Simulation of Subrapid Solidification and Secondary Cooling for the Strip Casting of IF Steel
Abstract
:1. Introduction
2. Experimental Details
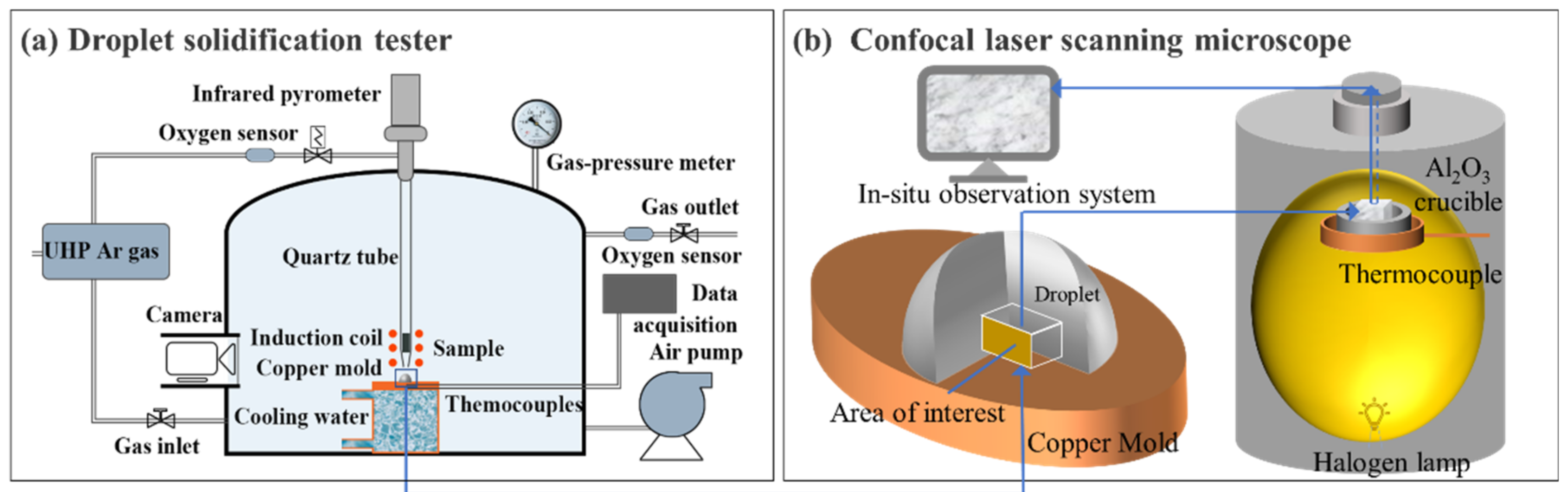
2.1. Experimental Apparatus
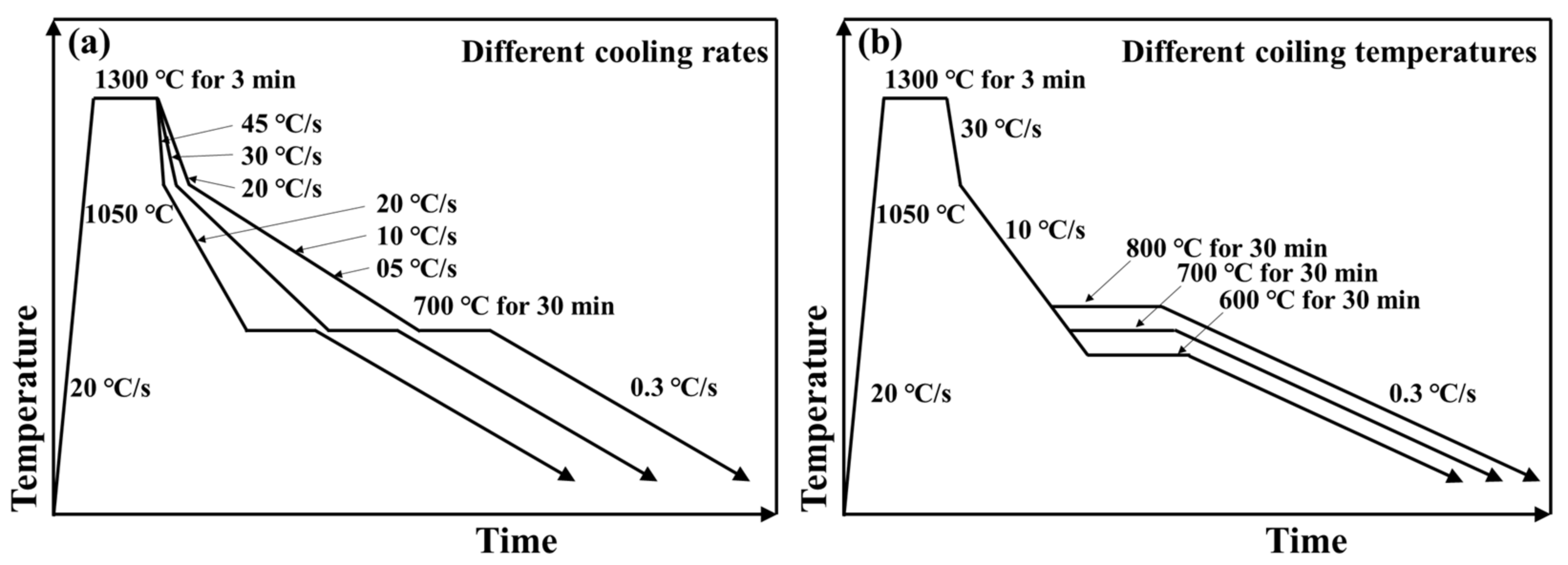
2.2. Experimental Procedure
2.3. Analytical Methods
3. Results and Discussion
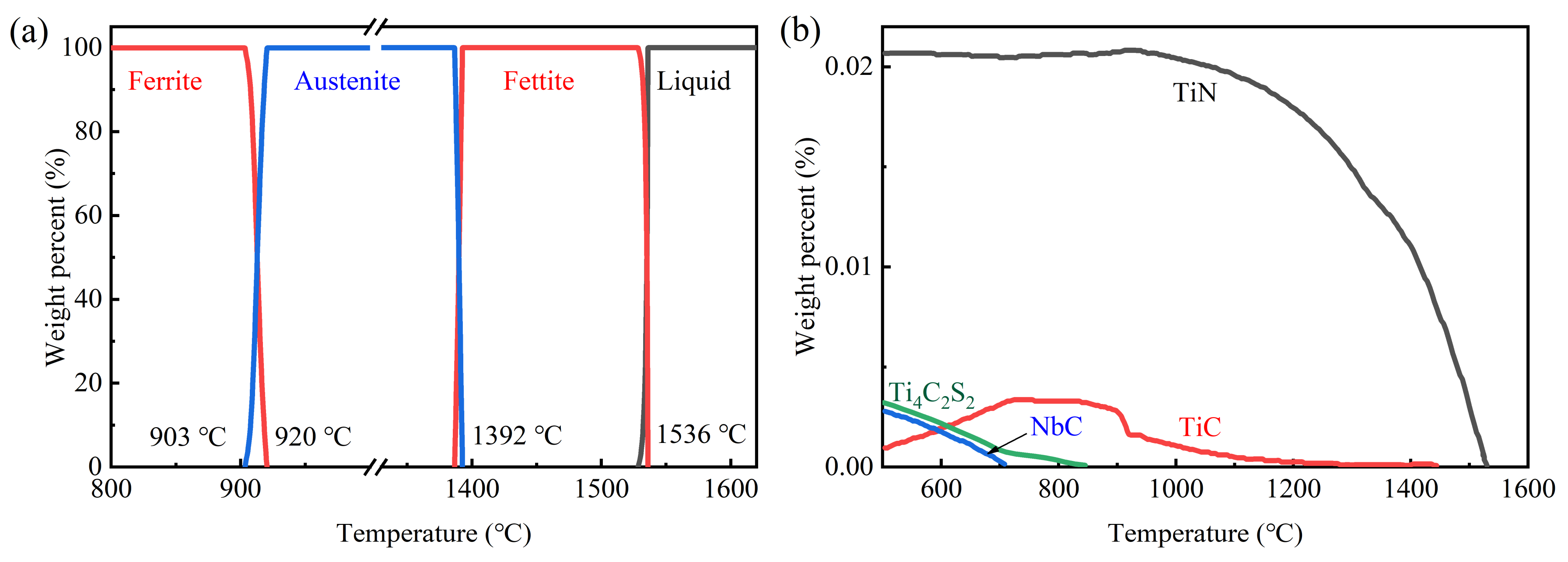
3.1. Thermodynamics Calculation and Microstructures of IF Steel Samples
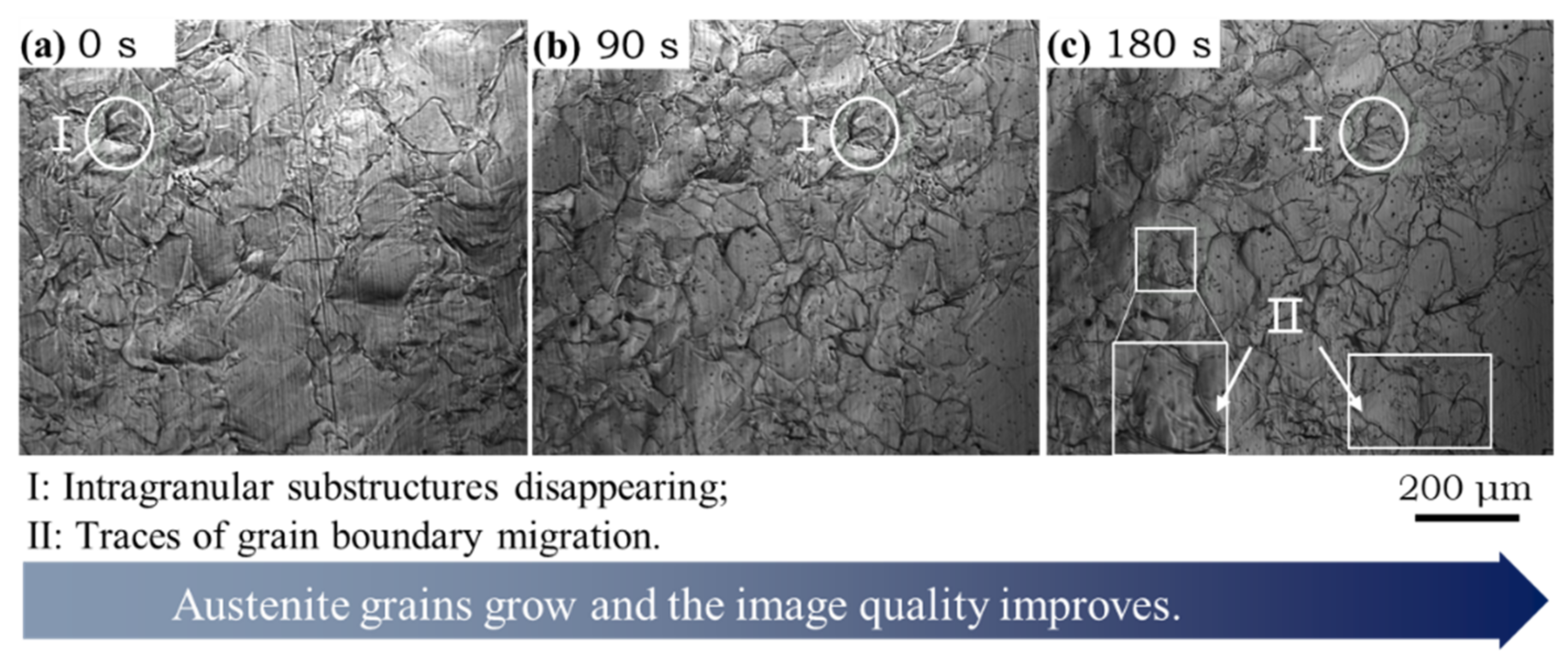
3.2. Austenite Transformation and Growth during Heating and Isothermal Processes
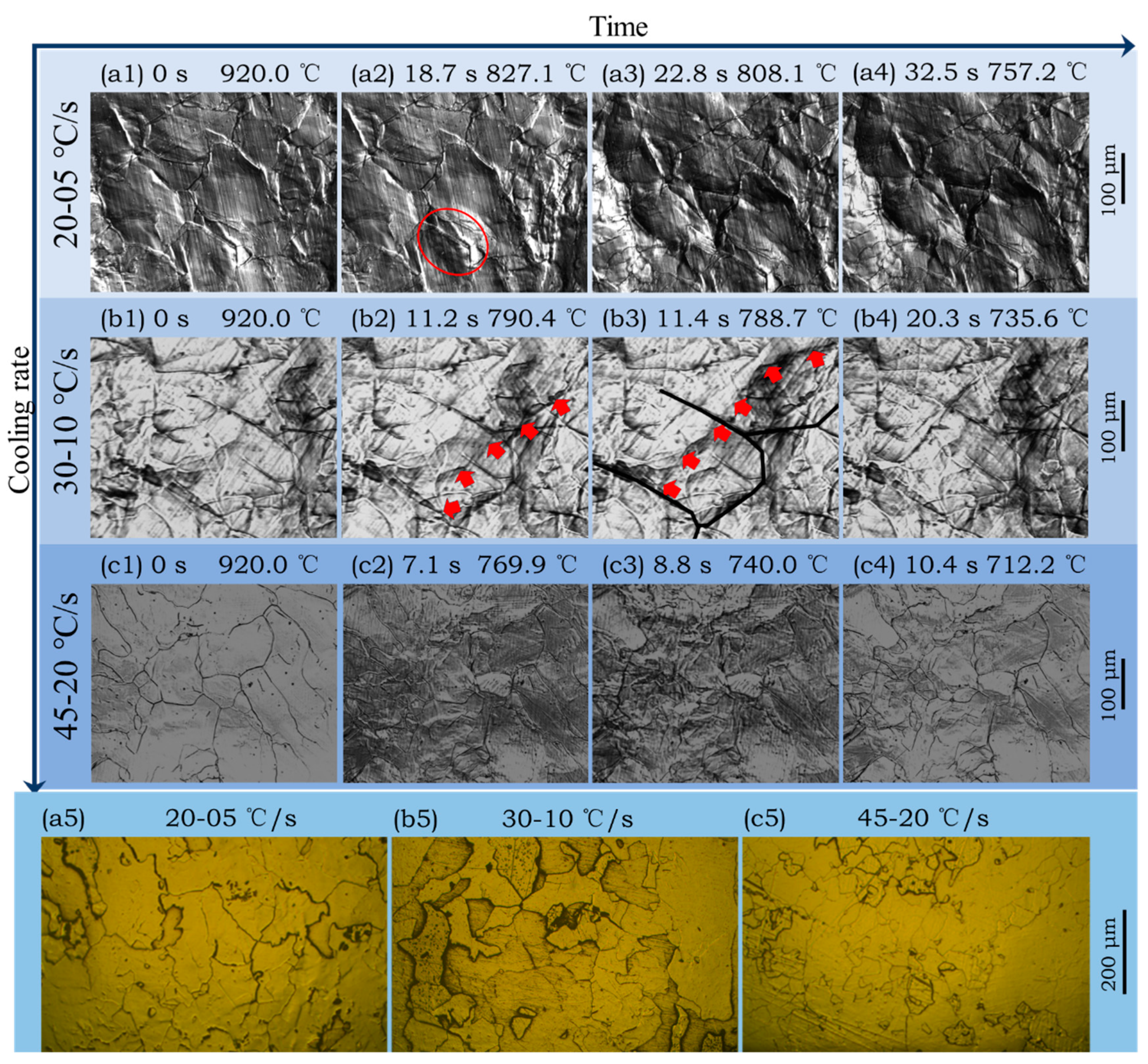
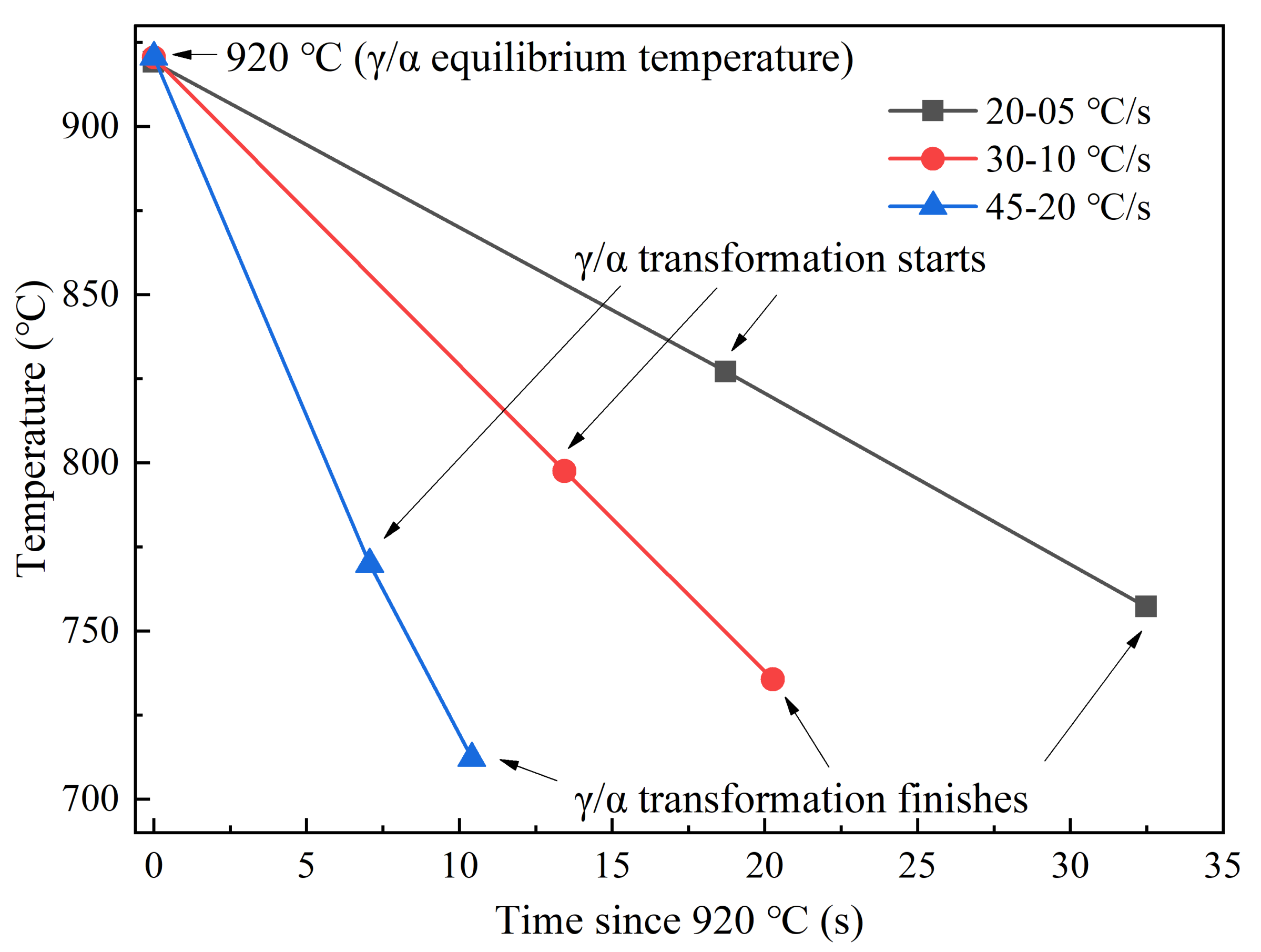
3.3. Ferrite Transformation of Subrapid Solidified IF Steel at Different Cooling Rates
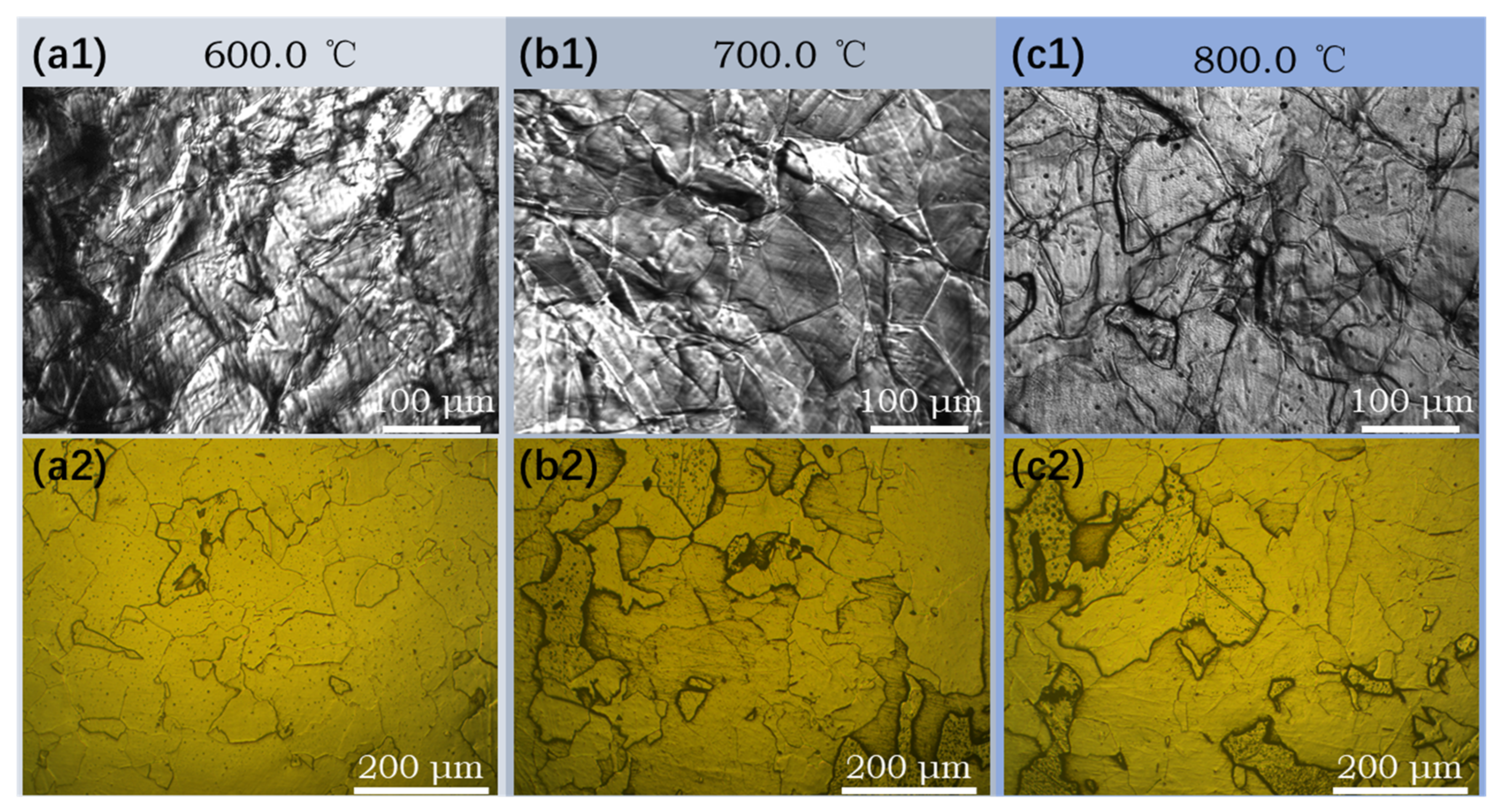
3.4. Effect of Coiling Temperature on the Microstructure of Subrapid Solidified IF Steel
3.5. Relationship between Mechanical Properties and Microstructure
4. Conclusions
- (1)
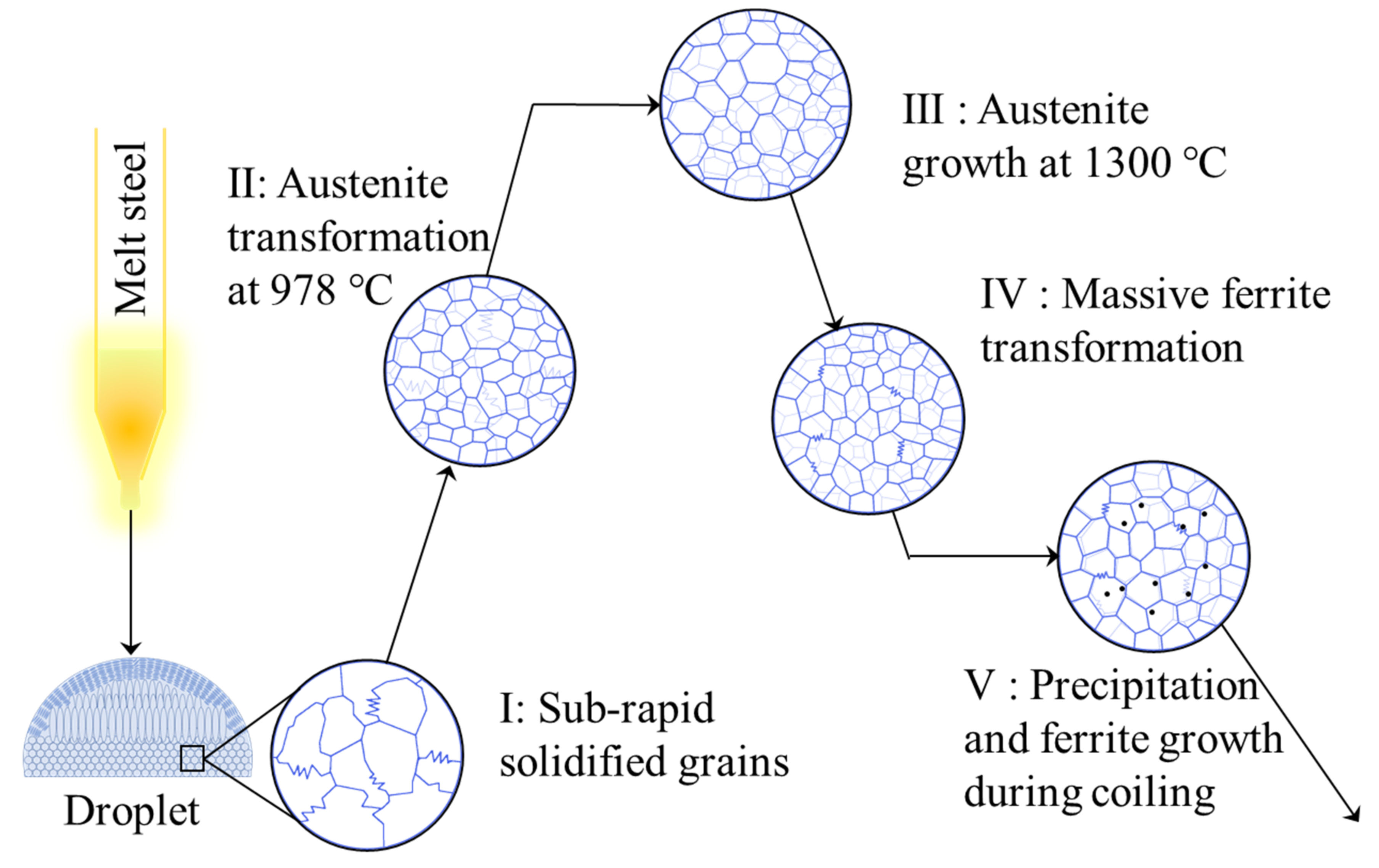
- The droplet has a hierarchical structure: fine diffusionless zone at the bottom (~2.5 mm), columnar zone in the center (~2.0 mm), and equiaxial zone at the top (~0.5 mm). By measuring dendrite arm spacing in columnar zone, the solidification rate at the bottom is indirectly estimated to be higher than 1000 °C/s, confirming that the droplet tester can simulate subrapid solidification successfully. Fine grains with irregular boundaries were formed in the bottom part of droplet during subrapid solidification. Its hardness is 84.8 HV, higher than that of the initial IF slab (77.7 HV).
