SBA-15 with Crystalline Walls Produced via Thermal Treatment with the Alkali and Alkali Earth Metal Ions
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Synthesis of SBA-15
2.3. Synthesis of SBA-15 with Crystalline Walls
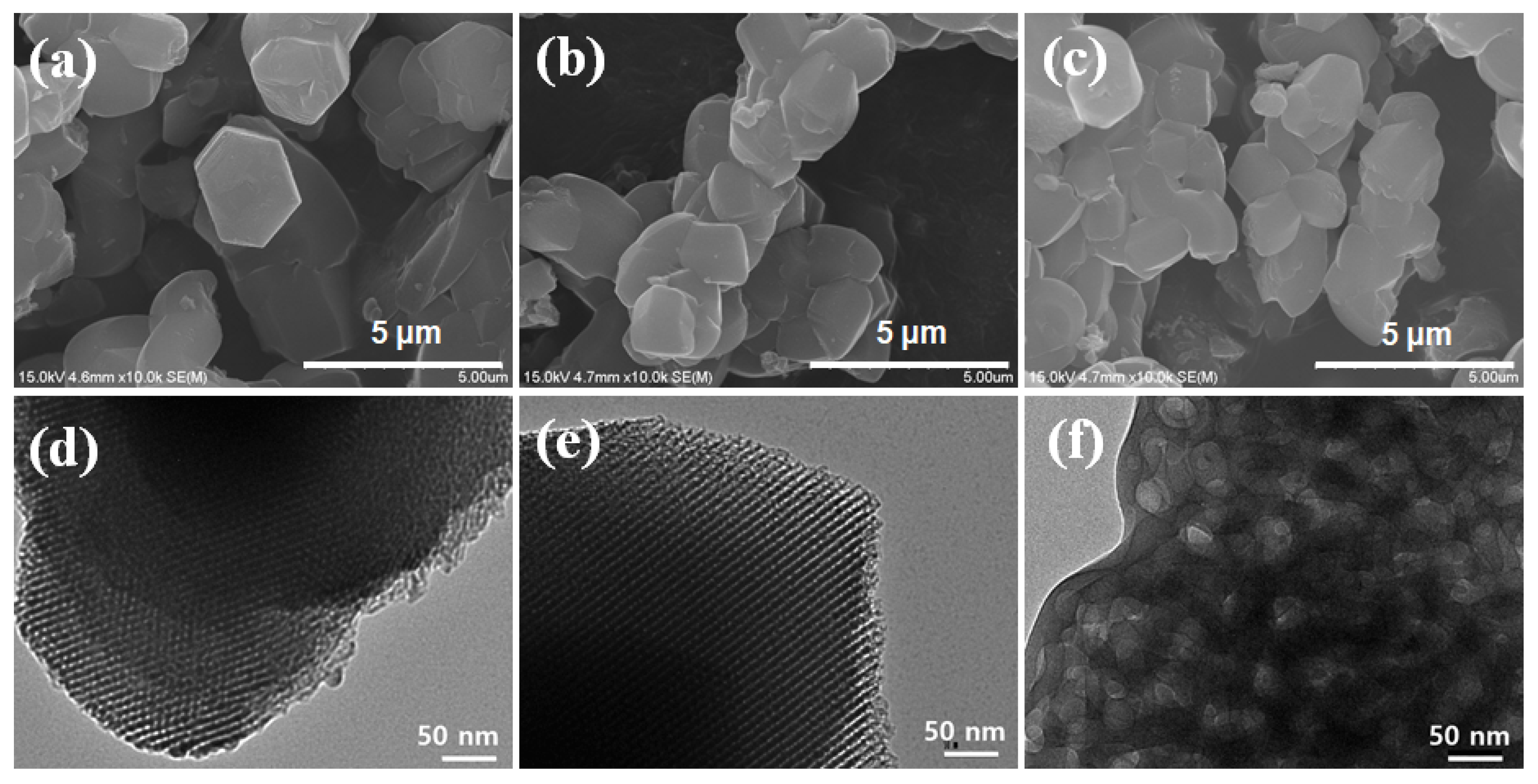
2.4. Characterization
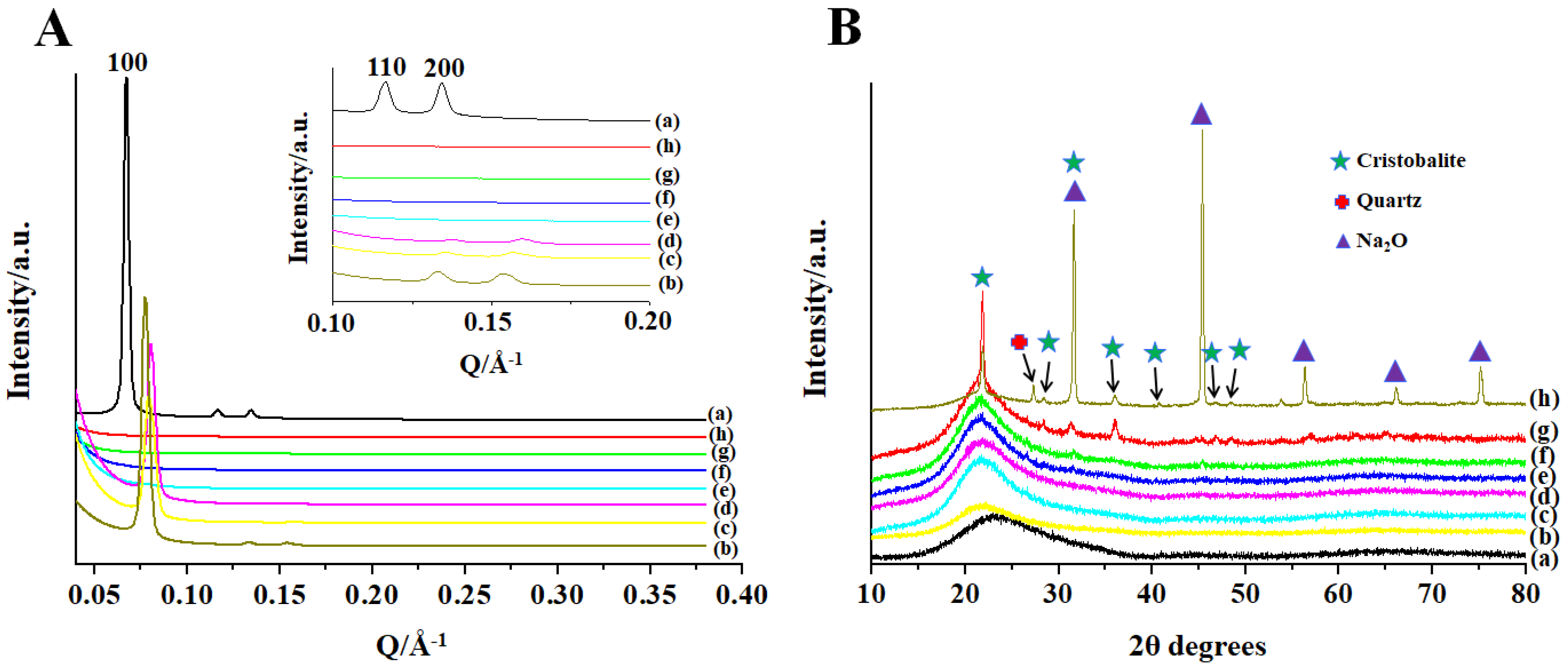
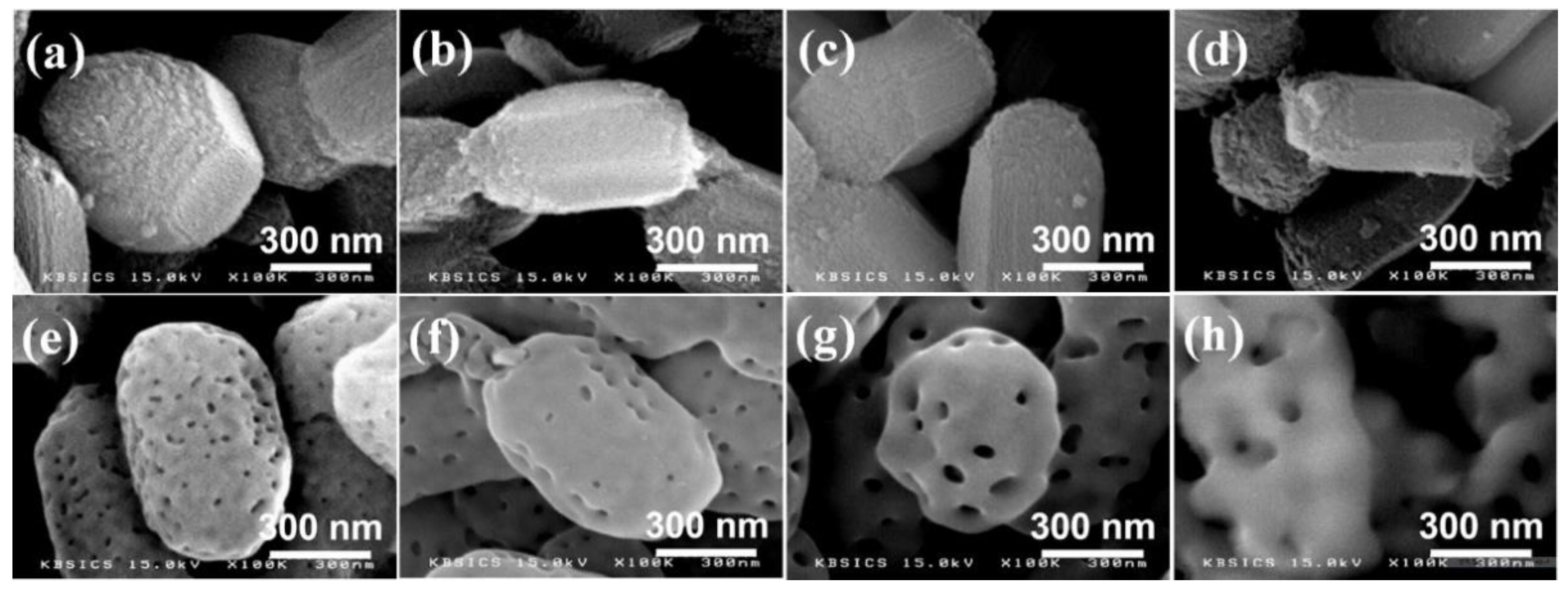
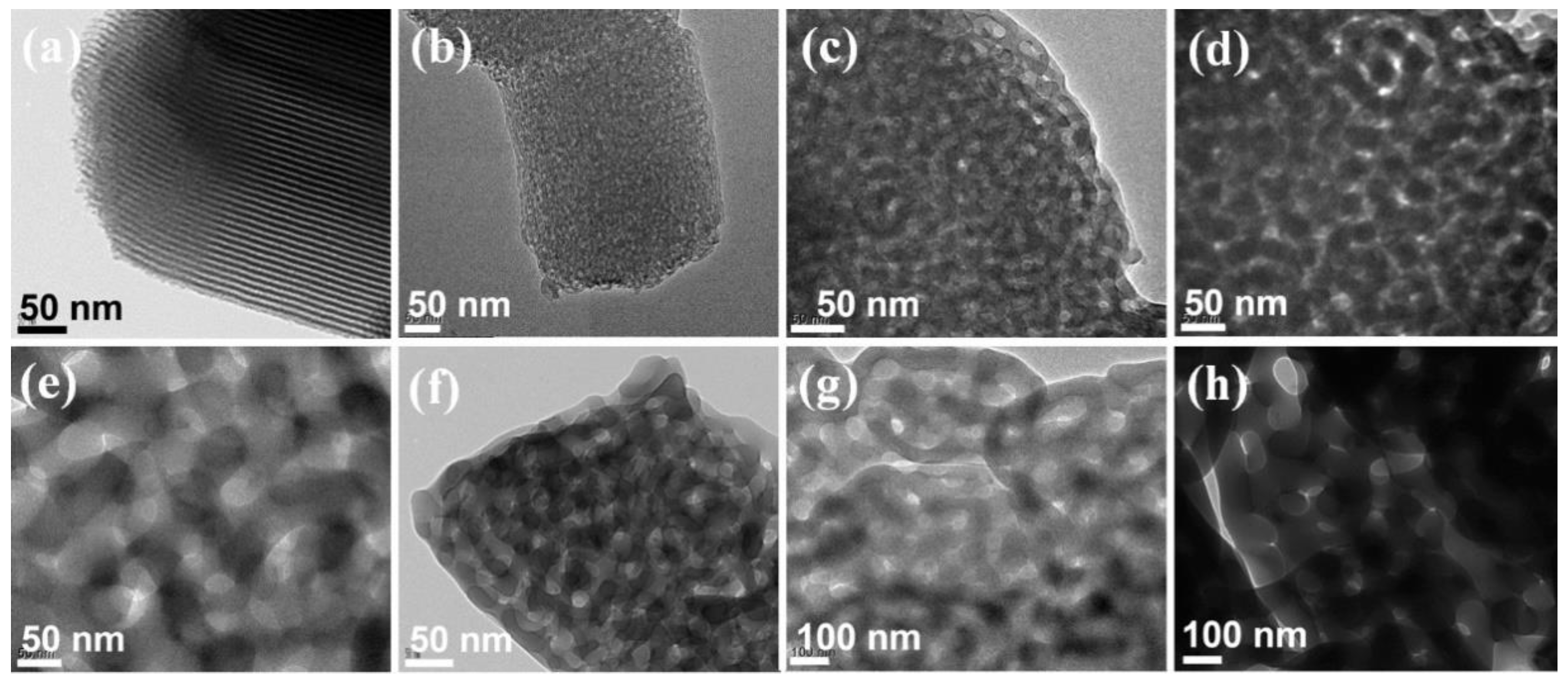
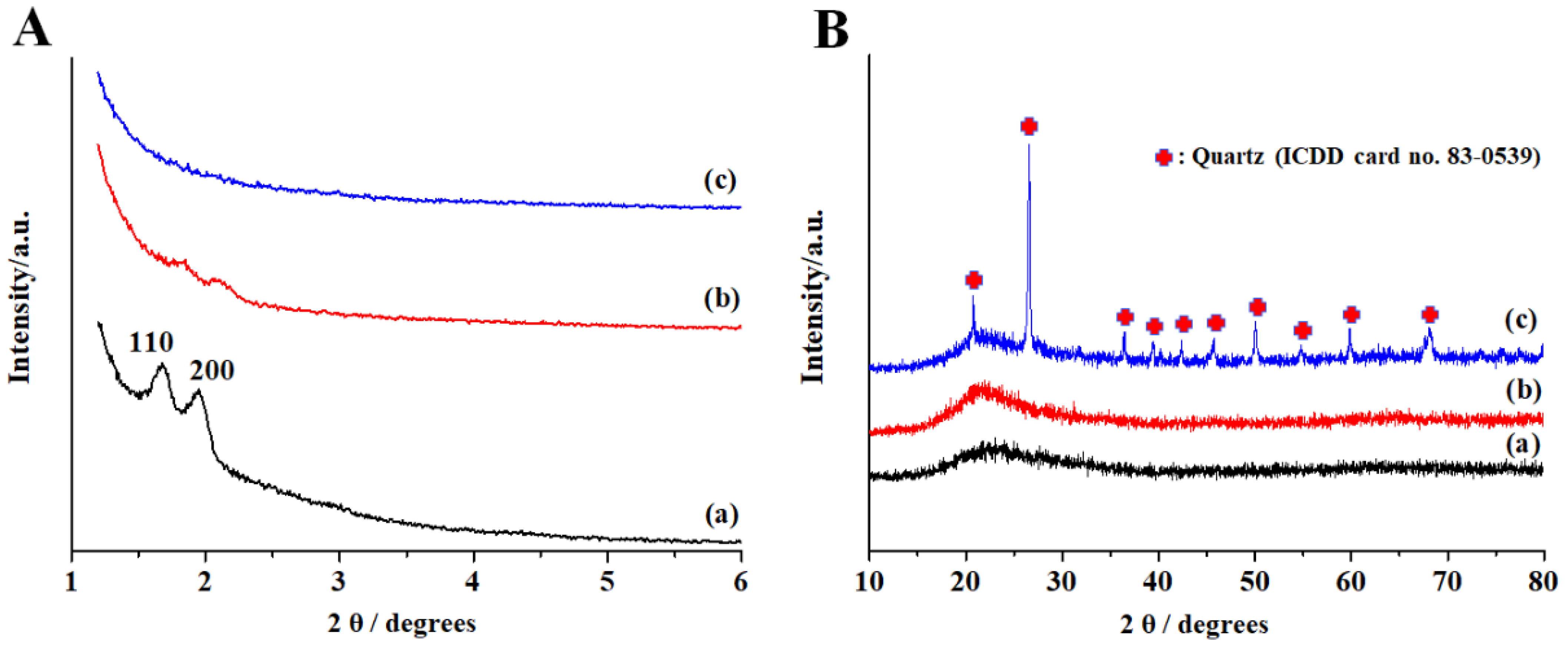
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kresge, C.T.; Leonowicz, M.E.; Roth, W.J.; Vartuli, J.C.; Beck, J.S. Ordered mesoporous molecular sieves synthesized by a liquid-crystal template mechanism. Nature 1992, 359, 710–712. [Google Scholar] [CrossRef]
- Zhao, D.; Feng, J.; Huo, Q.; Melosh, N.; Fredrickson, G.H.; Chmelka, B.F.; Stucky, G.D. Triblock copolymer syntheses of mesoporous silica with periodic 50 to 300 angstrom pores. Science 1998, 279, 548–552. [Google Scholar] [CrossRef] [Green Version]
- Innocenzi, P.; Malfatti, L. Mesoporous thin films: Properties and applications. Chem. Soc. Rev. 2013, 42, 4198–4216. [Google Scholar] [CrossRef] [Green Version]
- Yang, H.; Coombs, N.; Ozin, G.A. Morphogenesis of shapes and surface patterns in mesoporous silica. Nature 1997, 386, 692–695. [Google Scholar] [CrossRef]
- Pal, N.; Bhaumik, A. Soft templating strategies for the synthesis of mesoporous materials: Inorganic, organic-inorganic hybrid and purely organic solids. Adv. Colloid Interface Sci. 2013, 189–190, 21–41. [Google Scholar] [CrossRef]
- Zhao, D.; Wan, Y.; Zhou, W. Ordered Mesoporous Materials; Wiley-VCH Verlag & Co. KGaA Boschstr: Weinheim, Germany, 2013; pp. 219–506. [Google Scholar]
- Shylesh, S.; Samuel, P.P.; Sisodiya, S.; Singh, A.P. Periodic mesoporous silicas and organosilicas: An overview towards catalysis. Catal. Surv. Asia 2008, 12, 266–282. [Google Scholar] [CrossRef]
