High-Temperature Properties and Applications of Si-Based Polymer-Derived Ceramics: A Review
Abstract
:1. Introduction
2. Applications
2.1. Fibers and Matrices
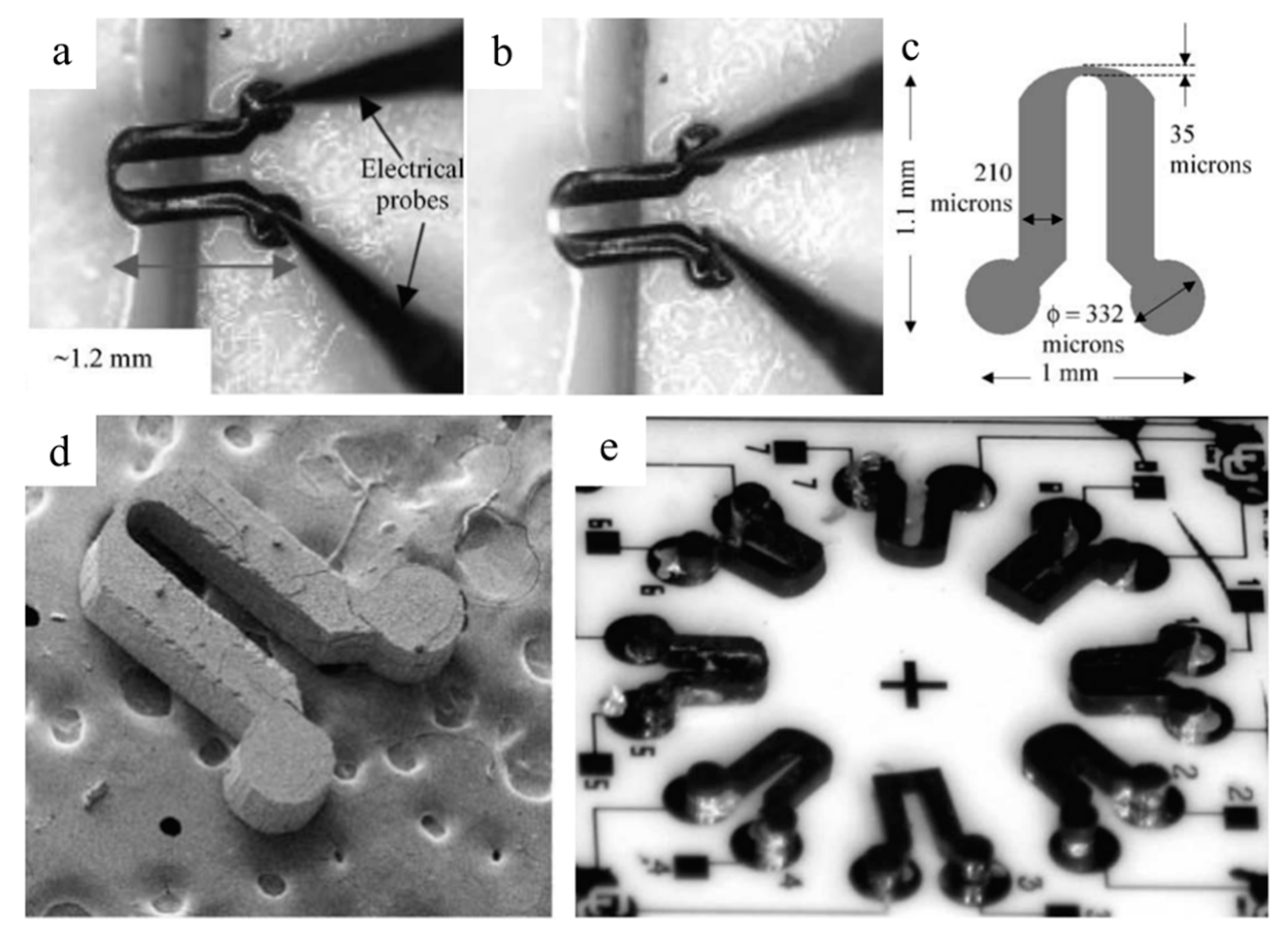
2.2. Microelectromechanical Systems and Semiconductors
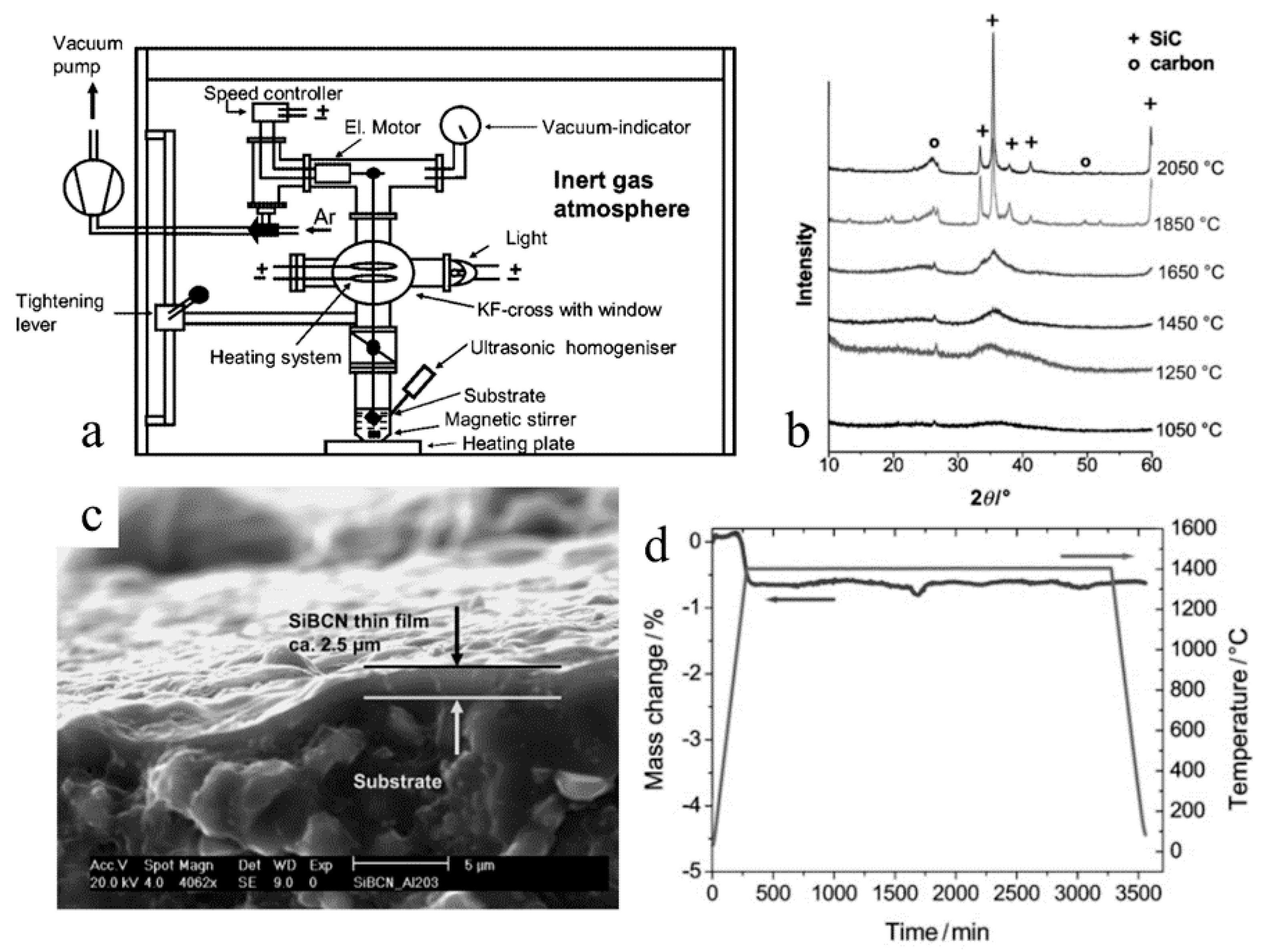
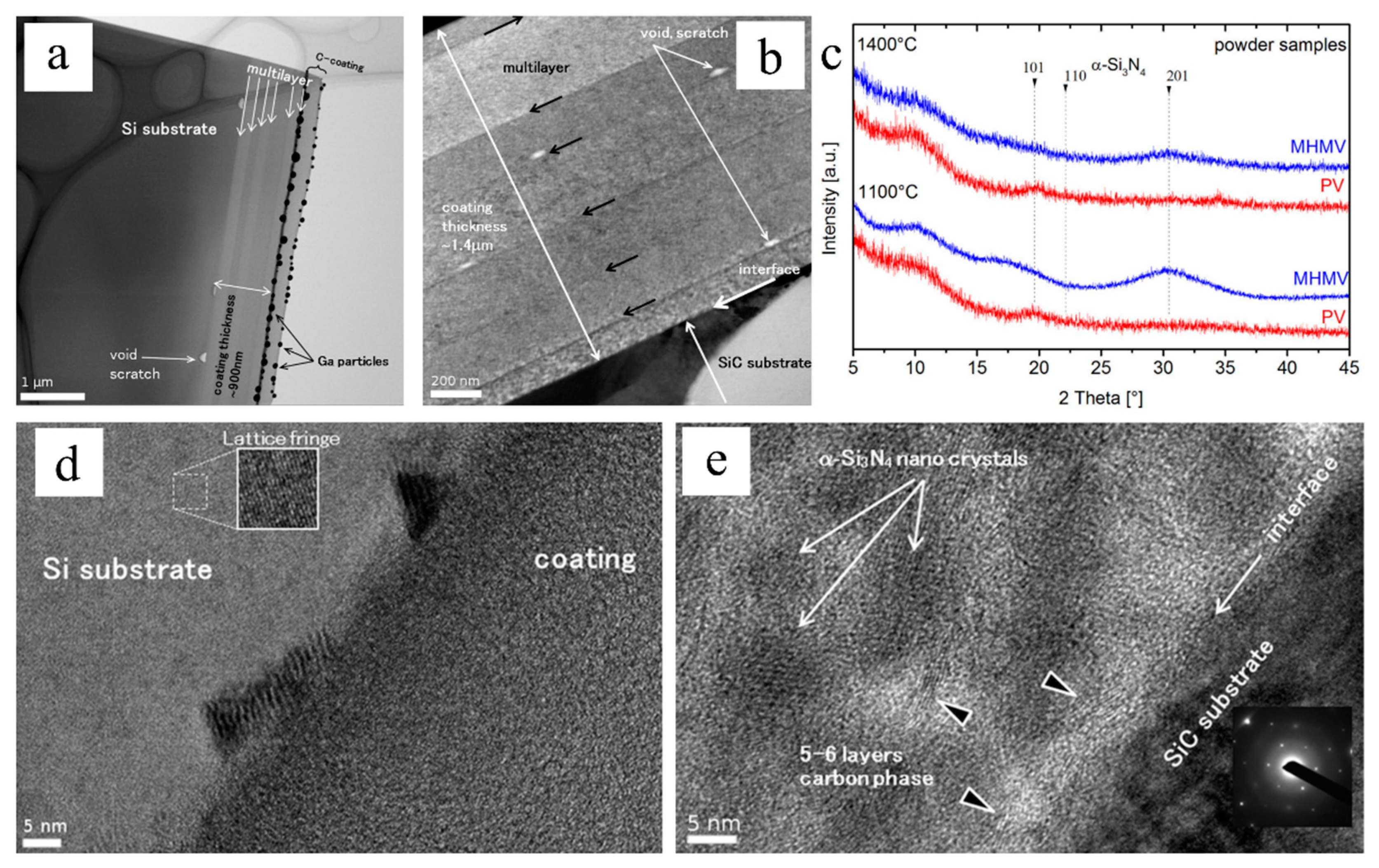
2.3. Membranes, Coatings and Adhesives
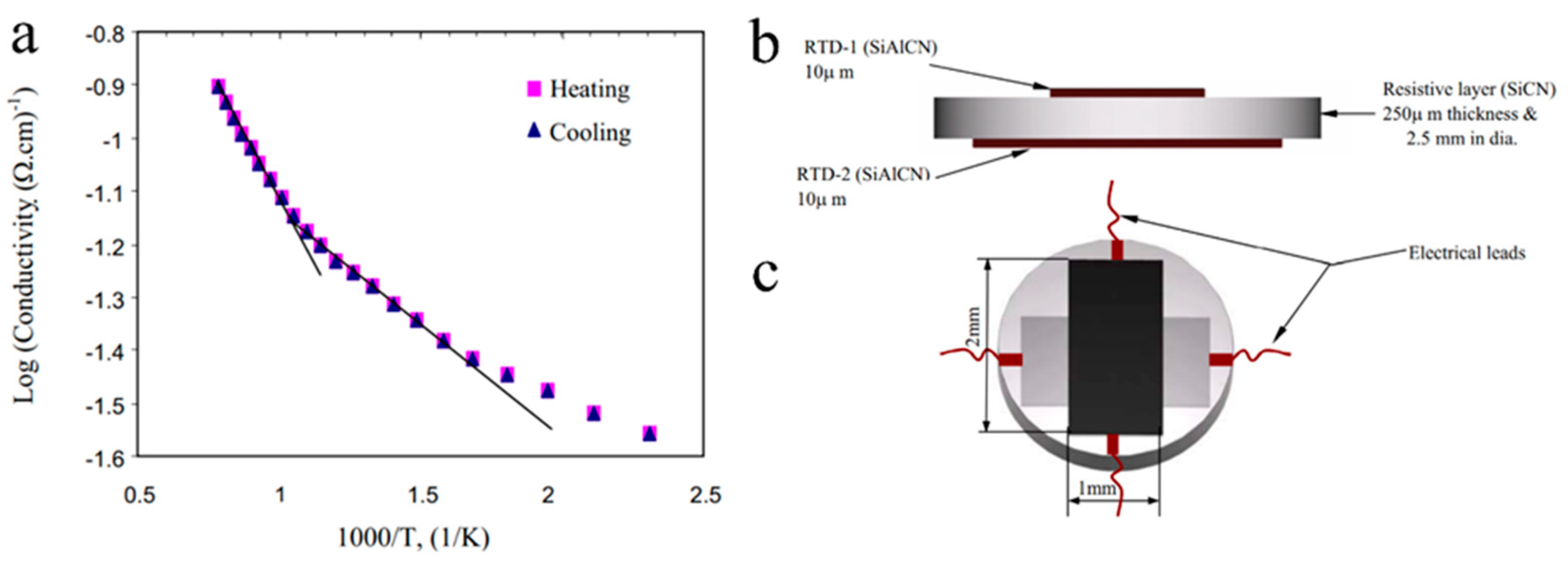
2.4. Sensors
3. Conclusions and Outlook
Author Contributions
Funding
Conflicts of Interest
References
- Greil, P. Near Net Shape Manufacturing of Polymer Derived Ceramics. J. Eur. Ceram. Soc. 1998, 18, 1905–1914. [Google Scholar] [CrossRef]
- Riedel, R. From molecules to materials—A novel route for the synthesis of advanced ceramics. Naturwissenschaften 1995, 82, 12–20. [Google Scholar] [CrossRef]
- Bill, J.; Aldinger, F. Precursor-derived Covalent Ceramics. Adv. Mater. 1995, 7, 775–787. [Google Scholar] [CrossRef]
- Ionescu, E.; Linck, C.; Fasel, C.; Müller, M.; Kleebe, H.; Riedel, R.; Müller, M. Polymer-Derived SiOC/ZrO2Ceramic Nanocomposites with Excellent High-Temperature Stability. J. Am. Ceram. Soc. 2010, 93, 241–250. [Google Scholar] [CrossRef]
- Wen, Q.; Yu, Z.; Riedel, R. The fate and role of in situ formed carbon in polymer-derived ceramics. Prog. Mater. Sci. 2020, 109, 100623. [Google Scholar] [CrossRef]
- Eckel, Z.C.; Zhou, C.; Martin, J.H.; Jacobsen, A.J.; Carter, W.B.; Schaedler, T.A. Additive manufacturing of polymer-derived ceramics. Science 2016, 351, 58–62. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Colombo, P.; Mera, G.; Riedel, R.; Sorarù, G.D. Polymer-Derived Ceramics: 40 Years of Research and Innovation in Advanced Ceramics. J. Am. Ceram. Soc. 2010, 93, 1805–1837. [Google Scholar] [CrossRef]