- (2)
- After reheating to 1300 °C at the rate of 20 °C/s and held for 3 min, the average austenite grain size of subrapid solidified sample reached ~120 μm. Upon this microstructure, secondary cooling and hot coiling is simulated. With the help of CLSM, interface-controlled massive transformation is directly observed. Ferrite mainly nucleates at the austenite grain boundaries, and the ferrite grains grow quickly (~350 μm/s).
- (3)
- With the increase of secondary cooling rate, the γ/α transformation temperature decreases, and the incubation period and phase transformation duration are reduced. As a result, the hardness shows a slight increase due to fine-grain strengthening.
- (4)
- With coiling temperature increasing from 600 °C to 800 °C, the grain size becomes larger, precipitates such as TiN and TiC become coarser, and lattice defects of grain decrease. Consequently, the hardness of solidified sample decreases.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zapuskalov, N. Comparison of continuous strip casting with conventional technology. ISIJ Int. 2003, 43, 1115–1127. [Google Scholar] [CrossRef] [Green Version]
- Ge, S.; Isac, M.; Guthrie, R.I.L. Progress of strip casting technology for steel; historical developments. ISIJ Int. 2012, 52, 2109–2122. [Google Scholar] [CrossRef] [Green Version]
- Ge, S.; Isac, M.; Guthrie, R.I.L. Progress in strip casting technologies for steel; technical developments. ISIJ Int. 2013, 53, 729–742. [Google Scholar] [CrossRef] [Green Version]
- Guthrie, R.I.L.; Isac, M.M. Conventional and near net shape casting options for steel Sheet. Ironmak. Steelmak. 2016, 43, 650–658. [Google Scholar] [CrossRef]
- Ibarrondo, I. Review of the continuous casting of steel by strip casting technology. Twin roll method system. Rev. Metal. 2008, 44, 521–539. [Google Scholar] [CrossRef] [Green Version]
- Maleki, A.; Taherizadeh, A.; Hosseini, N. Twin roll casting of steels: An overview. ISIJ Int. 2017, 57, 1–14. [Google Scholar] [CrossRef] [Green Version]
- Chen, W.; Zhang, Y.Z.; Zhang, C.J.; Zhu, L.G.; Lu, W.G.; Wang, B.X.; Ma, J.H. Thermo-mechanical simulation and parameters optimization for beam blank continuous casting. Mater. Sci. Eng. A 2009, 499, 58–63. [Google Scholar] [CrossRef]
- Ramirez, A.; Carrillo, F.; Gonzalez, J.L.; Lopez, S. Stochastic simulation of grain growth during continuous casting. Mater. Sci. Eng. A 2006, 421, 208–216. [Google Scholar] [CrossRef]
- Zhong, H.G.; Chen, X.R.; Han, Q.Y.; Han, K.; Zhai, Q.J. A thermal simulation method for solidification process of steel slab in continuous casting. Metall. Mater. Trans. B 2016, 47, 2963–2970. [Google Scholar] [CrossRef]
- Strezov, L.; Herbertson, J.; Belton, G.R. Mechanisms of initial melt/substrate heat transfer pertinent to strip casting. Metall. Mater. Trans. B 2000, 31, 1023–1030. [Google Scholar] [CrossRef]