- Singh, B.; Na, J.; Konarova, M.; Wakihara, T.; Yamauchi, Y.; Salomon, C.; Gawande, M.B. Functional mesoporous silica nanomaterials for catalysis and environmental applications. Bull. Chem. Soc. Jpn. 2020, 93, 1459–1496. [Google Scholar] [CrossRef]
- Park, S.S.; Moorthy, M.S.; Ha, C.-S. Periodic mesoporous organosilicas for advanced applications. NPG Asia Mater. 2014, 6, e96. [Google Scholar] [CrossRef]
- Park, S.S.; Moorthy, M.S.; Ha, C.-S. Periodic mesoporous organosilica (PMO) for catalytic applications. Korean J. Chem. Eng. 2014, 31, 1707–1719. [Google Scholar] [CrossRef]
- Popat, A.; Hartono, S.B.; Stahr, F.; Liu, J.; Qiao, S.Z.; Lu, G.Q. Mesoporous silicananoparticles for bioadsorption, enzyme immobilisation, and delivery carriers. Nanoscale 2011, 3, 2801–2818. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Simoes-Cardoso, J.C.; Kojo, H.; Yoshimoto, N.; Yamamoto, S. Microcalorimetric analysis of the adsorption of lysozyme and cytochrome c onto cation-exchange chromatography resins: Influence of temperature on retention. Langmuir 2020, 36, 3336–3345. [Google Scholar] [CrossRef] [PubMed]
- Walcarius, A.; Mercier, L. Mesoporous organosilica adsorbents: Nanoengineered materials for removal of organic and inorganic pollutants. J. Mater. Chem. 2010, 20, 4478–4511. [Google Scholar] [CrossRef]
- Azmi, A.A.; Aziz, M.A.A. Mesoporous adsorbent for CO2 capture application under mild condition: A review. J. Environ. Chem. Eng. 2019, 7, 103022. [Google Scholar] [CrossRef]
- Gibson, L.T. Mesosilica materials and organic pollutant adsorption: Part A removal from air. Chem. Soc. Rev. 2014, 43, 5163–5172. [Google Scholar] [CrossRef]
- Da’na, E. Adsorption of heavy metals on functionalized-mesoporous silica: A review. Micropor. Mesopor. Mater. 2017, 247, 145–157. [Google Scholar] [CrossRef]
- Putz, A.-M.; Ciopec, M.; Negrea, A.; Grad, O.; Ianăşi, C.; Ivankov, O.I.; Milanović, M.; Stijepovíc, I.; Almásy, L. Comparison of structure and adsorption properties of mesoporous silica functionalized with aminopropyl groups by the co-condensation and the post grafting methods. Materials 2021, 14, 628. [Google Scholar] [CrossRef]