- Müller, A.; Peng, J.; Seifert, H.J.; Bill, J.; Aldinger, F. Si−B−C−N Ceramic Precursors Derived from Dichlorodivinylsilane and Chlorotrivinylsilane. 2. Ceramization of Polymers and High-Temperature Behavior of Ceramic Materials. Chem. Mater. 2002, 14, 3406–3412. [Google Scholar] [CrossRef]
- Zimmermann, A.; Bauer, A.; Christ, M.; Cai, Y.; Aldinger, F. High-temperature deformation of amorphous Si–C–N and Si–B–C–N ceramics derived from polymers. Acta Mater. 2002, 50, 1187–1196. [Google Scholar] [CrossRef]
- Hauser, R.; Nahar-Borchard, S.; Riedel, R.; Ikuhara, Y.H.; Iwamoto, Y. Polymer-Derived SiBCN Ceramic and their Potential Application for High Temperature Membranes. J. Ceram. Soc. Jpn. 2006, 114, 524–528. [Google Scholar] [CrossRef] [Green Version]
- Xie, S.; Wang, Y.; Lei, Y.; Wang, B.; Wu, N.; Gou, Y.; Fang, D. A simply prepared flexible SiBOC ultrafine fiber mat with enhanced high-temperature stability and chemical resistance. RSC Adv. 2015, 5, 64911–64917. [Google Scholar] [CrossRef]
- Lyu, Y.; Tang, H.; Zhao, G. Effect of Hf and B incorporation on the SiOC precursor architecture and high-temperature oxidation behavior of SiHfBOC ceramics. J. Eur. Ceram. Soc. 2020, 40, 324–332. [Google Scholar] [CrossRef]
- Plachký, T.; Lenčéš, Z.; Hric, L.; Šajgalík, P.; Baláž, P.; Riedel, R.; Kleebe, H.-J. Processing and mechanical properties of Si3N4 composites employing polymer-derived SiAlOC as sintering aid. J. Eur. Ceram. Soc. 2010, 30, 759–767. [Google Scholar] [CrossRef]
- Harshe, R.; Balan, C.; Riedel, R. Amorphous Si(Al)OC ceramic from polysiloxanes: Bulk ceramic processing, crystallization behavior and applications. J. Eur. Ceram. Soc. 2004, 24, 3471–3482. [Google Scholar] [CrossRef]
- Wang, Y.; Fan, Y.; Zhang, L.; Zhang, W.; An, L. Polymer-derived SiAlCN ceramics resist oxidation at 1400 °C. Scr. Mater. 2006, 55, 295–297. [Google Scholar] [CrossRef]
