Effect of Sc and Zr Additions on Recrystallization Behavior and Intergranular Corrosion Resistance of Al-Zn-Mg-Cu Alloys
Abstract
:1. Introduction
2. Materials and Methods
3. Results
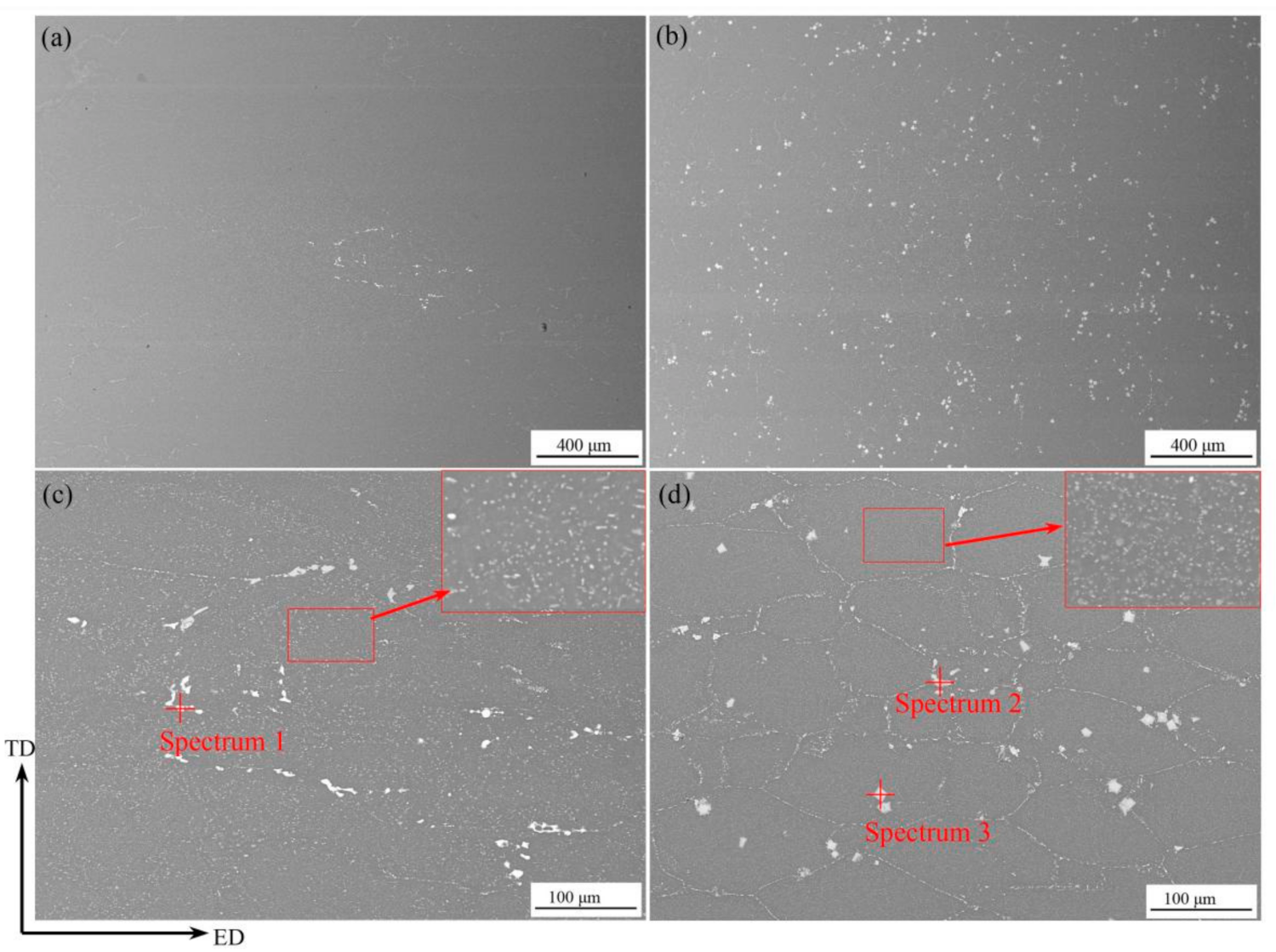
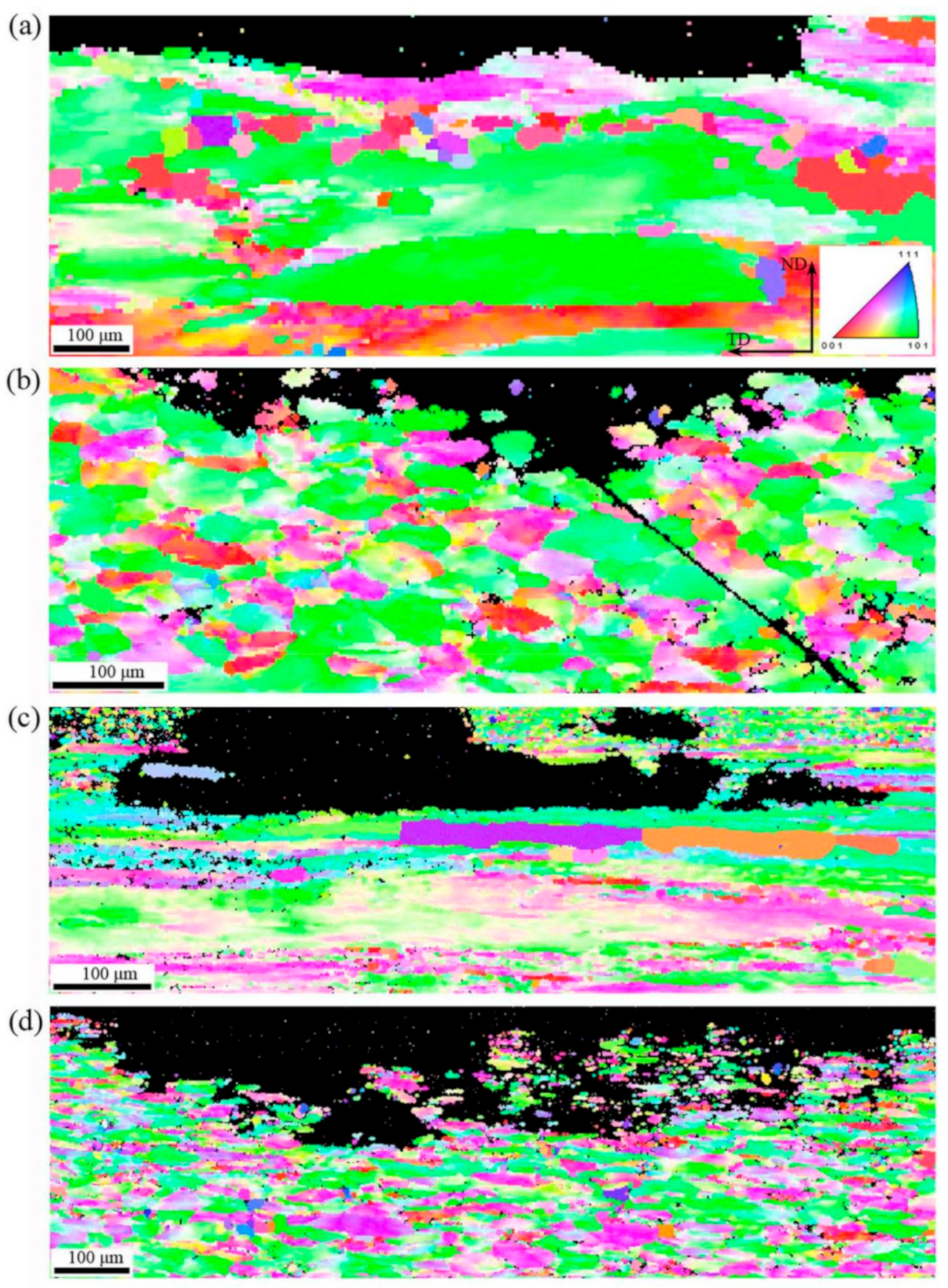
3.1. Microstructure
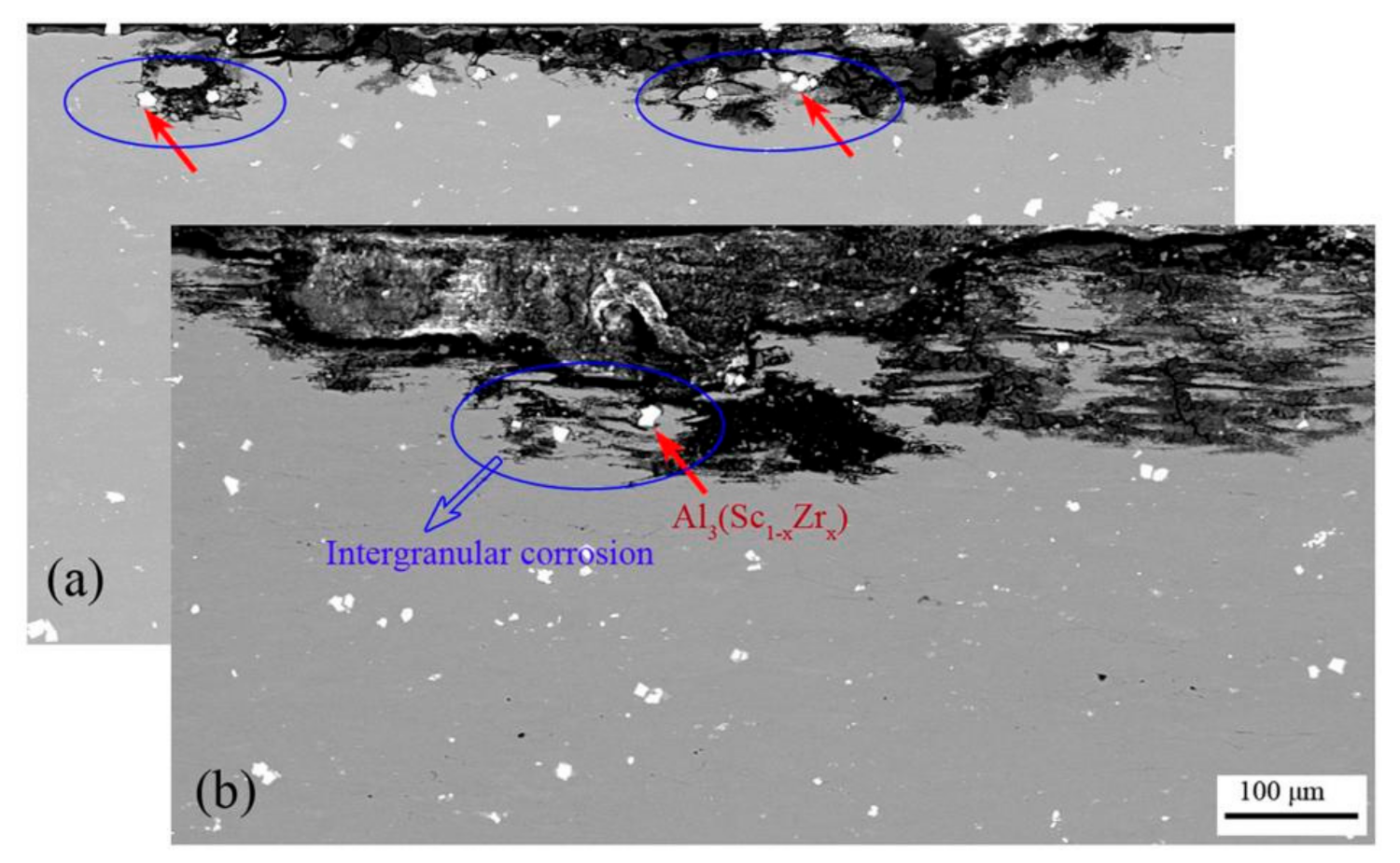
3.2. IGC Behavior
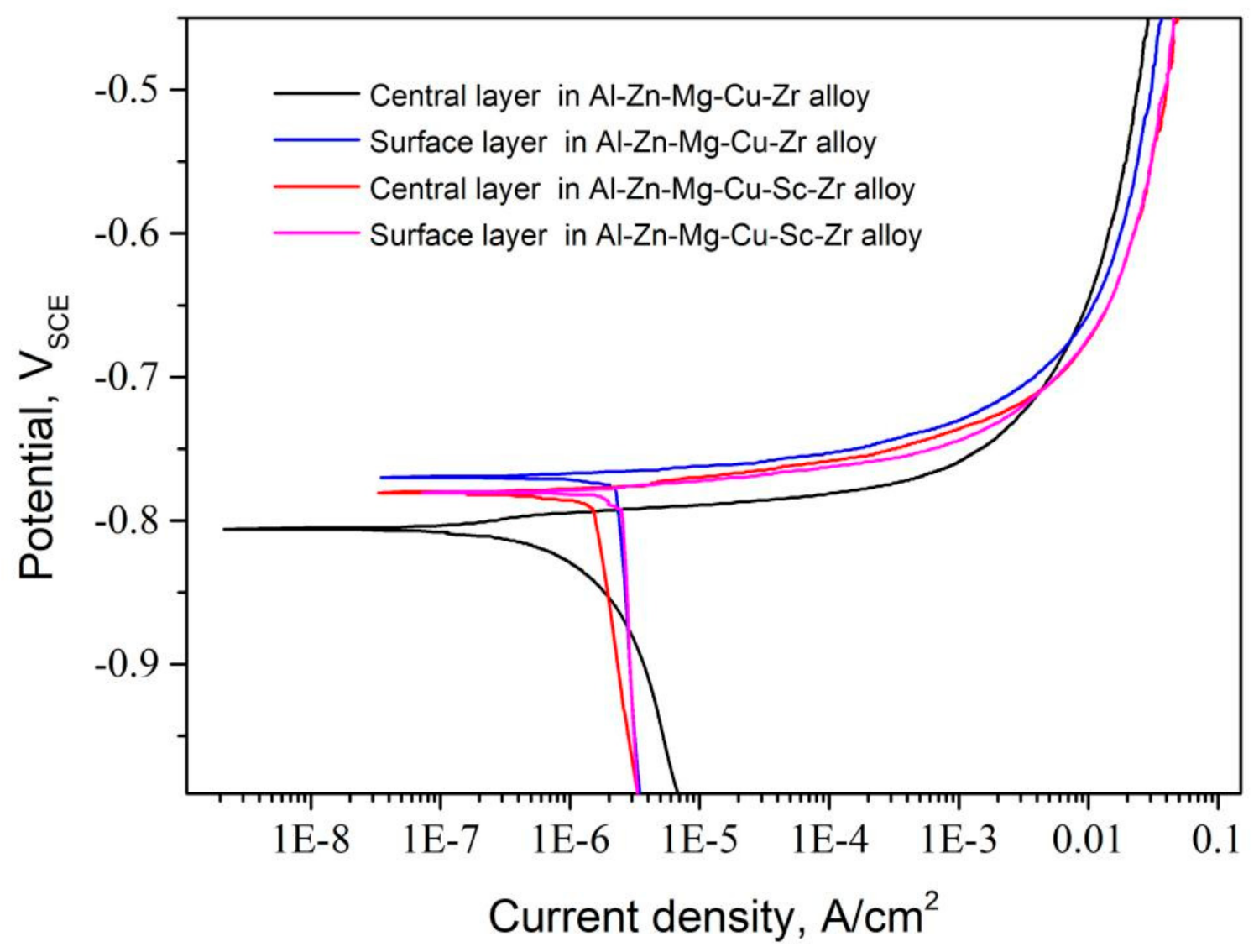
3.3. Electrochemical Characteristics
4. Discussion
4.1. Microstructures and Recrystallization Behavior
4.2. Intergranular Corrosion Behavior
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Heinz, A.; Haszler, A.; Keidel, C.; Moldenhauer, S.; Benedictus, R.; Miller, W.S. Recent development in aluminium alloys for aerospace applications. Mater. Sci. Eng. A 2000, 280, 102–110. [Google Scholar] [CrossRef]
- Marlaud, T.; Deschamps, A.; Bley, F.; Lefebvre, W.; Barouxa, B. Evolution of precipitate microstructures during the retrogression and re-ageing heat treatment of an Al-Zn-Mg-Cu alloy. Acta Mater. 2010, 58, 4814–4826. [Google Scholar] [CrossRef]
- Wen, K.; Fan, Y.; Wang, G.; Jin, L.; Li, X.; Li, Z.; Zhang, Y.; Xiong, B. Aging behavior and precipitate characterization of a high Zn-containing Al-Zn-Mg-Cu alloy with various tempers. Mater. Des. 2016, 101, 16–23. [Google Scholar] [CrossRef]
- Song, R.G.; Dietzel, W.; Zhang, B.J.; Liu, W.J.; Tseng, M.K.; Airens, A. Stress corrosion cracking and hydrogen embritlement of all Al-Zn-Mg-Cu alloy. Acta Mater. 2004, 52, 4727–4743. [Google Scholar] [CrossRef]
- Sun, X.; Zhang, B.; Lin, H.; Zhou, Y.; Sun, L.; Wang, J.; Han, E.-H.; Ke, W. Correlations between stress corrosion cracking susceptibility and grain boundary microstructures for an Al-Zn-Mg alloy. Corros. Sci. 2013, 77, 103–112. [Google Scholar] [CrossRef]
