pH-Triggered Release Performance of Microcapsule-Based Inhibitor and Its Inhibition Effect on the Reinforcement Embedded in Mortar
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Methods
2.2.1. Compressive Strength and Flexural Strength
2.2.2. Release Amount of BTA Encapsulated in MCI
2.2.3. Morphology and Composition of the Mortar Samples
2.2.4. Electrochemical Behavior of Reinforced Mortar
2.2.5. Surface Analysis of the Reinforcement Embedded in Mortar
3. Results
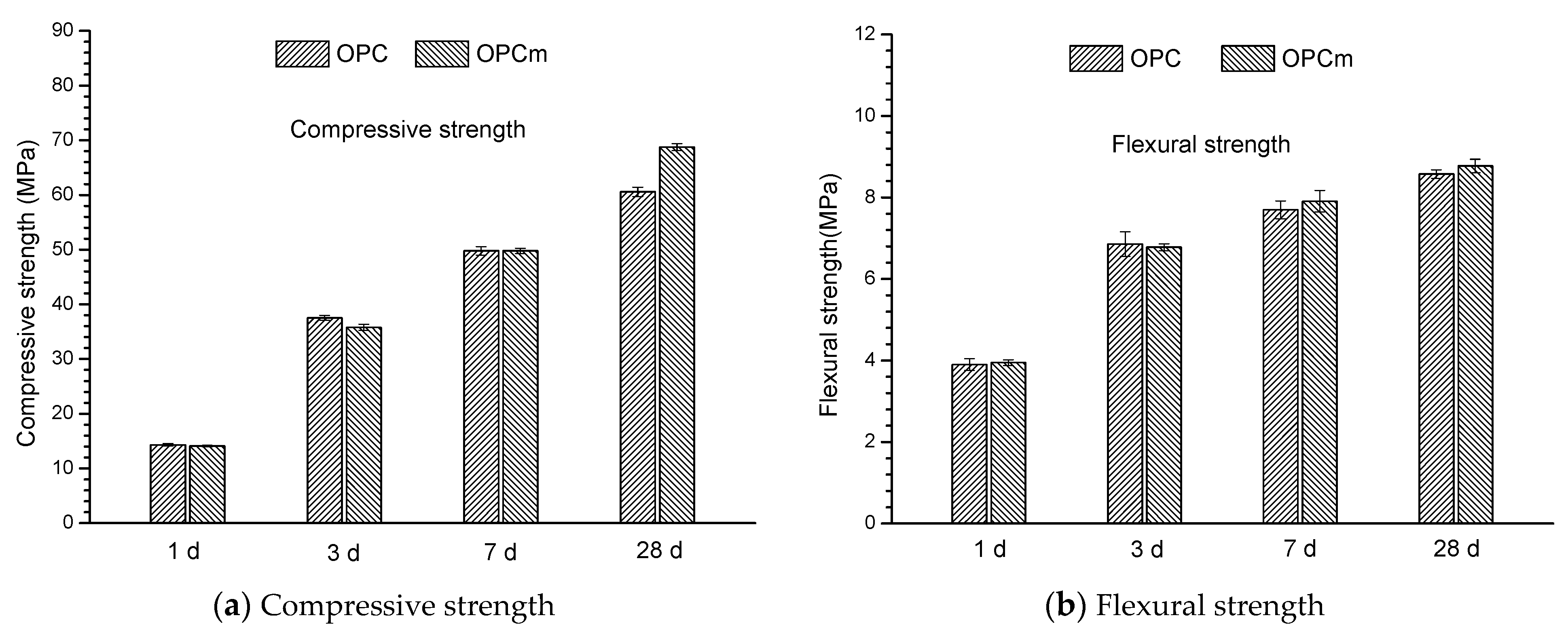
3.1. Mechanical Properties of Mortar Sample in the Presence of MCI
3.2. Release amount of BTA Encapsulated in MCI from Mortar Sample
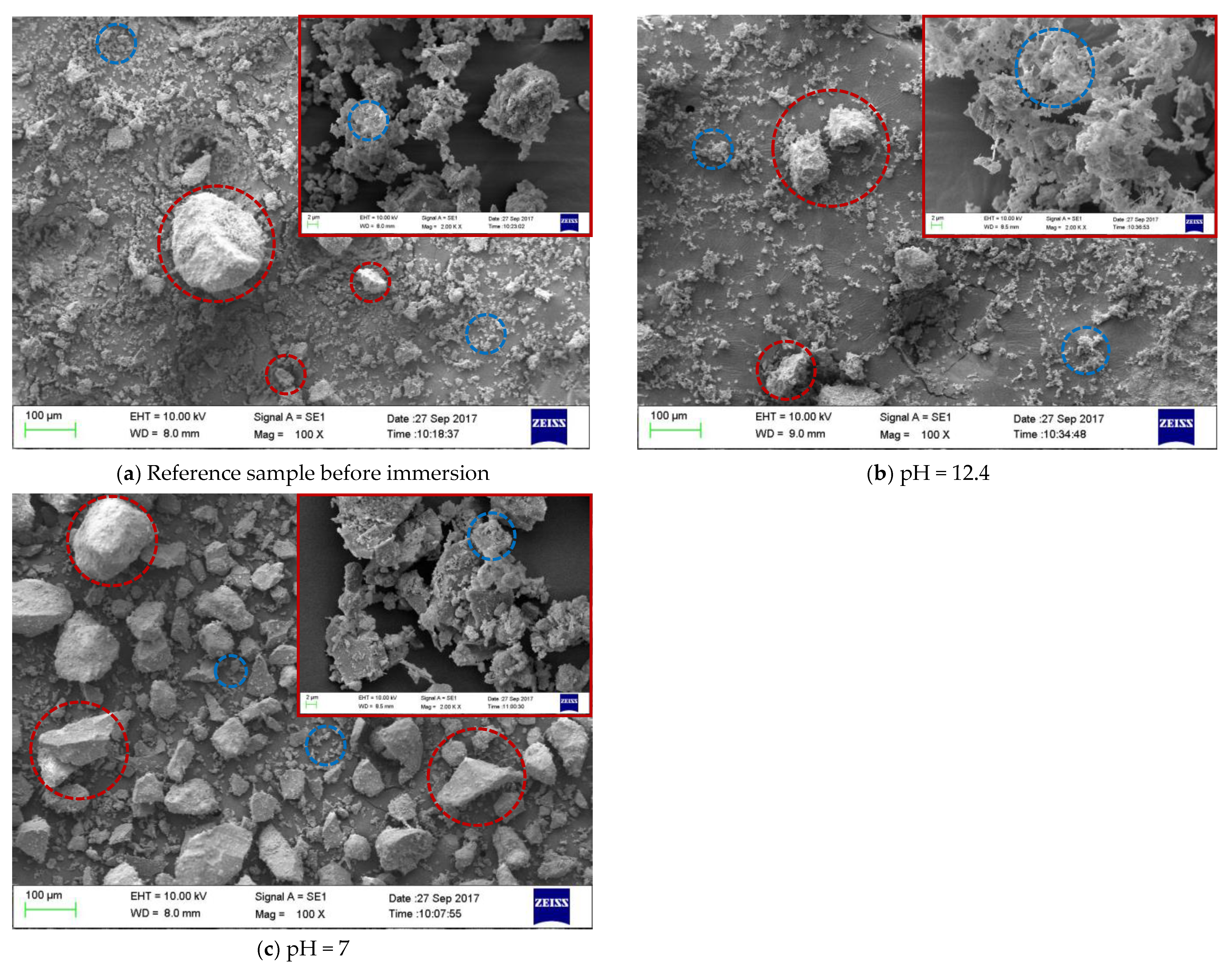
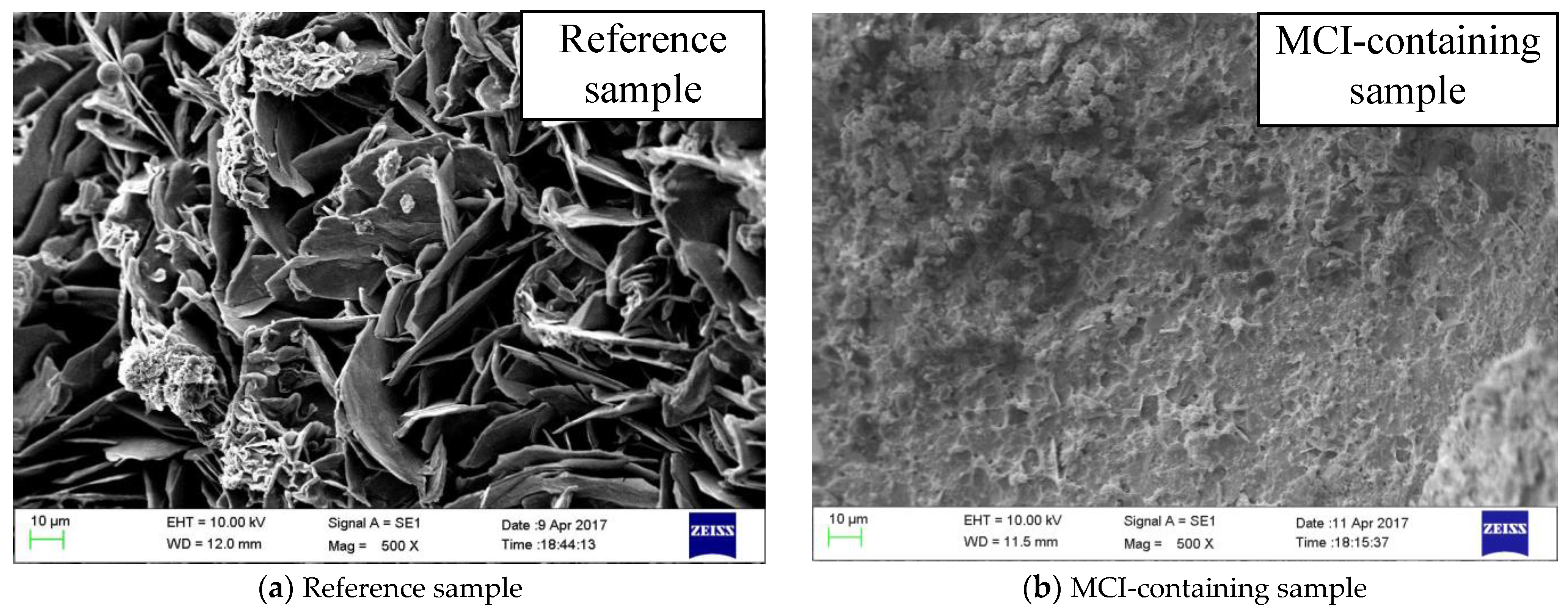
3.3. Morphology Alterations of the Mortar Samples
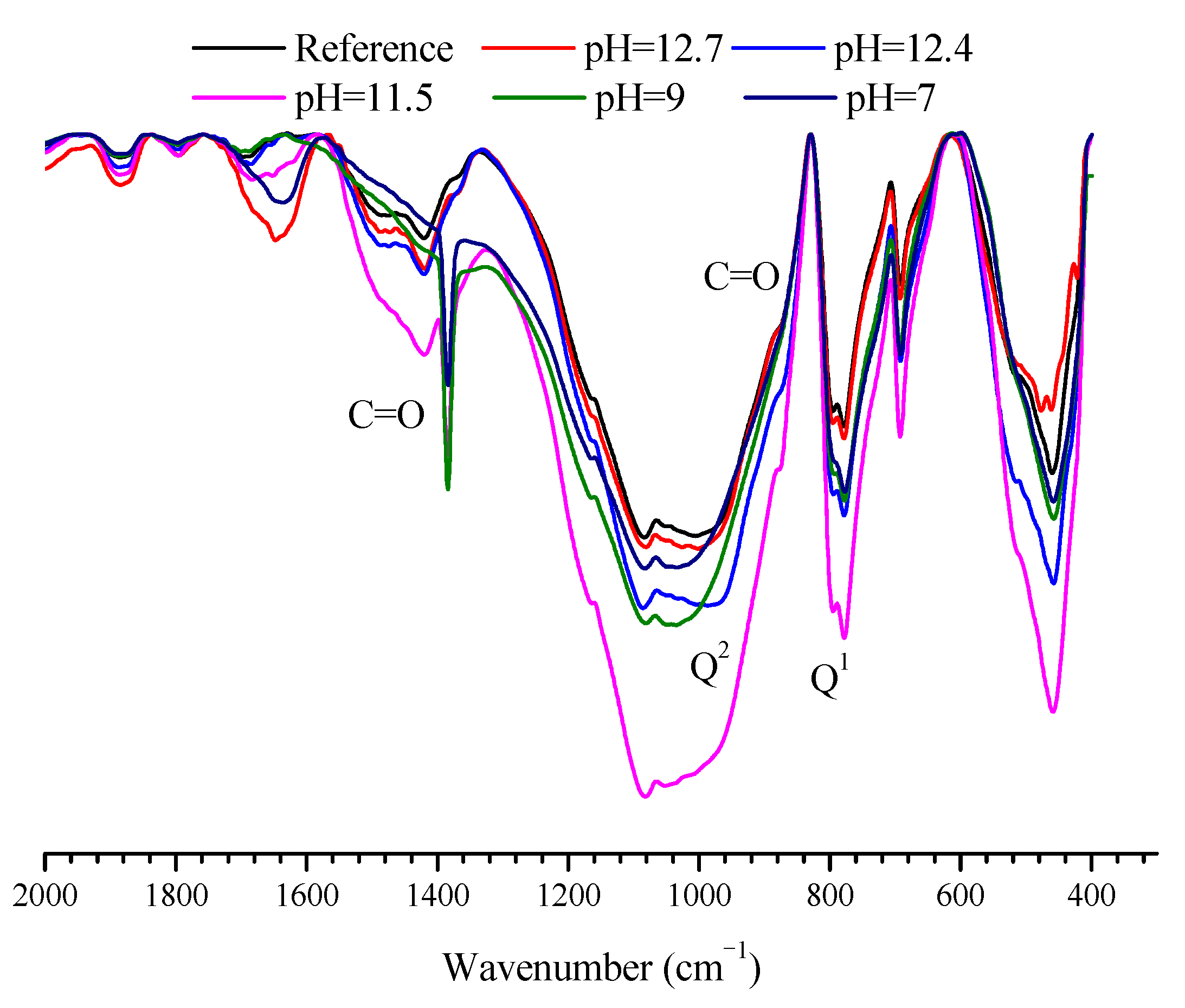
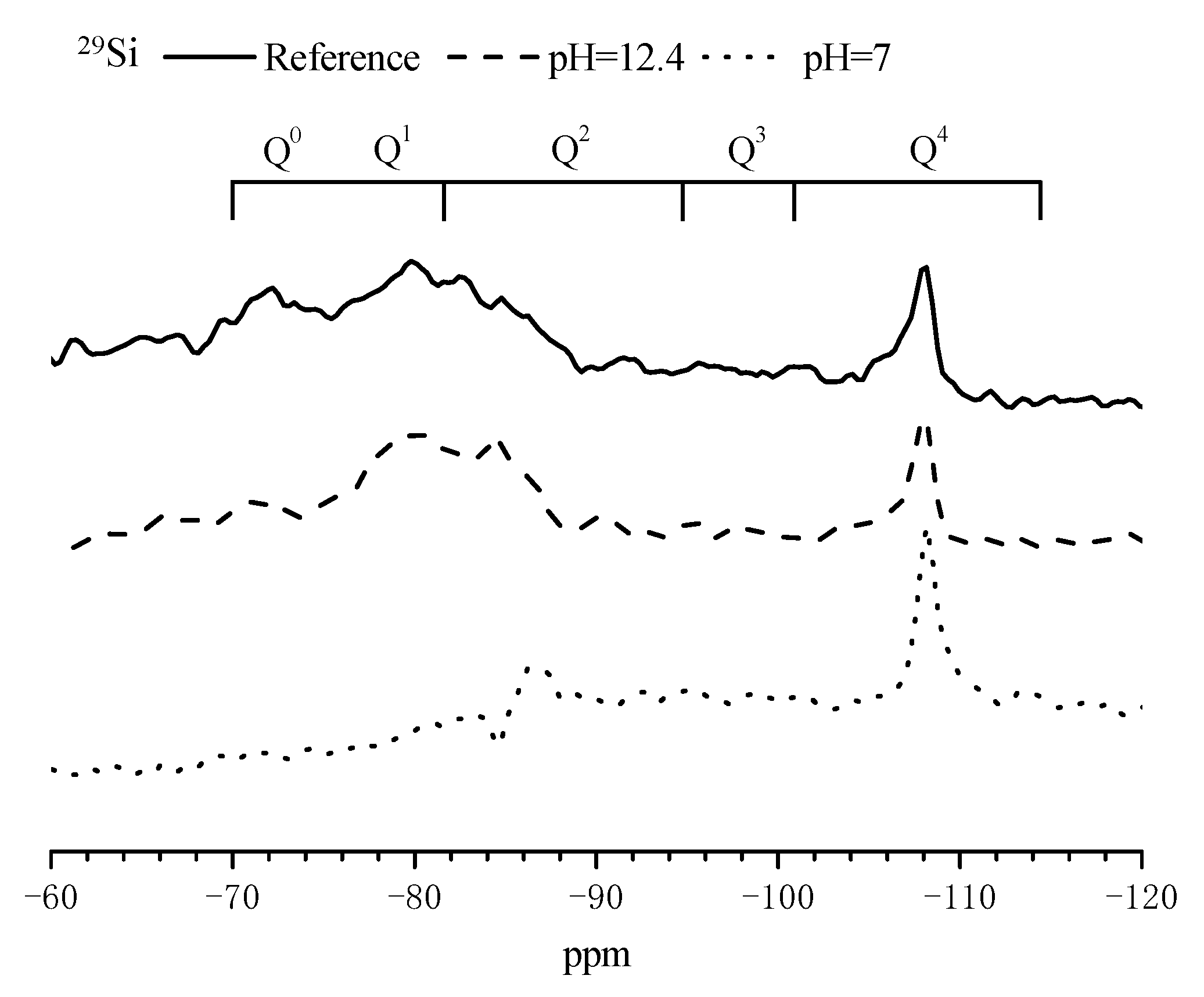
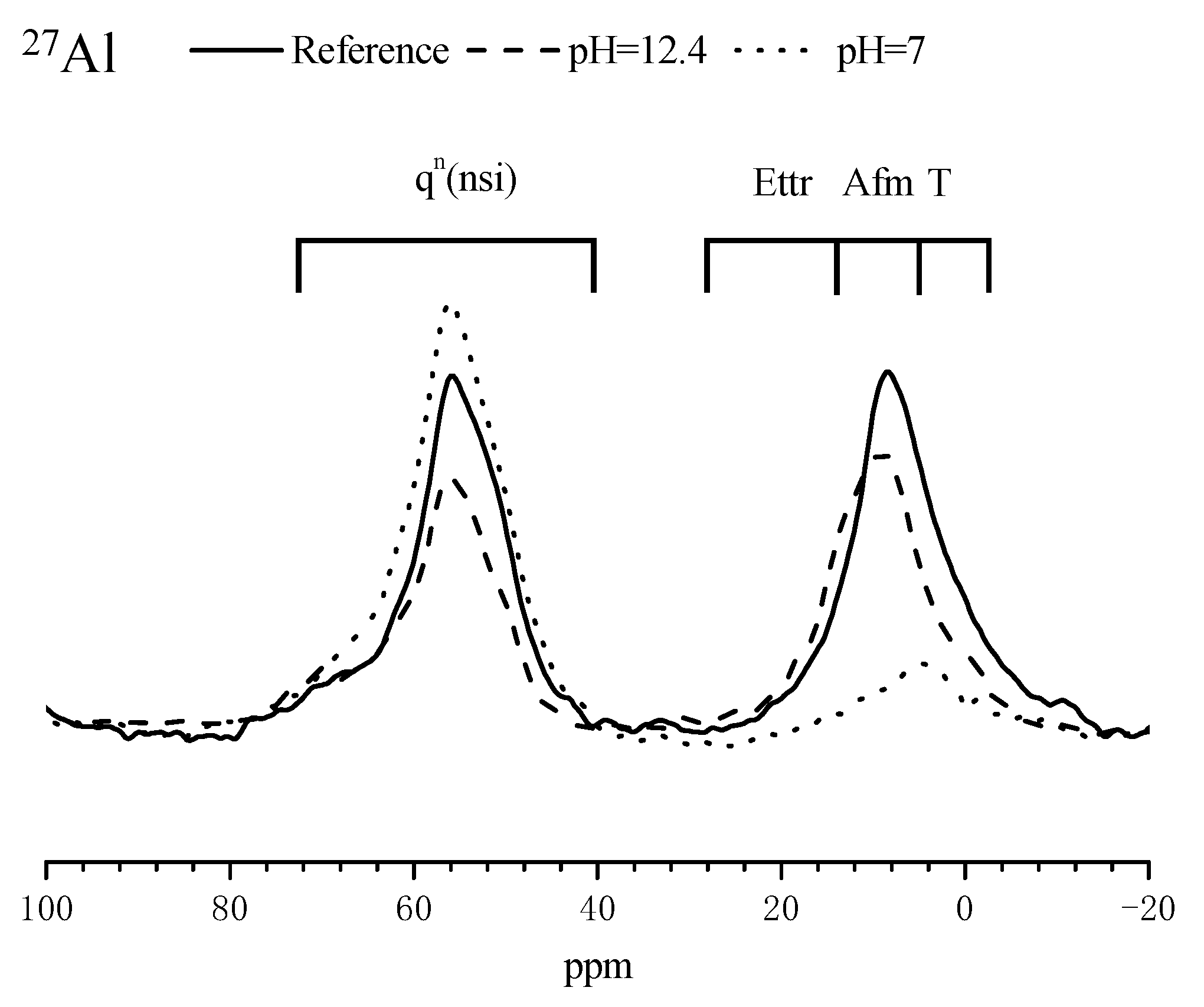
3.4. Composition Alterations of the Hydration Products in Mortar Samples after Immersed in SPS
3.5. Corrosion Inhibition Effect of MCI on the Reinforcement Embedded in Mortar
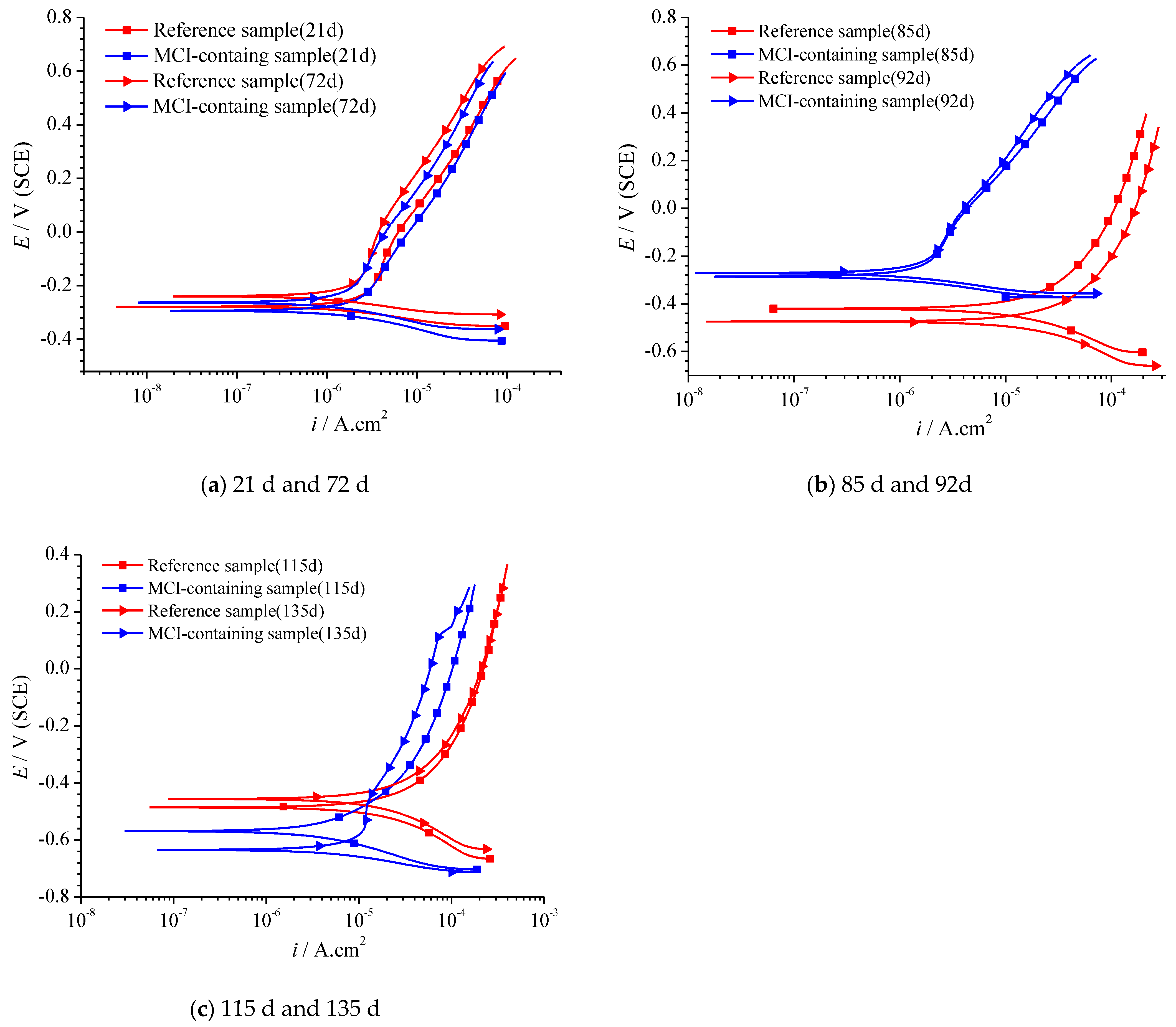
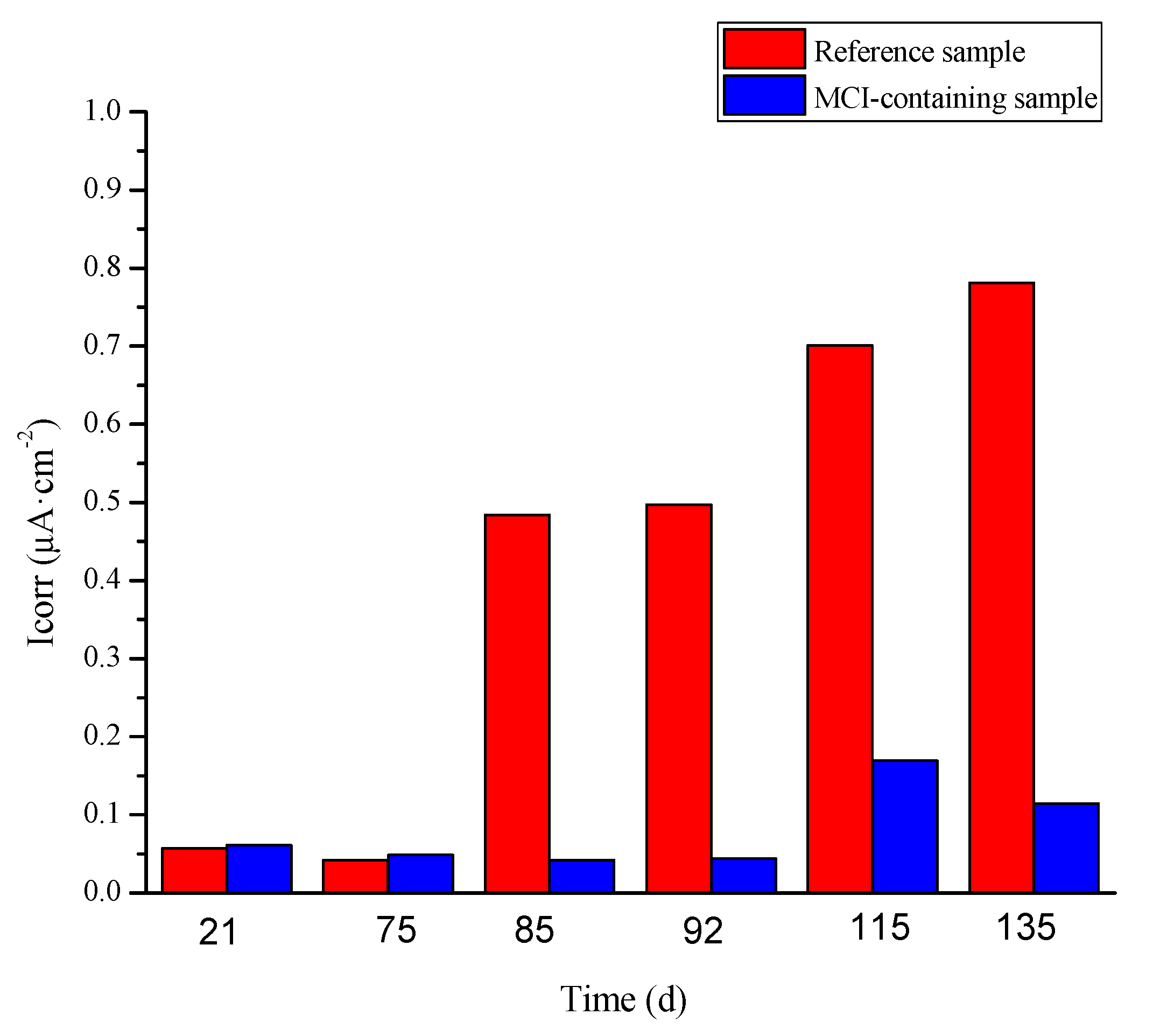
3.5.1. Influence of MCI on the Electrochemical Behavior of the Reinforcement Embedded in Mortar
3.5.2. Influence of MCI on the Morphology and Composition of Corrosion Products
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Stefanoni, M.; Angst, U.; Elsener, B. Corrosion rate of carbon steel in carbonated concrete—A critical review. Cem. Concr. Res. 2018, 103, 35–48. [Google Scholar] [CrossRef]