- Thiyagarajan, G.B.; Koroleva, E.; Filimonov, A.; Vakhrushev, S.; Kumar, R. Tailorable Dielectric Performance of Niobium-Modified Poly(hydridomethylsiloxane) Precursor-Derived Ceramic Nanocomposites. Phys. Status Solidi 2020, 217, 2000417. [Google Scholar] [CrossRef]
- Su, D.; Yan, X.; Liu, N.; Li, X.; Ji, H. Preparation and characterization of continuous SiZrOC fibers by polyvinyl pyrrolidone-assisted sol–gel process. J. Mater. Sci. 2016, 51, 1418–1427. [Google Scholar] [CrossRef]
- Anand, R.; Nayak, B.B.; Behera, S.K. Coarsening kinetics of nanostructured ZrO2 in Zr-doped SiCN ceramic hybrids. J. Alloys Compd. 2019, 811, 151939. [Google Scholar] [CrossRef]
- Hering, N.; Schreiber, K.; Riedel, R.; Lichtenberger, O. Synthesis of polymeric precursors for the formation of nanocrystalline Ti-C-N/amorphous Si-C-N composites. Appl. Organomet. Chem. 2001, 15, 879–886. [Google Scholar] [CrossRef]
- Yu, Y.; Guo, Y.; Cheng, X.; Zhang, Y. Preparation of TiO2/SiO2 composite fiber by thermal decomposition of polycarbosilane–tetrabutyl titanate hybrid precursor. J. Mater. Chem. 2009, 19, 5637–5642. [Google Scholar] [CrossRef]
- Tang, X.; Yu, Y.; Yang, D. SiO2/TiO2 fibers from titanium-modified polycarbosilane. J. Mater. Sci. 2010, 45, 2670–2674. [Google Scholar] [CrossRef]
- Sujith, R.; Zimmermann, A.; Kumar, R. Crack Evolution and Estimation of Fracture Toughness of HfO2/SiCN(O) Polymer Derived Ceramic Nanocomposites. Adv. Eng. Mater. 2015, 17, 1265–1269. [Google Scholar] [CrossRef]
- Yan, X.; Su, D.; Duan, H.; Zhang, F. Preparation of SiOC/HfO2 fibers from silicon alkoxides and tetrachloride hafnium by a sol–gel process. Mater. Lett. 2015, 148, 196–199. [Google Scholar] [CrossRef]