- Tian, W.; Li, S.; Wang, B.; Liu, J.; Yu, M. Pitting corrosion of naturally aged AA 7075 aluminum alloys with bimodal grain size. Corros. Sci. 2016, 113, 1–16. [Google Scholar] [CrossRef]
- Kannan, M.B.; Raja, V.S.; Raman, R.; Mukhopadhyay, A.K. Influence of multi-step aging on the stress corrosion cracking behavior of 7010 Al Alloy. Corros. Sci. 2003, 59, 881–889. [Google Scholar] [CrossRef]
- Li, J.-F.; Peng, Z.-W.; Li, C.-X.; Jia, Z.-Q.; Chen, W.-J.; Zheng, Z.-Q. Mechanical properties, corrosion behaviors and microstructures of 7075 aluminium alloy with various aging treatments. Trans. Nonferrous Met. Soc. China 2008, 18, 755–762. [Google Scholar] [CrossRef]
- Peng, G.; Chen, K.; Chen, S.; Fang, H. Influence of repetitious-RRA treatment on the strength and SCC resistance of Al-Zn-Mg-Cu alloy. Mater. Sci. Eng. A 2011, 528, 4014–4018. [Google Scholar] [CrossRef]
- Song, J.; Curtin, W.A. Ananoscale mechanism of hydrogen embrittlement in metals. Acta Mater. 2011, 59, 1557–1569. [Google Scholar] [CrossRef]
- Bobby Kannan, M.; Raja, V.S. Enhancing stress corrosion cracking resistance in Al-Zn-Mg-Cu-Zr alloy through inhibiting recrystallization. Eng. Fract. Mech. 2010, 77, 249–256. [Google Scholar] [CrossRef]
- Fang, H.C.; Chao, H.; Chen, K.H. Effect of recrystallization on intergranular fracture and corrosion of Al-Zn-Mg-Cu-Zr alloy. J. Alloys Compd. 2015, 622, 166–173. [Google Scholar] [CrossRef]
- Deng, Y.; Yin, Z.M.; Zhao, K.; Duan, J.Q.; Hu, J.; He, Z.B. Effects of Sc and Zr microalloying dditions and aging time at 120 °C on the corrosion behaviour of an Al-Zn-Mg alloy. Corros. Sci. 2012, 65, 288–298. [Google Scholar] [CrossRef]
- Shi, Y.J.; Pan, Q.L.; Li, M.J.; Huang, X.; Li, B. Effect of Sc and Zr additions on corrosion behaviour of Al-Zn-Mg-Cu alloys. J. Alloys Compd. 2014, 612, 42–50. [Google Scholar] [CrossRef]