- Cashin, V.B.; Eldridge, D.S.; Yu, A.; Zhao, D. Surface functionalization and manipulation of mesoporous silica adsorbents for improved removal of pollutants: A review. Environ. Sci. Water Res. Technol. 2018, 4, 110–128. [Google Scholar] [CrossRef]
- Kumar, P.; Guliants, V.V. Periodic mesoporous organic–inorganic hybrid materials: Applications in membrane separations and adsorption. Microporous Mesoporous Mater. 2010, 132, 1–14. [Google Scholar] [CrossRef]
- Li, L.; Wang, T.; Liu, Q.; Cao, Y.; Qiu, J. A high CO2 permselective mesoporous silica/carbon composite membrane for CO2 separation. Carbon 2012, 50, 5186–5195. [Google Scholar] [CrossRef]
- Mekawy, M.M.; Yamaguchi, A.; El-Safty, S.A.; Itoh, T.; Teramae, N. Mesoporous silica hybrid membranes for precise size-exclusive separation of silver nanoparticles. J. Colloid Interface Sci. 2011, 355, 348–358. [Google Scholar] [CrossRef]
- Li, J.; Zhu, K.; Shang, J.; Wang, D.; Nie, Z.; Guo, R.; Liu, C.; Wang, Z.; Li, X.; Liu, J. Fluorescent functionalized mesoporous silica for radioactive material extraction. Sep. Sci. Technol. 2012, 47, 1507–1513. [Google Scholar] [CrossRef]
- Yang, J.; Lin, G.-S.; Mou, C.-Y.; Tung, K.-L. Mesoporous silica thin membrane with tunable pore size for ultrahigh permeation and precise molecular separation. ACS Appl. Mater. Interfaces 2020, 12, 7459–7465. [Google Scholar] [CrossRef]
- Das, T.; Singha, D.; Pal, A.; Nandi, M. Mesoporous silica based recyclable probe for colorimetric detection and separation of ppb level Hg2+ from aqueous medium. Sci. Rep. 2019, 9, 19378. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tao, Z. Mesoporous silica-based nanodevices for biological applications. RSC Adv. 2014, 4, 18961–18980. [Google Scholar] [CrossRef]