- Sujith, R.; Kousaalya, A.B.; Kumar, R. Synthesis and phase stability of precursor derived HfO2/Si–C–N–O nanocomposites. Ceram. Int. 2012, 38, 1227–1233. [Google Scholar] [CrossRef]
- Sun, J.; Wen, Q.B.; Li, T.; Wiehl, L.; Fasel, C.; Feng, Y.; De Carolis, D.; Yu, Z.J.; Fu, Q.G.; Riedel, R. Phase evolution of SiOC-based ceramic nanocomposites derived from a polymethylsiloxane modified by Hf-and Ti-alkoxides. J. Am. Ceram. Soc. 2020, 103, 1436–1445. [Google Scholar] [CrossRef]
- Greil, P. Polymer derived engineering ceramics. Adv. Eng. Mater. 2000, 2, 339–348. [Google Scholar] [CrossRef]
- Riedel, R.; Mera, G.; Hauser, R.; Klonczynski, A. Silicon-Based Polymer-Derived Ceramics: Synthesis Properties and Applications—A Review. J. Ceram. Soc. Jpn. 2006, 114, 425–444. [Google Scholar] [CrossRef] [Green Version]
- Miele, P.; Bernard, S.; Cornu, D.; Toury, B. Recent Developments in Polymer-Derived Ceramic Fibers (PDCFs): Preparation, Properties and Applications—A Review. Soft Mater. 2007, 4, 249–286. [Google Scholar] [CrossRef]
- Flores, O.; Bordia, R.K.; Nestler, D.; Krenkel, W.; Motz, G. Ceramic Fibers Based on SiC and SiCN Systems: Current Research, Development, and Commercial Status. Adv. Eng. Mater. 2014, 16, 621–636. [Google Scholar] [CrossRef]
- Ichikawa, H. Polymer-Derived Ceramic Fibers. Annu. Rev. Mater. Res. 2016, 46, 335–356. [Google Scholar] [CrossRef]
- Viard, A.; Fonblanc, D.; Lopez-Ferber, D.; Schmidt, M.; Lale, A.; Durif, C.; Balestrat, M.; Rossignol, F.; Weinmann, M.; Riedel, R.; et al. Polymer Derived Si-B-C-N Ceramics: 30 Years of Research. Adv. Eng. Mater. 2018, 20, 1800360. [Google Scholar] [CrossRef]
- Fu, S.; Zhu, M.; Zhu, Y. Organosilicon polymer-derived ceramics: An overview. J. Adv. Ceram. 2019, 8, 457–478. [Google Scholar] [CrossRef] [Green Version]