- Liu, L.; Jia, Y.-Y.; Jiang, J.-T.; Zhang, B.; Li, G.-A.; Shao, W.-Z.; Zhen, L. The effect of Cu and Sc on the localized corrosion resistance of Al-Zn-Mg-X alloys. J. Alloys Compd. 2019, 799, 1–14. [Google Scholar] [CrossRef]
- Sun, F.; Nash, G.L.; Li, Q.; Liu, E.; He, C.; Shi, C.; Zhao, N. Effect of Sc and Zr additions on microstructures and corrosion behavior of Al-Cu-Mg-Sc-Zr alloys. J. Mater. Sci. Technol. 2017, 33, 1015–1022. [Google Scholar] [CrossRef]
- Liu, J.; Yao, P.; Zhao, N.Q.; Shi, C.S.; Li, H.J.; Li, X.; Xi, D.S.; Yang, S. Effect of minor Sc and Zr on recrystallization behavior and mechanical properties of novel Al-Zn-Mg-Cu alloys. J. Alloys Compd. 2016, 657, 717–725. [Google Scholar] [CrossRef]
- Li, Z.; Jiang, H.; Wang, Y.; Zhang, D.; Yan, D.; Rong, L. Effect of minor Sc addition on microstructure and stress corrosion cracking behavior of medium strength Al-Zn-Mg alloy. J. Mater. Sci. Technol. 2018, 34, 1172–1179. [Google Scholar] [CrossRef]
- Senkov, O.N.; Shagiev, M.R.; Senkova, S.V.; Miracle, D.B. Precipitation of Al3 (Sc,Zr) particles in an Al-Zn-Mg-Cu-Sc-Zr alloy during conventional solution heat treatment and its effect on tensile properties. Acta Mater. 2008, 56, 3723–3738. [Google Scholar] [CrossRef]
- Huang, X.; Pan, Q.L.; Li, B.; Liu, Z.M.; Huang, Z.Q.; Yin, Z.M. Effect of minor Sc and Zr on recrystallization behavior and mechanical properties of novel Al-Zn-Mg-Cu alloys. J. Alloys Compd. 2015, 650, 805–820. [Google Scholar] [CrossRef]
- Li, G.; Zhao, N.Q.; Liu, T.; Li, J.J.; He, C.N.; Shi, C.S.; Liu, E.Z.; Sha, J.W. Effect of Sc/Zr ratio on the microstructure and mechanical properties of new type of Al-Zn-Mg-Sc-Zr alloys. Mater. Sci. Eng. A 2014, 617, 219–227. [Google Scholar] [CrossRef]