- Lyu, P.S.; Wang, W.L.; Qian, H.R.; Wu, J.C.; Fang, Y. Formation of naturally deposited film and its effect on interfacial heat transfer during strip casting of martensitic steel. JOM 2020, 72, 1910–1919. [Google Scholar] [CrossRef]
- Lyu, P.S.; Wang, W.L.; Wang, C.H.; Zhou, L.J.; Fang, Y.; Wu, J.C. Effect of sub-rapid solidification and secondary cooling on microstructure and properties of strip cast low-carbon bainitic-martensitic steel. Metall. Mater. Trans. A 2021, 52, 1–16. [Google Scholar] [CrossRef]
- Wang, W.L.; Qian, H.R.; Cai, D.W.; Zhou, L.J.; Mao, S.; Lyu, P.S. Microstructure and magnetic properties of 6.5 Wt Pct Si steel strip produced by simulated strip casting process. Metall. Mater. Trans. A 2021, 52, 1799–1811. [Google Scholar] [CrossRef]
- Nolli, P.; Cramb, A.W.; Choo, D.K. The effect of surface oxide films on heat transfer behavior in the strip casting process. Iron Steel Technol. 2004, 1, 117–123. [Google Scholar]
- Wang, W.L.; Zhu, C.Y.; Lu, C.; Yu, J.; Zhou, L.J. Study of the heat transfer behavior and naturally deposited films in strip casting by using droplet solidification technique. Metall. Mater. Trans. A 2018, 49, 5524–5534. [Google Scholar] [CrossRef]
- Zhu, C.Y.; Wang, W.L.; Zeng, J.; Lu, C.; Zhou, L.J.; Chang, J. Interactive relationship between the superheat, interfacial heat transfer, deposited film and microstructure in strip casting of duplex stainless steel. ISIJ Int. 2019, 59, 880–888. [Google Scholar] [CrossRef] [Green Version]