- Shah, S.R.; Raj, R. Mechanical properties of a fully dense polymer derived ceramic made by a novel pressure casting process. Acta Mater. 2002, 50, 4093–4103. [Google Scholar] [CrossRef]
- Cross, T.; Raj, R.; Prasad, S.V.; Buchheit, T.; Tallant, D.R. Mechanical and Tribological Behavior of Polymer-Derived Ceramics Constituted from SiCxOyNz. J. Am. Ceram. Soc. 2006, 89, 3706–3714. [Google Scholar] [CrossRef]
- Barroso, G.; Li, Q.; Bordia, R.K.; Motz, G. Polymeric and ceramic silicon-based coatings—A review. J. Mater. Chem. A 2019, 7, 1936–1963. [Google Scholar] [CrossRef]
- Sujith, R.; Jothi, S.; Zimmermann, A.; Aldinger, F.; Kumar, R. Mechanical behaviour of polymer derived ceramics—A review. Int. Mater. Rev. 2020, 1–24. [Google Scholar] [CrossRef]
- Ricohermoso, E.; Rosenburg, F.; Klug, F.; Nicoloso, N.; Schlaak, H.F.; Riedel, R.; Ionescu, E. Piezoresistive carbon-containing ceramic nanocomposites—A review. Open Ceram. 2021, 5, 100057. [Google Scholar] [CrossRef]
- Bhandavat, R.; Pei, Z.; Singh, G. Polymer-derived ceramics as anode material for rechargeable Li-ion batteries: A review. Nanomater. Energy 2012, 1, 324–337. [Google Scholar] [CrossRef] [Green Version]
- Vakifahmetoglu, C.; Zeydanli, D.; Colombo, P. Porous polymer derived ceramics. Mater. Sci. Eng. R Rep. 2016, 106, 1–30. [Google Scholar] [CrossRef]
- Barrios, E.; Zhai, L. A review of the evolution of the nanostructure of SiCN and SiOC polymer derived ceramics and the impact on mechanical properties. Mol. Syst. Des. Eng. 2020, 5, 1606–1641. [Google Scholar] [CrossRef]
- Campbell, F.C. Structural Composite Materials; ASM international: Cleveland, OH, USA, 2010. [Google Scholar]
- Ji, X.; Wang, S.; Shao, C.; Wang, H. High-Temperature Corrosion Behavior of SiBCN Fibers for Aerospace Applications. ACS Appl. Mater. Interfaces 2018, 10, 19712–19720. [Google Scholar] [CrossRef]