- Deng, Y.; Yin, Z.; Zhao, K.; Duan, J.; He, Z. Effects of Sc and Zr microalloying additions on the microstructure and mechanical properties of new Al-Zn-Mg alloys. J. Alloys Compd. 2012, 530, 71–80. [Google Scholar] [CrossRef]
- Mukhopadhyay, A.K.; Kumar, A.; Raveendra, S.; Samajdar, I. Development of grain structure during superplastic deformation of an Al-Zn-Mg-Cu-Zr alloy containing Sc. Scr. Mater. 2011, 64, 386–389. [Google Scholar] [CrossRef]
- Shi, Y.; Pan, Q.; Li, M.; Huang, X.; Li, B. Influence of alloyed Sc and Zr, and heat treatment on microstructures and stress corrosion cracking of Al-Zn-Mg-Cu alloys. Mater. Sci. Eng. A 2015, 621, 173–181. [Google Scholar] [CrossRef]
- Van Geertruyden, W.H.; Browne, H.M.; Misiolek, W.Z.; Wang, P.T. Evolution of surface recrystallization during indirect extrusion of 6xxx aluminum alloys. Met. Mater. Trans. A 2004, 36, 1049–1056. [Google Scholar] [CrossRef]
- Van Geertruyden, W.H.; Misiolek, W.Z.; Wang, P.T. Surface grain structure development during indirect extrusion of 6xxx aluminum alloys. J. Mater. Sci. 2005, 40, 3861–3863. [Google Scholar] [CrossRef]
- Knipling, K.E.; Seidman, D.N.; Dunand, D.C. Ambient- and high-temperature mechanical properties of isochronally aged Al-0.06Sc, Al-0.06Zr and Al-0.06Sc-0.06Zr (at.%) alloys. Acta Mater. 2011, 59, 943–954. [Google Scholar] [CrossRef] [Green Version]
- Geist, D.; Ii, S.; Tsuchiya, K.; Karnthaler, H.; Stefanov, G.; Rentenberger, C. Nanocrystalline Zr3Al made through amorphization by repeated cold rolling and followed by crystallization. J. Alloys Compd. 2011, 509, 1815–1818. [Google Scholar] [CrossRef] [Green Version]
- GB 7998-2005. National Standard of China. Test Method for Inter-Granular Corrosion of Aluminum Alloys; Standardization Administration of People’s Republic of China: Beijing, China, 2005. [Google Scholar]