- Wang, G.X.; Matthys, E.F. Experimental determination of the interfacial heat transfer during cooling and solidification of molten metal droplets impacting on a metallic substrate: Effect of roughness and superheat. Int. J. Heat Mass Transf. 2002, 45, 4967–4981. [Google Scholar] [CrossRef]
- Hoile, S. Processing and properties of mild interstitial free steels. MST Mater. Sci. Technol. 2000, 16, 1079. [Google Scholar] [CrossRef]
- Bhagat, A.N.; Singh, A.; Gope, N.; Venugopalan, T. Development of cold-rolled high-strength formable steel for automotive applications. Mater. Manuf. Process. 2010, 25, 202–205. [Google Scholar] [CrossRef]
- Han, D.; Zhu, X.; Li, S.; Liao, X.; Ai, X. Study on cleanliness of interstitial-free (If) steel continuous casting slab. Metalurgija 2021, 60, 11–14. [Google Scholar]
- Deng, X.X.; Ji, C.X.; Cui, Y.; Tian, Z.H.; Yin, X.; Shao, X.J.; Yang, Y.D.; McLean, A. Formation and evolution of macro inclusions in if steels during continuous casting. Ironmak. Steelmak. 2017, 44, 739–749. [Google Scholar] [CrossRef]
- Santos, A.P.D.; da Mota, T.C.; Segundo, H.V.G.; de Almeida, L.H.; Araujo, L.S.; Rocha, A.D. Texture, microstructure and anisotropic properties of if-steels with different additions of titanium, niobium and phosphorus. J. Mater. Res. Technol. JMRT 2018, 7, 331–336. [Google Scholar] [CrossRef]