- Park, S.S.; Moorthy, M.S.; Chu, S.-W.; Dong, F.; Guo, W.; Ha, C.-S. Controlled drug delivery of hollow mesostructured materials. Adv. Porous Mater. 2013, 1, 4–33. [Google Scholar] [CrossRef]
- Castillo, R.R.; Lozano, D.; Vallet-Regí, M. Mesoporous silica nanoparticles as carriers for therapeutic biomolecules. Pharmaceutics 2020, 12, 432. [Google Scholar] [CrossRef]
- Xu, C.; Lei, C.; Yu, C. Mesoporous silica nanoparticles for protein protection and delivery. Front. Chem. 2019, 7, 290. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Manzano, M.; Vallet-Regí, M. Mesoporous silica nanoparticles for drug delivery. Adv. Funct. Mater. 2020, 30, 1902634. [Google Scholar] [CrossRef]
- Pu, X.; Li, J.; Qiao, P.; Li, M.; Wang, H.; Zong, L.; Yuan, Q.; Duan, S. Mesoporous silica nanoparticles as a prospective and promising approach for drug delivery and biomedical applications. Curr. Cancer Drug Targets 2019, 19, 285–295. [Google Scholar] [CrossRef]
- Abdullah, N.; Hossain, S.; Konstantinov, K.; Tanabe, H. Tuning wall thicknesses in mesoporous silica films for optimization of optical anti-reflective properties. J. Nanosci. Nanotechnol. 2018, 18, 100–103. [Google Scholar] [CrossRef]
- Schulz-Ekloff, G.; Wöhrle, D.; Duffel, B.; van Schoonheydt, R.A. Chromophores in porous silicas and minerals: Preparation and optical properties. Microporous Mesoporous Mater. 2002, 51, 91–138. [Google Scholar] [CrossRef]
- Wagner, T.; Haffer, S.; Weinberger, C.; Klaus, D.; Tiemann, M. Mesoporous materials as gas sensors. Chem. Soc. Rev. 2013, 42, 4036–4053. [Google Scholar] [CrossRef] [PubMed]
- Melde, B.J.; Johnson, B.J.; Charles, P.T. Mesoporous silicate materials in sensing. Sensors 2008, 8, 5202–5228. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, G.; Sun, S. Fabrication of a mesoporous silica film based optical waveguide sensor for detection of small molecules. J. Appl. Opt. 2020, 59, 3933–3941. [Google Scholar] [CrossRef]