- Bertolini, L.; Elsener, B.; Pedeferri, P.; Redaelli, E.; Polder, R.B. Corrosion of Steel in Concrete-Prevention, Diagnosis, Repair Second Edition in Chinese Language; John Wiley & Sons: Hoboken, NJ, USA, 2019. [Google Scholar]
- Mennucci, M.; Banczek, E.; Rodrigues, P.; Costa, I. Evaluation of benzotriazole as corrosion inhibitor for carbon steel in simulated pore solution. Cem. Concr. Compos. 2009, 31, 418–424. [Google Scholar] [CrossRef]
- Fan, L.; Bao, Y.; Meng, W.; Chen, G. In-situ monitoring of corrosion-induced expansion and mass loss of steel bar in steel fiber reinforced concrete using a distributed fiber optic sensor. Compos. Part B Eng. 2019, 165, 679–689. [Google Scholar] [CrossRef]
- Söylev, T.A.; Richardson, M. Corrosion inhibitors for steel in concrete: State-of-the-art report. Constr. Build. Mater. 2008, 22, 609–622. [Google Scholar] [CrossRef]
- Cabrini, M.; Fontana, F.; Lorenzi, S.; Pastore, T.; Pellegrini, S. Effect of Organic Inhibitors on Chloride Corrosion of Steel Rebars in Alkaline Pore Solution. J. Chem. 2015, 2015, 1–10. [Google Scholar] [CrossRef] [Green Version]
- Diamanti, M.; Rosales, E.P.; Raffaini, G.; Ganazzoli, F.; Brenna, A.; Pedeferri, M.; Ormellese, M. Molecular modelling and electrochemical evaluation of organic inhibitors in concrete. Corros. Sci. 2015, 100, 231–241. [Google Scholar] [CrossRef] [Green Version]
- Dong, Z.H.; Shi, W.; Zhang, G.A.; Guo, X.P. The role of inhibitors on the repassivation of pitting corrosion of carbon steel in synthetic carbonated concrete pore solution. Electrochimica Acta 2011, 56, 5890–5897. [Google Scholar] [CrossRef]