- Liu, Y.; Chen, K.; Dong, F.; Peng, S.; Cui, Y.; Zhang, C.; Han, K.; Yu, M.; Zhang, H. Effects of hydrolysis of precursor on the structures and properties of polymer-derived SiBN ceramic fibers. Ceram. Int. 2018, 44, 10199–10203. [Google Scholar] [CrossRef]
- Takeda, M.; Kagawa, Y.; Mitsuno, S.; Imai, Y.; Ichikawa, H. Strength of a Hi-Nicalon™/Silicon-Carbide-Matrix Composite Fabricated by the Multiple Polymer Infiltration-Pyrolysis Process. J. Am. Ceram. Soc. 2004, 82, 1579–1581. [Google Scholar] [CrossRef]
- Naslain, R.R. SiC-Matrix Composites: Nonbrittle Ceramics for Thermo-Structural Application. Int. J. Appl. Ceram. Technol. 2005, 2, 75–84. [Google Scholar] [CrossRef]
- Schmidt, S.; Beyer, S.; Knabe, H.; Immich, H.; Meistring, R.; Gessler, A. Advanced ceramic matrix composite materials for current and future propulsion technology applications. Acta Astronaut. 2004, 55, 409–420. [Google Scholar] [CrossRef]
- Yang, X.; Hu, H.-F.; Zhang, Y.; Chen, Z.-H. Thermal shock properties of 3D-C/SiC composites prepared via polymer infiltration pyrolysis (PIP). Ceram. Int. 2014, 40, 9087–9094. [Google Scholar] [CrossRef]
- Mainzer, B.; Lin, C.; Jemmali, R.; Frieß, M.; Riedel, R.; Koch, D. Characterization and application of a novel low viscosity polysilazane for the manufacture of C- and SiC-fiber reinforced SiCN ceramic matrix composites by PIP process. J. Eur. Ceram. Soc. 2019, 39, 212–221. [Google Scholar] [CrossRef]
- Fabrication of Ceramic Matrix Composites by Polymer Infiltration and Pyrolysis (PIP). Available online: https://www.substech.com/dokuwiki/doku.php?id=fabrication_of_ceramic_matrix_composites_by_polymer_infiltration_and_pyrolysis_pip (accessed on 2 June 2012).