- Liu, Y.; Jiang, D.M.; Xie, W.L.; Hu, J.; Ma, B.R. Solidification phases and their evolution during homogenization of a DC cast Al-8.35Zn-2.5Mg-2.25Cu alloy. Mater. Char. 2014, 93, 173–183. [Google Scholar] [CrossRef]
- Liu, S.D.; Yuan, Y.B.; Li, C.B.; You, J.H.; Zhang, X.M. Influence of cooling rate after homogenization on microstructure and mechanical properties of aluminum alloy 7050. Met. Mater. Int. 2012, 18, 679–683. [Google Scholar] [CrossRef]
- Wen, K.; Xiong, B.Q.; Zhang, Y.A.; Wang, G.J.; Li, X.W.; Li, Z.H.; Huang, S.H.; Liu, H.W. Microstructure evolution of a high zinc containing Al-Zn-Mg-Cu alloy during homogenization. Rare Metal Mat. Eng. 2017, 46, 928–934. [Google Scholar]
- Li, J.; Wiessner, M.; Albu, M.; Wurster, S.; Sartory, B.; Hofer, F.; Schumacher, P. Correlative characterization of primary Al3 (Sc,Zr) phase in an Al-Zn-Mg based alloy. Mater. Charact. 2015, 102, 62–70. [Google Scholar] [CrossRef]
- Wloka, J.; Virtanen, S. Influence of scandium on the pitting behaviour of Al-Zn-Mg-Cu alloys. Acta Mater. 2007, 55, 6666–6672. [Google Scholar] [CrossRef]
- Ryu, J.H.; Lee, D.N. The effect of precipitation on the evolution of recrystallization texture in AA8011 aluminum alloy sheet. Mater. Sci. Eng. A 2002, 336, 225–232. [Google Scholar] [CrossRef]
- Tang, J.; Wang, J.H.; Teng, J.; Wang, G.; Fu, D.F.; Zhang, H.; Jiang, F.L. Effect of Zn content on the dynamic softening of Al-Zn-Mg-Cu alloys during hot compression deformation. Vacuum 2021, 184, 109941. [Google Scholar] [CrossRef]
- Fang, H.; Luo, F.; Chen, K. Effect of intermetallic phases and recrystallization on the corrosion and fracture behavior of an Al-Zn-Mg-Cu-Zr-Yb-Cr alloy. Mater. Sci. Eng. A 2017, 684, 480–490. [Google Scholar] [CrossRef]