- Farias, F.; Balbi, M.; Batista, M.N.; Alvarez-Armas, I. Characterization of a continuously cooled dual-phase steel microstructure. Metall. Mater. Trans. A 2018, 49, 6010–6021. [Google Scholar] [CrossRef]
- Massalski, T.B. Distinguishing features of massive transformations. Metall. Trans. A 1984, 15, 421–425. [Google Scholar] [CrossRef]
- Massalski, T.B. Massive transformations revisited. Metall. Mater. Trans. A 2002, 33, 2277–2283. [Google Scholar] [CrossRef]
- Perepezko, J.H.; Massalski, T.B. Rapid growth of single crystals in the solid state utilizing a massive transformation. J. Mater. Sci. 1974, 9, 899–910. [Google Scholar] [CrossRef]
- Krauss, G.; Thompson, S.W. Ferritic microstructures in continuously cooled low—And ultralow-carbon steels. ISIJ Int. 1995, 35, 937–945. [Google Scholar] [CrossRef]
- Lee, J.M.; Shibata, K.; Asakura, K.; Masumoto, Y. Observation of γ→α transformation in ultralow-carbon steel under a high temperature optical microscope. ISIJ Int. 2002, 42, 1135–1143. [Google Scholar] [CrossRef]
- Xiong, Z.P.; Kostryzhev, A.G.; Stanford, N.E.; Pereloma, E.V. Microstructures and mechanical properties of dual phase steel produced by laboratory simulated strip casting. Mater. Des. 2015, 88, 537–549. [Google Scholar] [CrossRef]
- Xiong, Z.P.; Kostryzhev, A.G.; Stanford, N.E.; Pereloma, E.V. Effect of deformation on microstructure and mechanical properties of dual phase steel produced via strip casting simulation. Mater. Sci. Eng. A 2016, 651, 291–305. [Google Scholar] [CrossRef] [Green Version]