- Lee, S.H.; Weinmann, M.; Aldinger, F. Processing and properties of C/Si–B–C–N fiber–reinforced ceramic matrix composites prepared by precursor impregnation and pyrolysis. Acta Mater. 2008, 56, 1529–1538. [Google Scholar] [CrossRef]
- Ryu, H.-Y.; Wang, Q.; Raj, R. Ultrahigh-Temperature Semiconductors Made from Polymer-Derived Ceramics. J. Am. Ceram. Soc. 2010, 93, 1668–1676. [Google Scholar] [CrossRef]
- Terauds, K.; Sanchez-Jimenez, P.; Raj, R.; Vakifahmetoglu, C.; Colombo, P. Giant piezoresistivity of polymer-derived ceramics at high temperatures. J. Eur. Ceram. Soc. 2010, 30, 2203–2207. [Google Scholar] [CrossRef]
- Seo, D.; Jung, S.; Lombardo, S.J.; Feng, Z.; Chen, J.; Zhang, Y. Fabrication and electrical properties of polymer-derived ceramic (PDC) thin films for high-temperature heat flux sensors. Sens. Actuators A Phys. 2011, 165, 250–255. [Google Scholar] [CrossRef]
- Grossenbacher, J.; Gullo, M.R.; Bakumov, V.; Blugan, G.; Kuebler, J.; Brugger, J. On the micrometre precise mould filling of liquid polymer derived ceramic precursor for 300-µm-thick high aspect ratio ceramic MEMS. Ceram. Int. 2015, 41, 623–629. [Google Scholar] [CrossRef]
- Mehregany, M.; Zorman, C.A.; Rajan, N.; Wu, C.H. Silicon carbide MEMS for harsh environments. Proc. IEEE. 1998, 86, 1594–1609. [Google Scholar] [CrossRef]
- Mehregany, M.; Zorman, C.A. SiC MEMS: Opportunities and challenges for applications in harsh environments. Thin Solid Films 1999, 355, 518–524. [Google Scholar] [CrossRef]
- An, L.; Zhang, W.; Bright, V.M.; Dunn, M.L.; Raj, R. Development of injectable polymer-derived ceramics for high temperature MEMS. In Proceedings of the IEEE Thirteenth Annual International Conference on Micro Electro Mechanical Systems (Cat. No.00CH36308), Miyazaki, Japan, 23–27 January 2000; pp. 619–623. [Google Scholar] [CrossRef]
- Liew, L.-A.; Zhang, W.; Bright, V.M.; An, L.; Dunn, M.L.; Raj, R. Fabrication of SiCN ceramic MEMS using injectable polymer-precursor technique. Sens. Actuators A Phys. 2001, 89, 64–70. [Google Scholar] [CrossRef]
- Alvi, S.; Akhtar, F. High temperature tribology of polymer derived ceramic composite coatings. Sci. Rep. 2018, 8, 15105. [Google Scholar] [CrossRef] [Green Version]
- Klausmann, A.; Morita, K.; Johanns, K.E.; Fasel, C.; Durst, K.; Mera, G.; Riedel, R.; Ionescu, E. Synthesis and high-temperature evolution of polysilylcarbodiimide-derived SiCN ceramic coatings. J. Eur. Ceram. Soc. 2015, 35, 3771–3780. [Google Scholar] [CrossRef]
- Nagaiah, N.R.; Kapat, J.S.; An, L.; Chow, L. Novel polymer derived ceramic-high temperature heat flux sensor for gas turbine environment. In Proceedings of the Journal of Physics: Conference Series; IOP Publishing: Bristol, UK, 2006; Volume 34, pp. 458–463. [Google Scholar]
- Li, N.; Cao, Y.; Zhao, R.; Xu, Y.; An, L. Polymer-derived SiAlOC ceramic pressure sensor with potential for high-temperature application. Sens. Actuators A Phys. 2017, 263, 174–178. [Google Scholar] [CrossRef]
- Zhao, R.; Shao, G.; Cao, Y.; An, L.; Xu, C. Temperature sensor made of polymer-derived ceramics for high-temperature applications. Sens. Actuators A Phys. 2014, 219, 58–64. [Google Scholar] [CrossRef] [Green Version]
- Yu, Y.; Huang, Q.; Rhodes, S.; Fang, J.; An, L. SiCNO-GO composites with the negative temperature coefficient of resistance for high-temperature sensor applications. J. Am. Ceram. Soc. 2017, 100, 592–601. [Google Scholar] [CrossRef]
- Hou, Y.; Xiao, B.; Sun, Z.; Yang, W.; Wu, S.; Qi, S.; Wen, G.; Huang, X. High temperature anti-oxidative and tunable wave absorbing SiC/Fe3Si/CNTs composite ceramic derived from a novel polysilyacetylene. Ceram. Int. 2019, 45, 16369–16379. [Google Scholar] [CrossRef]