- She, H.; Shu, D.; Dong, A.; Wang, J.; Sun, B.; Lai, H. Relationship of particle stimulated nucleation, recrystallization and mechanical properties responding to Fe and Si contents in hot-extruded 7055 aluminum alloys. J. Mater. Sci. Technol. 2019, 35, 2570–2581. [Google Scholar] [CrossRef]
- Adam, K.F.; Long, Z.; Field, D. Analysis of particle-stimulated nucleation (PSN)-dominated recrystallization for hot-rolled 7050 aluminum alloy. Met. Mater. Trans. A 2017, 48, 2062–2076. [Google Scholar] [CrossRef]
- Fang, H.C.; Chen, K.H.; Chen, X.; Chao, H.; Peng, G.S. Effect of Cr, Yb and Zr additions on localized corrosion of Al-Zn-Mg-Cu alloy. Corros. Sci. 2009, 51, 2872–2877. [Google Scholar] [CrossRef]
- Conde, A.; de Damborenea, J. Electrochemical modelling of exfoliation corrosion behaviour of 8090 alloy. Electrochim. Acta 1998, 43, 849–860. [Google Scholar] [CrossRef]
- Xiao, Y.P.; Pan, Q.L.; Li, W.B.; Liu, X.Y.; He, Y.B. Influence of retrogression and reaging treatment on corrosion behaviour of an Al-Zn-Mg-Cu alloy. Mater. Des. 2011, 32, 2149–2156. [Google Scholar] [CrossRef]
- Deng, Y.-L.; Zhang, Y.-Y.; Wan, L.; Zhu, A.A.; Zhang, X.-M. Three-stage homogenization of Al-Zn-Mg-Cu alloys containing trace Zr. Met. Mater. Trans. A 2013, 44, 2470–2477. [Google Scholar] [CrossRef]
- Schöbel, M.; Pongratz, P.; Degischer, H. Coherency loss of Al3 (Sc,Zr) precipitates by deformation of an Al-Zn-Mg alloy. Acta Mater. 2012, 60, 4247–4254. [Google Scholar] [CrossRef]
- Jiang, J.; Jiang, F.; Zhang, M.; Tang, Z.; Tong, M. Recrystallization behavior of Al-Mg-Mn-Sc-Zr alloy based on two different deformation ways. Mater. Lett. 2020, 265, 127455. [Google Scholar] [CrossRef]
- Robson, J. Modelling the evolution of particle size distribution during nucleation, growth and coarsening. Mater. Sci. Technol. 2004, 20, 441–448. [Google Scholar] [CrossRef]