- Luo, L.; De Schutter, G. Influence of corrosion inhibitors on concrete transport properties. Mater. Struct. 2008, 41, 1571–1579. [Google Scholar] [CrossRef]
- Dong, B.; Wang, Y.; Fang, G.; Han, N.; Xing, F.; Lu, Y. Smart releasing behavior of a chemical self-healing microcapsule in the stimulated concrete pore solution. Cem. Concr. Compos. 2015, 56, 46–50. [Google Scholar] [CrossRef]
- Wang, Y.; Fang, G.; Ding, W.; Han, N.; Xing, F.; Dong, B. Self-immunity microcapsules for corrosion protection of steel bar in reinforced concrete. Sci. Rep. 2015, 5, 18484. [Google Scholar] [CrossRef]
- Han, N.-X.; Xing, F. A Comprehensive Review of the Study and Development of Microcapsule Based Self-Resilience Systems for Concrete Structures at Shenzhen University. Materials 2016, 10, 2. [Google Scholar] [CrossRef]
- Xiong, W.; Tang, J.; Zhu, G.; Han, N.; Schlangen, E.; Dong, B.; Wang, X.; Xing, F. A novel capsule-based self-recovery system with a chloride ion trigger. Sci. Rep. 2015, 5, 10866. [Google Scholar] [CrossRef] [Green Version]
- Wang, Y.; Ding, W.; Fang, G.; Liu, Y.; Xing, F.; Dong, B. Feasibility study on corrosion protection of steel bar in a self-immunity system based on increasing OH− content. Constr. Build. Mater. 2016, 125, 742–748. [Google Scholar] [CrossRef]
- Dong, B.; Ding, W.; Qin, S.; Han, N.; Fang, G.; Liu, Y.; Xing, F.; Hong, S. Chemical self-healing system with novel microcapsules for corrosion inhibition of rebar in concrete. Cem. Concr. Compos. 2018, 85, 83–91. [Google Scholar] [CrossRef]