- Allain, S.; Chateau, J.P.; Bouaziz, O.; Migot, S.; Guelton, N. Correlations between the Calculated stacking fault energy and the plasticity mechanisms in Fe-Mn-C Alloys. Mater. Sci. Eng. A 2004, 387, 158–162. [Google Scholar] [CrossRef]
- Hua, M.; Garcia, C.I.; DeArdo, A.J. Precipitation behavior in ultra-low-carbon steels containing titanium and niobium. Metall. Mater. Trans. A 1997, 28, 1769–1780. [Google Scholar] [CrossRef]
- Jacobi, H.; Schwerdtfeger, K. Dendrite morphology of steady state unidirectionally solidified steel. Metall. Trans. A 1976, 7, 811–820. [Google Scholar] [CrossRef]
- Carpenter, K.R.; Killmore, C.R. The effect of Nb on the continuous cooling transformation curves of ultra-thin strip castrip((C)) steels. Metals 2015, 5, 1857–1877. [Google Scholar] [CrossRef]
- Liu, F.; Sommer, F.; Mittemeijer, E.J. Analysis of the kinetics of phase transformations; roles of nucleation index and temperature dependent site saturation, and recipes for the extraction of kinetic parameters. J. Mater. Sci. 2007, 42, 573–587. [Google Scholar] [CrossRef]
- Liu, B.P.-H.; Chung, T.-F.; Yang, J.-R.; Fu, J.; Chen, C.-Y.; Wang, S.-H.; Tsai, M.-C.; Huang, C.-Y. Microstructure characterization of massive ferrite in laser-weldments of interstitial-free steels. Metals 2020, 10, 898. [Google Scholar] [CrossRef]
- Yang, G.W.; Sun, X.J.; Li, Z.D.; Li, X.X.; Yong, Q.L. Effects of vanadium on the microstructure and mechanical properties of a high strength low alloy martensite steel. Mater. Des. 2013, 50, 102–107. [Google Scholar] [CrossRef]