- Zhao, J.H.; Deng, Y.L.; Tang, J.G.; Zhang, J. Effect of gradient grain structures on corrosion resistance of extruded Al-Zn-Mg-Cu alloy. J. Alloys Compd. 2020, 832, 154911. [Google Scholar] [CrossRef]
- Cavanaugh, M.; Birbilis, N.; Buchheit, R.; Bovard, F. Investigating localized corrosion susceptibility arising from Sc containing intermetallic Al3Sc in high strength Al-alloys. Scr. Mater. 2007, 56, 995–998. [Google Scholar] [CrossRef]
- Li, J.H.; Oberdorfer, B.; Wurster, S.; Schumacher, P. Impurity effects on the nucleation and growth of primary Al3 (Sc,Zr) phase in Al alloys. J. Mater. Sci. 2014, 49, 5961–5977. [Google Scholar] [CrossRef]
- Dang, J.-Z.; Huang, Y.-F.; Cheng, J. Effect of Sc and Zr on microstructures and mechanical properties of as-cast Al-Mg-Si-Mn alloys. Trans. Nonferrous Met. Soc. China 2009, 19, 540–544. [Google Scholar] [CrossRef]
- Dong, Q.S.; Howells, A.; Lloyd, D.J.; Gallerneault, M.; Fallah, V. Effect of solidification cooling rate on kinetics of continuous/discontinuous Al3 (Sc,Zr) precipitation and the subsequent age-hardening response in cold-rolled AlMgSc (Zr) sheets. Mater. Sci. Eng. A 2020, 772, 138693. [Google Scholar] [CrossRef]








| Alloys | Cu | Mg | Zn | Zr | Sc | Si | Fe | Al |
|---|---|---|---|---|---|---|---|---|
| Al-Zn-Mg-Cu-Zr | 1.8 | 2.2 | 8.9 | 0.16 | - | 0.03 | 0.03 | Bal. |
| Al-Zn-Mg-Cu-Sc-Zr | 1.8 | 2.3 | 8.8 | 0.16 | 0.30 | 0.03 | 0.03 | Bal. |
| Spectrum Locations | Mg | Al | Cu | Zn | Sc | Zr |
|---|---|---|---|---|---|---|
| Spectrum 1 | 33.06 | 25.64 | 16.47 | 24.82 | 0 | 0 |
| Spectrum 2 | 19.50 | 55.79 | 9.40 | 15.31 | 0 | 0 |
| Spectrum 3 | 0 | 73.23 | 0 | 2.17 | 14.74 | 9.86 |
| Samples | Depth/Width, μm/μm | ||
|---|---|---|---|