- Liu, W.; Li, J.; Huang, X.; Bi, J. Corrosion Protection of Q235 Steel Using Epoxy Coatings Loaded with Calcium Carbonate Microparticles Modified by Sodium Lignosulfonate in Simulated Concrete Pore Solutions. Materials 2021, 14, 1982. [Google Scholar] [CrossRef] [PubMed]
- Ress, J.; Martin, U.; Bosch, J.; Bastidas, D.M. pH-Triggered Release of NaNO2 Corrosion Inhibitors from Novel Colophony Microcapsules in Simulated Concrete Pore Solution. ACS Appl. Mater. Interfaces 2020, 12, 46686–46700. [Google Scholar] [CrossRef] [PubMed]
- Liang, Z.; Wang, Q.; Dong, B.; Jiang, B.; Xing, F. Ion-triggered calcium hydroxide microcapsules for enhanced corrosion resistance of steel bars. RSC Adv. 2018, 8, 39536–39544. [Google Scholar] [CrossRef] [Green Version]
- Zhu, Y.; Ma, Y.; Yu, Q.; Wei, J.; Hu, J. Preparation of pH-sensitive core-shell organic corrosion inhibitor and its release behavior in simulated concrete pore solutions. Mater. Des. 2017, 119, 254–262. [Google Scholar] [CrossRef]
- Zhu, Y.; Ma, Y.; Hu, J.; Zhang, Z.; Huang, J.; Wang, Y.; Wang, H.; Cai, W.; Huang, H.; Yu, Q.; et al. Adsorption of organic core-shell corrosion inhibitors on cement particles and their influence on early age properties of fresh cement paste. Cem. Concr. Res. 2020, 130, 106000. [Google Scholar] [CrossRef]
- Bogue, R.H. Calculation of the Compounds in Portland Cement. Ind. Eng. Chem. Anal. Ed. 1929, 1, 192–197. [Google Scholar] [CrossRef]
- Kocaba, V.; Gallucci, E.; Scrivener, K. Methods for determination of degree of reaction of slag in blended cement pastes. Cem. Concr. Res. 2012, 42, 511–525. [Google Scholar] [CrossRef]