| C | N | Si | Mn | P | S | Al | Ti | Nb |
|---|---|---|---|---|---|---|---|---|
| 0.0008 | 0.0050 | 0.005 | 0.107 | 0.0113 | 0.0041 | 0.0335 | 0.0189 | 0.0112 |
| Samples | Vickers Hardness | Estimated Yield Strength (MPa) | Estimated Ultimate Tensile Strength (MPa) |
|---|---|---|---|
| Initial IF slab | 77.7 ± 3.4 | 132.8 | 190.4 |
| As-cast subrapid solidified sample | 84.8 ± 3.2 | 153.1 | 216.8 |
| Cooling-20–05 °C/s | 78.7 ± 3.5 | 135.7 | 194.2 |
| Coiling-600 °C | 90.1 ± 3.8 | 168.3 | 236.5 |
| Cooling-30–10 °C/s (Coiling-700 °C) | 81.1 ± 4.2 | 142.7 | 203.2 |
| Coiling-800 °C | 80.4 ± 1.8 | 140.4 | 200.3 |
| Cooling-45–20 °C/s | 82.4 ± 1.2 | 146.4 | 208.0 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, W.; Mao, S.; Zhang, H.; Lu, C.; Lyu, P. Simulation of Subrapid Solidification and Secondary Cooling for the Strip Casting of IF Steel. Materials 2021, 14, 5274. https://doi.org/10.3390/ma14185274
Wang W, Mao S, Zhang H, Lu C, Lyu P. Simulation of Subrapid Solidification and Secondary Cooling for the Strip Casting of IF Steel. Materials. 2021; 14(18):5274. https://doi.org/10.3390/ma14185274
Chicago/Turabian StyleWang, Wanlin, Song Mao, Hualong Zhang, Cheng Lu, and Peisheng Lyu. 2021. "Simulation of Subrapid Solidification and Secondary Cooling for the Strip Casting of IF Steel" Materials 14, no. 18: 5274. https://doi.org/10.3390/ma14185274






