 3.5 wt.% NaCl. Corros. Sci. 2008, 50, 823–834. [Google Scholar] [CrossRef]
- Hur, B.Y.; Kim, K.W. A New Method for Evaluation of Pitting Corrosion Resistance of Mg Alloys. Corros. Rev. 1998, 16, 85–94. [Google Scholar] [CrossRef]
- Dabah, E.; Ben-Hamu, G.; Lisitsyn, V.; Eliezer, D.; Shin, K.S. The influence of Ca on the corrosion behavior of new die cast Mg-Al-based alloys for elevated temperature applications. J. Mater. Sci. 2010, 45, 3007–3015. [Google Scholar] [CrossRef]
- Murray, R.C., Jr.; Rock., P.A. The determination of the ferrocyanide-ferricyanide standard electrode potential at 25 °C in cells without liquid junction using cation-sensitive glass electrodes. Elctrochim. Acta 1968, 13, 969–975. [Google Scholar] [CrossRef]

| Al | Ca | Mn | Fe | Mg | |
|---|---|---|---|---|---|
| AX33 | 3.20 | 3.30 | 0.70 | <0.001 | Bal. |
| AX44 | 4.20 | 4.30 | 0.70 | <0.001 | Bal. |
| AX55 | 5.10 | 5.20 | 0.70 | <0.001 | Bal. |
| Experiment Conditions | Units | AX33 | AX44 | AX55 | |
|---|---|---|---|---|---|
| σb/σs/δ | RT | MPa/MPa/% | 324/287/6 | 350/318/5 | 339/315/4 |
| σb/σs/δ | 423 K | MPa/MPa/% | 201/150/16 | 210/153/14 | 187/152/13 |
| σb/σs/δ | 448 K | MPa/MPa/% | 175/143/32 | 194/154/17 | 151/128/15 |
| Creep total-elongation | 423 K 60 MPa 100 h | % | 1.03 | 0.98 | 0.92 |
| Secondary creep rate | 423 K 60 MPa 100 h | ×10−8 s−1 | 2.05 | 1.95 | 1.98 |
| 423 K 70 MPa 100 h | ×10−8 s−1 | 3.04 | 2.89 | 2.68 | |
| 423 K 90 MPa 100 h | ×10−8 s−1 | 32.6 | 27.2 | 26.5 | |
| superplasticity elongation-to-failure δ/σb | 673 K 3.6 × 10−4 s−1 | %/MPa | 312/4.3 | 384/8.3 | 602/9.5 |
| 673 K 3.6 × 10−3 s−1 | %/MPa | 272/9.8 | 311/18.2 | 449/18.3 | |
| 623 K 3.6 × 10−4 s−1 | %/MPa | 213/17.9 | 263/20.3 | 352/20.5 | |
| 623 K 3.6 × 10−3 s−1 | %/MPa | 139/28.8 | 209/34.1 | 229/41.9 | |
| Ignition temperature | in air | k | 1292 | 1313 | 1344 |
| Corrosion rate | 298 K in 5% NaCl aq | g·mm−2·h−1 | 1.3125 | 1.4861 | 2.2492 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chu, A.; Zhao, Y.; ud-din, R.; Hu, H.; Zhi, Q.; Wang, Z. Microstructure and Properties of Mg-Al-Ca-Mn Alloy with High Ca/Al Ratio Fabricated by Hot Extrusion. Materials 2021, 14, 5230. https://doi.org/10.3390/ma14185230
Chu A, Zhao Y, ud-din R, Hu H, Zhi Q, Wang Z. Microstructure and Properties of Mg-Al-Ca-Mn Alloy with High Ca/Al Ratio Fabricated by Hot Extrusion. Materials. 2021; 14(18):5230. https://doi.org/10.3390/ma14185230
Chicago/Turabian StyleChu, Aimin, Yuping Zhao, Rafi ud-din, Hairong Hu, Qian Zhi, and Zerui Wang. 2021. "Microstructure and Properties of Mg-Al-Ca-Mn Alloy with High Ca/Al Ratio Fabricated by Hot Extrusion" Materials 14, no. 18: 5230. https://doi.org/10.3390/ma14185230