- Weiss, C.A.; Altaner, S.P.; Kirkpatrick, R.J. High-resolution 29Si NMR spectroscopy of 2:1 layer silicates; correlations among chemical shift, structural distortions, and chemical variations. Am. Mineral. 1987, 72, 935–942. [Google Scholar]
- Lippmaa, E.; Maegi, M.; Samoson, A.; Engelhardt, G.; Grimmer, A.R. Structural studies of silicates by solid-state high-resolution silicon-29 NMR. J. Am. Chem. Soc. 1980, 102, 4889–4893. [Google Scholar] [CrossRef]
- Yu, P.; Kirkpatrick, R.J.; Poe, B.; McMillan, P.F.; Cong, X. Structure of Calcium Silicate Hydrate (C-S-H): Near-, Mid-, and Far-Infrared Spectroscopy. J. Am. Ceram. Soc. 2004, 82, 742–748. [Google Scholar] [CrossRef]
- Del Bosque, I.S.; Martinez-Ramirez, S.; Blanco-Varela, M.T. FTIR study of the effect of temperature and nanosilica on the nano structure of C–S–H gel formed by hydrating tricalcium silicate. Constr. Build. Mater. 2014, 52, 314–323. [Google Scholar] [CrossRef] [Green Version]
- Puertas, F.; Palacios, M.; Manzano, H.; Dolado, J.S.; Rico, A.; Rodríguez, J. A model for the C-A-S-H gel formed in alkali-activated slag cements. J. Eur. Ceram. Soc. 2011, 31, 2043–2056. [Google Scholar] [CrossRef]
- Sykes, D.; Kubicki, J. A model for H2O solubility mechanisms in albite melts from infrared spectroscopy and molecular orbital calculations. Geochim. Cosmochim. Acta 1993, 57, 1039–1052. [Google Scholar] [CrossRef]
- Gutberlet, T.; Hilbig, H.; Beddoe, R. Acid attack on hydrated cement—Effect of mineral acids on the degradation process. Cem. Concr. Res. 2015, 74, 35–43. [Google Scholar] [CrossRef]