- Park, J.-W.; Park, S.S.; Kim, Y.; Kim, I.; Ha, C.-S. Mesoporous silica nanolayers infiltrated with hole-transporting molecules for hybrid organic light-emitting devices. ACS Nano 2008, 2, 1137–1142. [Google Scholar] [CrossRef]
- Sun, B.; Zhen, X.; Jiang, X. Development of mesoporous silica-based nanoprobes for optical bioimaging applications. Biomater. Sci. 2021, 9, 3603–3620. [Google Scholar] [CrossRef]
- Yuan, D.; Ellis, C.M.; Davis, J.J. Mesoporous silica nanoparticles in bioimaging. Materials 2020, 13, 3795. [Google Scholar] [CrossRef]
- Fujita, S.; Inagaki, S. Self-organization of organosilica solids with molecular-scale and mesoscale periodicities. Chem. Mater. 2008, 20, 891–908. [Google Scholar] [CrossRef]
- Park, S.S.; Park, D.H.; Ha, C.-S. Free-standing periodic mesoporous organosilica film with a crystal-like wall structure. Chem. Mater. 2007, 19, 2709–2711. [Google Scholar] [CrossRef]
- Ivanova, I.I.; Knyazeva, E.E. Micro-mesoporous materials obtained by zeolite recrystallization: Synthesis, characterization and catalytic applications. Chem. Soc. Rev. 2013, 42, 3671–3688. [Google Scholar] [CrossRef]
- Bai, R.; Song, Y.; Li, Y.; Yu, J. Creating hierarchical pores in zeolite catalysts. Trends Analyt. Chem. 2019, 1, 601–611. [Google Scholar] [CrossRef] [Green Version]
- Zhang, J.; Wang, L.; Ji, Y.; Chen, F.; Xiao, F.-S. Mesoporous zeolites for biofuel upgrading and glycerol conversion. Front. Chem. Sci. Eng. 2018, 12, 132–144. [Google Scholar] [CrossRef]
- Park, S.S.; Jung, Y.; Xue, C.; Che, R.; Zhao, D.; Ha, C.-S. Free-standing mesoporous silica/carbon composite films with crystalline silica wall from ethylene-bridged organosilane. Chem. Mater. 2010, 22, 18–26. [Google Scholar] [CrossRef]
- Zhao, L.; Li, N.; Langner, A.; Steinhart, M.; Tan, T.Y.; Pippel, E.; Hofmeister, H.; Tu, K.-N.; Gösele, U. Crystallization of amorphous SiO2 microtubes catalyzed by lithium. Adv. Funct. Mater. 2007, 17, 1952–1957. [Google Scholar] [CrossRef]