| Center layer in Al-Zn-Mg-Cu-Zr alloy | 287/875 | 145/2096 | 125/805 |
| Center layer in Al-Zn-Mg-Cu-Sc-Zr alloy | 117/742 | 82/524 | 75/751 |
| Surface layer in Al-Zn-Mg-Cu-Zr alloy | 166/626 | 108/893 | 85/1031 |
| Surface layer in Al-Zn-Mg-Cu-Sc-Zr alloy | 189/983 | 184/1222 | 175/1773 |
| Samples | Icorr/μA·cm−2 | Ecorr/VSCE |
|---|---|---|
| Center layer in Al-Zn-Mg-Cu-Zr alloy | 2.09 | −0.796 |
| Surface layer in Al-Zn-Mg-Cu-Zr alloy | 2.33 | −0.775 |
| Center layer in Al-Zn-Mg-Cu-Sc-Zr alloy | 1.50 | −0.785 |
| Surface layer in Al-Zn-Mg-Cu-Sc-Zr alloy | 2.51 | −0.783 |
| Samples | Rs | Rt | CPE1-T | CPE1-P |
|---|---|---|---|---|
| Center layer in Al-Zn-Mg-Cu-Zr alloy | 0.064 | 81.06 | 9.0036E-4 | 0.856 |
| Surface layer in Al-Zn-Mg-Cu-Zr alloy | 0.075 | 76.83 | 8.1234E-4 | 0.886 |
| Center layer in Al-Zn-Mg-Cu-Sc-Zr alloy | 0.063 | 97.29 | 8.4542E-4 | 0.876 |
| Surface layer in Al-Zn-Mg-Cu-Sc-Zr alloy | 0.105 | 57.19 | 1.5015E-4 | 0.886 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xia, P.; Wang, S.; Huang, H.; Zhou, N.; Song, D.; Jia, Y. Effect of Sc and Zr Additions on Recrystallization Behavior and Intergranular Corrosion Resistance of Al-Zn-Mg-Cu Alloys. Materials 2021, 14, 5516. https://doi.org/10.3390/ma14195516
Xia P, Wang S, Huang H, Zhou N, Song D, Jia Y. Effect of Sc and Zr Additions on Recrystallization Behavior and Intergranular Corrosion Resistance of Al-Zn-Mg-Cu Alloys. Materials. 2021; 14(19):5516. https://doi.org/10.3390/ma14195516
Chicago/Turabian StyleXia, Peng, Shuncheng Wang, Huilan Huang, Nan Zhou, Dongfu Song, and Yiwang Jia. 2021. "Effect of Sc and Zr Additions on Recrystallization Behavior and Intergranular Corrosion Resistance of Al-Zn-Mg-Cu Alloys" Materials 14, no. 19: 5516. https://doi.org/10.3390/ma14195516