- Bassuoni, M.; Nehdi, M. Resistance of self-consolidating concrete to sulfuric acid attack with consecutive pH reduction. Cem. Concr. Res. 2007, 37, 1070–1084. [Google Scholar] [CrossRef]
- Andersen, M.D.; Jakobsen, H.J.; Skibsted, J. A new aluminium-hydrate species in hydrated Portland cements characterized by 27Al and 29Si MAS NMR spectroscopy. Cem. Concr. Res. 2006, 36, 3–17. [Google Scholar] [CrossRef]
- Andersen, M.D.; Jakobsen, H.J.; Skibsted, J. Incorporation of Aluminum in the Calcium Silicate Hydrate (C−S−H) of Hydrated Portland Cements: A High-Field27Al and29Si MAS NMR Investigation. Inorg. Chem. 2003, 42, 2280–2287. [Google Scholar] [CrossRef]
- Wang, S.-D.; Scrivener, K. 29Si and 27Al NMR study of alkali-activated slag. Cem. Concr. Res. 2003, 33, 769–774. [Google Scholar] [CrossRef]
- Bastidas, D.; Criado, M.; Fajardo, S.; La Iglesia, A. Corrosion inhibition mechanism of phosphates for early-age reinforced mortar in the presence of chlorides. Cem. Concr. Compos. 2015, 61, 1–6. [Google Scholar] [CrossRef]
- Tang, Y.; Zhang, F.; Hu, S.; Cao, Z.; Wu, Z.; Jing, W. Novel benzimidazole derivatives as corrosion inhibitors of mild steel in the acidic media. Part I: Gravimetric, electrochemical, SEM and XPS studies. Corros. Sci. 2013, 74, 271–282. [Google Scholar] [CrossRef]
- Hu, J.; Koleva, D.; Petrov, P.; van Breugel, K. Polymeric vesicles for corrosion control in reinforced mortar: Electrochemical behavior, steel surface analysis and bulk matrix properties. Corros. Sci. 2012, 65, 414–430. [Google Scholar] [CrossRef]