- Deepak, F.L.; Gundiah, G.; Seikh, M.M.; Govindaraj, A.; Rao, C.N.R.J. Crystalline silica nanowires. Mater. Res. 2004, 19, 2216–2221. [Google Scholar] [CrossRef]
- Wagstaff, F.E.; Richards, K.J. Kinetics of crystallization of stoichiometric SiO2 glass in H2O atmospheres. J. Am. Ceram. Soc. 1966, 49, 118–121. [Google Scholar] [CrossRef]
- Pol, V.G.; Gedanken, A.; Calderon-Moreno, J. Deposition of gold nanoparticles on silica spheres: a sonochemical approach. J. Chem. Mater. 2003, 15, 1111–1118. [Google Scholar] [CrossRef]
- Garnica-Romo, M.G.; Gonzalez-Hernandez, J.; Hernandez-Landaverde, M.A.; Vorobiev, Y.V.; Ruiz, F.; Martinez, J.R. Structure of heat-treated sol-gel SiO2 glasses containing silver. J. Mater. Res. 2001, 16, 2007–2012. [Google Scholar] [CrossRef]
- Palermo, A.; Vazquez, J.P.H.; Lee, A.F.; Tikhov, M.S.; Lambert, R.M. Critical influence of the amorphous silica-to-cristobalite phase transition on the performance of Mn/Na2WO4/SiO2 catalysts for the oxidative coupling of methane. J. Catal. 1998, 177, 259–266. [Google Scholar] [CrossRef]
- Horii, N.; Kamide, M.; Inouye, A.; Kuzuu, N. Crystallization of silica glass upon heating by contact with a NaCl crystal grain. J. Ceram. Soc. Jpn. 2010, 118, 318–320. [Google Scholar] [CrossRef] [Green Version]
- Venezia, A.M.; La Parola, V.; Longo, A.; Martorana, A. Effect of alkali ions on the amorphous to crystalline phase transition of silica. J. Solid State Chem. 2001, 161, 373–378. [Google Scholar] [CrossRef]
- Shinohara, Y.; Kohyama, N. Quantitative analysis of tridymite and cristobalite crystallized in rice husk ash by heating. Ind. Health 2004, 42, 277–285. [Google Scholar] [CrossRef] [PubMed]
- Joni, I.M.; Nulhakim, L.; Vanitha, M.; Panatarani, C. Characteristics of crystalline silica (SiO2) particles prepared by simple solution method using sodium silicate (Na2SiO3) precursor. J. Phys. Conf. Ser. 2018, 1080, 012006. [Google Scholar] [CrossRef]