- Dubois, F.; Mendibide, C.; Pagnier, T.; Perrard, F.; Duret, C. Raman mapping of corrosion products formed onto spring steels during salt spray experiments. A correlation between the scale composition and the corrosion resistance. Corros. Sci. 2008, 50, 3401–3409. [Google Scholar] [CrossRef]
- Koleva, D.; Hu, J.; Fraaij, A.; Stroeven, P.; Boshkov, N.; de Wit, J. Quantitative characterisation of steel/cement paste interface microstructure and corrosion phenomena in mortars suffering from chloride attack. Corros. Sci. 2006, 48, 4001–4019. [Google Scholar] [CrossRef]
- Singh, J.; Singh, D. The nature of rusts and corrosion characteristics of low alloy and plain carbon steels in three kinds of concrete pore solution with salinity and different pH. Corros. Sci. 2012, 56, 129–142. [Google Scholar] [CrossRef]
- Shi, C.; Stegemann, J. Acid corrosion resistance of different cementing materials. Cem. Concr. Res. 2000, 30, 803–808. [Google Scholar] [CrossRef]
- Möschner, G.; Lothenbach, B.; Winnefeld, F.; Ulrich, A.; Figi, R.; Kretzschmar, R. Solid solution between Al-ettringite and Fe-ettringite (Ca6[Al1−xFex(OH)6]2(SO4)3·26H2O). Cem. Concr. Res. 2009, 39, 482–489. [Google Scholar] [CrossRef]
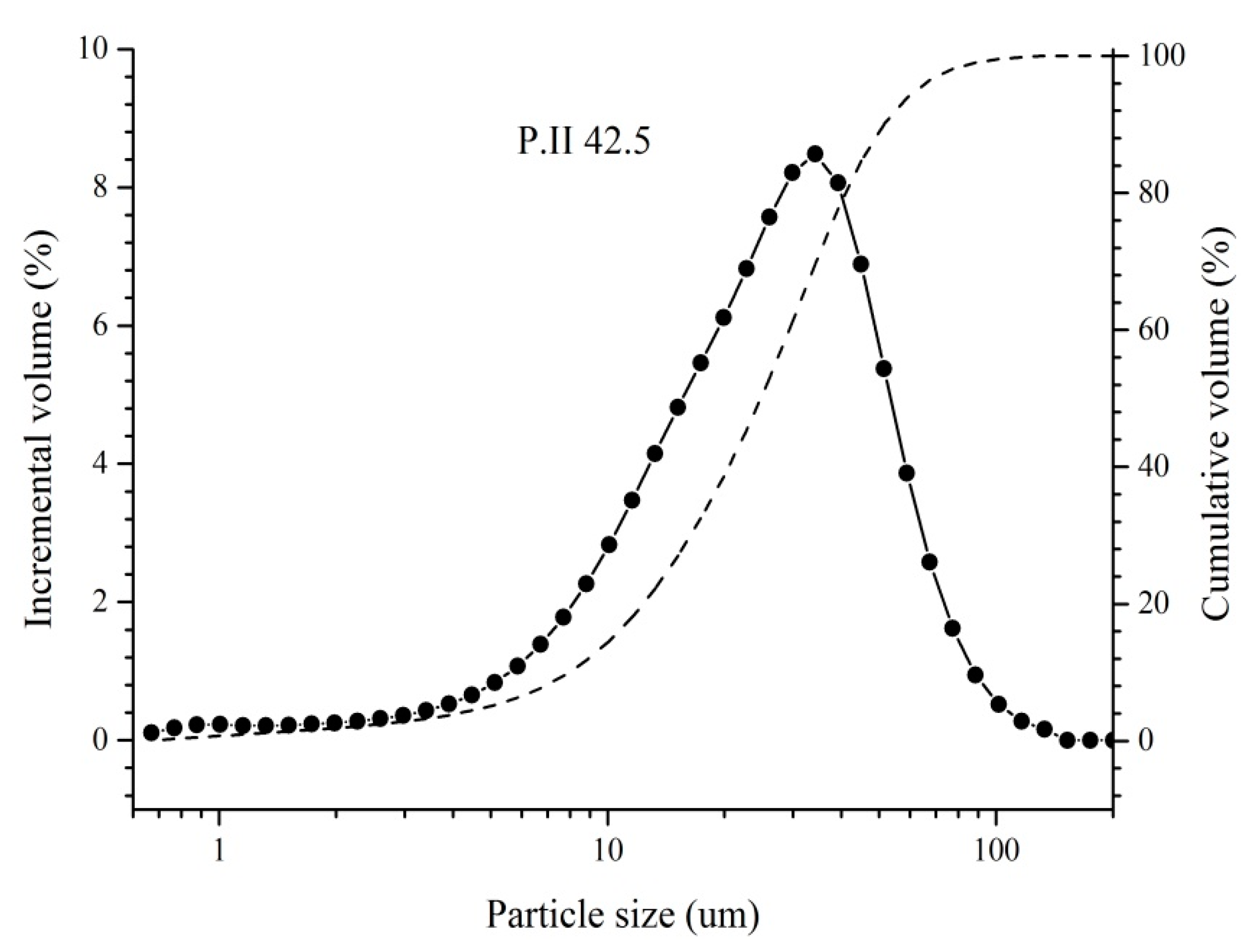






| SiO2 | Al2O3 | Fe2O3 | CaO | MgO | K2O | Na2O | SO3 | Others | LOI * |
|---|---|---|---|---|---|---|---|---|---|
| 21.60 | 4.35 | 2.95 | 63.81 | 1.76 | 0.51 | 0.16 | 2.06 | 1.61 | 1.19 |
| C3S * | C2S * | C3A * | C4AF * | CaSO4·2H2O | CaCO3 |
|---|---|---|---|---|---|
| 56.20 | 19.61 | 6.54 | 8.97 | 4.33 | 4.35 |
| Elements Composition (wt. %) | |||||
|---|---|---|---|---|---|
| C | Si | Mn | S | P | Fe |
| 0.18 | 0.28 | 0.55 | 0.04 | 0.04 | 98.91 |