- Santoro, M.; Gorelli, F.A.; Bini, R.; Salamat, A.; Garbarino, G.; Levelut, C.; Cambon, O.; Haines, J. Carbon enters silica forming a cristobalite-type CO2–SiO2 solid solution. Nat. Commun. 2014, 5, 3761. [Google Scholar] [CrossRef] [PubMed]
- Tsuchida, Y.; Yagi, T. New pressure-induced transformations of silica at room temperature. Nature 1990, 347, 267–269. [Google Scholar] [CrossRef]
- Hu, Q.Y.; Shu, J.-F.; Cadien, A.; Meng, Y.; Yang, W.G.; Sheng, H.W.; Mao, H.-K. Polymorphic phase transition mechanism of compressed coesite. Nat. Commun. 2015, 6, 6630. [Google Scholar] [CrossRef]
- Huang, L.; Durandurdu, M.; Kieffer, J. Transformation pathways of silica under high pressure. Nat. Mater. 2006, 5, 977–981. [Google Scholar] [CrossRef] [Green Version]
- Drisko, G.L.; Carretero-Genevrier, A.; Perrot, A.; Gich, M.; Gàzquez, J.; Rodriguez-Carvajal, J.; Favre, L.; Grosso, D.; Boissièreah, C.; Sanchez, C. Crystallization of hollow mesoporous silica nanoparticles. Chem. Commun. 2015, 51, 4164–4167. [Google Scholar] [CrossRef]
- Park, S.S.; Moorthy, M.S.; Song, H.-J.; Ha, C.-S. Functionalized mesoporous silicas with crown ether moieties for selective adsorption of lithium ions in artificial sea water. J. Nanosci. Nanotechnol. 2014, 14, 8845–8851. [Google Scholar] [CrossRef]
- Marcus, Y. A simple empirical model describing the thermodynamics of hydration of ions of widely varying charges, sizes, and shapes. Biophys. Chem. 1994, 51, 111–127. [Google Scholar] [CrossRef]
- Sugiura, Y.; Saito, Y.; Endo, T.; Makita, Y. Effect of the ionic radius of alkali metal ions on octacalcium phosphate formation via different substitution modes. Cryst. Growth Des. 2019, 19, 4162–4171. [Google Scholar] [CrossRef]
- Lina, C.-C.; Chen, S.-F.; Liu, L.-G.; Li, C.-C. Size effects of modifying cations on the structure and elastic properties of Na2O–MO–SiO2 glasses (M = Mg, Ca, Sr, Ba). Mater. Chem. Phys. 2010, 123, 569–580. [Google Scholar] [CrossRef]