| Oxides | Reference | pH = 12.7 | pH = 12.4 | pH = 11.5 | pH = 9.0 | pH = 7.0 |
|---|---|---|---|---|---|---|
| SiO2 | 82.1 | 82.7 | 85.9 | 89.0 | 89.1 | 92.0 |
| CaO | 12.3 | 12.2 | 8.8 | 6.0 | 5.9 | 2.9 |
| Al2O3 | 2.9 | 2.7 | 2.9 | 3.0 | 3.0 | 2.9 |
| Fe2O3 | 1.1 | 1.0 | 1.1 | 1.0 | 1.0 | 1.0 |
| SO3 | 0.6 | 0.7 | 0.5 | 0.3 | 0.4 | 0.4 |
| K2O | 0.6 | 0.6 | 0.5 | 0.5 | 0.6 | 0.6 |
| TiO2 | 0.1 | 0.1 | 0.1 | 0.1 | 0.0 | 0.0 |
| Others | 0.3 | 0.0 | 0.2 | 0.1 | 0.0 | 0.2 |
| Sites | Chemical Shift (ppm) |
|---|---|
| C2S-Q0 | −71 |
| C3S-Q0 | −69 to −73 |
| CASH-Q1 | −79 |
| CASH-Q2(1Al) | −82 |
| CASH-Q2 | −85 |
| Q2(1Al) | −86 |
| Q2 | −92 |
| Q3(1Al) | −95 |
| Q3 | −101 |
| Q4(1Al) | −104 |
| Q4 | −110 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Huang, J.; Zhu, Y.; Ma, Y.; Hu, J.; Huang, H.; Wei, J.; Yu, Q. pH-Triggered Release Performance of Microcapsule-Based Inhibitor and Its Inhibition Effect on the Reinforcement Embedded in Mortar. Materials 2021, 14, 5517. https://doi.org/10.3390/ma14195517
Huang J, Zhu Y, Ma Y, Hu J, Huang H, Wei J, Yu Q. pH-Triggered Release Performance of Microcapsule-Based Inhibitor and Its Inhibition Effect on the Reinforcement Embedded in Mortar. Materials. 2021; 14(19):5517. https://doi.org/10.3390/ma14195517
Chicago/Turabian StyleHuang, Jinzhen, Yangyang Zhu, Yuwei Ma, Jie Hu, Haoliang Huang, Jiangxiong Wei, and Qijun Yu. 2021. "pH-Triggered Release Performance of Microcapsule-Based Inhibitor and Its Inhibition Effect on the Reinforcement Embedded in Mortar" Materials 14, no. 19: 5517. https://doi.org/10.3390/ma14195517