| Sample Name | Si/Na Ratios | d100 (a) (Å) | ao (b) (Å) | SBET (c) (m2·g−1) | Vtotal (d) (cm3·g−1) | D (e) (Å) |
|---|---|---|---|---|---|---|
| SBA-15 | ∞ (pristine SBA-15) | 93.6 | 108.0 | 713 | 0.92 | 66.7 |
| SBA15-Na-80-700 | 80 | 81.2 | 93.8 | 201 | 0.35 | 48.3 |
| SBA15-Na-60-700 | 60 | 79.3 | 91.6 | 169 | 0.30 | 48.6 |
| SBA15-Na-50-700 | 50 | 79.2 | 91.4 | 149 | 0.27 | 48.1 |
| SBA15-Na-40-700 | 40 | - | - | 34 | 0.23 | 983.7 |
| SBA15-Na-30-700 | 30 | - | - | 27 | 0.24 | 898.4 |
| SBA15-Na-20-700 | 20 | - | - | 16 | 0.05 | 1779.9 |
| SBA15-Na-2.1-700 | 2.1 | - | - | 11 | 0.03 | 18.3, 972.7 |
| Sample Name | Treated Temp. (°C) | d100 (a) (Å) | a0 (b) (Å) | SBET (c) (m2·g−1) | Vtotal (d) (cm3·g−1) | D (e) (Å) |
|---|---|---|---|---|---|---|
| SBA15-Na-40-500 | 500 | 91 | 105 | 477 | 0.73 | 78.5 |
| SBA15-Na-40-600 | 600 | 86 | 99 | 112 | 0.26 | 66 |
| SBA15-Na-40-650 | 650 | - | - | 25 | 0.04 | 119, 364 |
| Sample Name | Treated Temp. (°C) | SBET (a) (m2·g−1) | Vtotal (b) (cm3·g−1) | D (c) (Å) |
|---|---|---|---|---|
| SBA15-Li-40-700 | 700 | 230 | 0.34 | 79.6 |
| SBA15-K-40-700 | 700 | 210 | 0.32 | 74.7 |
| SBA15-Ca-40-700 | 700 | 35 | 0.27 | 802.5 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Park, S.S.; Chu, S.-W.; Shi, L.; Yuan, S.; Ha, C.-S. SBA-15 with Crystalline Walls Produced via Thermal Treatment with the Alkali and Alkali Earth Metal Ions. Materials 2021, 14, 5270. https://doi.org/10.3390/ma14185270
Park SS, Chu S-W, Shi L, Yuan S, Ha C-S. SBA-15 with Crystalline Walls Produced via Thermal Treatment with the Alkali and Alkali Earth Metal Ions. Materials. 2021; 14(18):5270. https://doi.org/10.3390/ma14185270
Chicago/Turabian StylePark, Sung Soo, Sang-Wook Chu, Liyi Shi, Shuai Yuan, and Chang-Sik Ha. 2021. "SBA-15 with Crystalline Walls Produced via Thermal Treatment with the Alkali and Alkali Earth Metal Ions" Materials 14, no. 18: 5270. https://doi.org/10.3390/ma14185270