- Flores, O.; Schmalz, T.; Krenkel, W.; Heymann, L.; Motz, G. Selective cross-linking of oligosilazanes to tailored meltable polysilazanes for the processing of ceramic SiCN fibres. J. Mater. Chem. A 2013, 1, 15406. [Google Scholar] [CrossRef]
- Zhang, C.; Liu, Y.; Cui, Y.; Chen, K.; Peng, S.; Zhang, H.; Han, K.; Yu, M. Comparison of effects of nitrogen sources on the structures and properties of SiBNC ceramic fiber precursors. Ceram. Int. 2018, 44, 14878–14883. [Google Scholar] [CrossRef]
- Wu, N.; Wan, Y.; Wang, Y.; Ko, F. Conversion of hydrophilic SiOC nanofibrous membrane to robust hydrophobic materials by introducing palladium. Appl. Surf. Sci. 2017, 425, 750–757. [Google Scholar] [CrossRef]
- Su, D.; Wang, L.; Liang, K.; Zhang, F.; Lin, D.; Hu, Y.; Ji, H.; Li, X.; Chen, S.; Yan, X. Silicon oxycarbide/titanium dioxide fibers with wrinkle-like surface by electrospinning. Mater. Lett. 2016, 172, 202–206. [Google Scholar] [CrossRef]
- Xu, Y.; Su, D.; Feng, H.; Yan, X.; Liu, N.; Sun, Y. Continuous sol–gel derived SiOC/HfO2 fibers with high strength. RSC Adv. 2015, 5, 35026–35032. [Google Scholar] [CrossRef]
- Pillai, S.K.T.; Kretschmer, W.P.; Denner, C.; Motz, G.; Hund, M.; Fery, A.; Trebbin, M.; Förster, S.; Kempe, R. SiCN Nanofibers with a Diameter Below 100 nm Synthesized via Concerted Block Copolymer Formation, Microphase Separation, and Crosslinking. Small 2012, 9, 984–989. [Google Scholar] [CrossRef]
- Yan, C.; Liu, R.; Cao, Y.; Zhang, C.; Zhang, D. Ablation behavior and mechanism of C/ZrC, C/ZrC–SiC and C/SiC composites fabricated by polymer infiltration and pyrolysis process. Corros. Sci. 2014, 86, 131–141. [Google Scholar] [CrossRef]
- Laadoua, H.; Pradeilles, N.; Lucas, R.; Foucaud, S.; Clegg, W.J. Preparation of ZrC/SiC composites by using polymer-derived ceramics and spark plasma sintering. J. Eur. Ceram. Soc. 2020, 40, 1811–1819. [Google Scholar] [CrossRef]
- Wang, Y.; Tian, H.; Quan, D.; Guo, L.; Ouyang, J.; Zhou, Y.; Jia, D. Preparation, characterization and infrared emissivity properties of polymer derived coating formed on 304 steel. Surf. Coatings Technol. 2012, 206, 3772–3776. [Google Scholar] [CrossRef]
- Liu, J.; Zhang, L.; Hu, F.; Yang, J.; Cheng, L.; Wang, Y. Polymer-derived yttrium silicate coatings on 2D C/SiC composites. J. Eur. Ceram. Soc. 2013, 33, 433–439. [Google Scholar] [CrossRef]
- Jia, Y.; Ajayi, T.D.; Roberts, M.A., Jr.; Chung, C.-C.; Xu, C. Ultrahigh-Temperature Ceramic–Polymer-Derived SiOC Ceramic Composites for High-Performance Electromagnetic Interference Shielding. ACS Appl. Mater. Interfaces 2020, 12, 46254–46266. [Google Scholar] [CrossRef] [PubMed]
- Biesuz, M.; Zera, E.; Tomasi, M.; Jana, P.; Ersen, O.; Baaziz, W.; Lindemann, A.; Sorarù, G.D. Polymer-derived Si3N4 nanofelts for flexible, high temperature, lightweight and easy-manufacturable super-thermal insulators. Appl. Mater. Today 2020, 20, 100648. [Google Scholar] [CrossRef]
- Luan, X.; Chang, S.; Riedel, R.; Cheng, L. An air stable high temperature adhesive from modified SiBCN precursor synthesized via polymer-derived-ceramic route. Ceram. Int. 2018, 44, 8476–8483. [Google Scholar] [CrossRef]
- Wang, X.; Shi, J.; Wang, H. Preparation, properties, and structural evolution of a novel polyborosilazane adhesive, temperature-resistant to 1600 °C for joining SiC ceramics. J. Alloys Compd. 2019, 772, 912–919. [Google Scholar] [CrossRef]
- Luan, X.; Chang, S.; Riedel, R.; Cheng, L. The improvement in thermal and mechanical properties of TiB2 modified adhesive through the polymer-derived-ceramic route. Ceram. Int. 2018, 44, 19505–19511. [Google Scholar] [CrossRef]
- Luan, X.; Chang, S.; Yu, R.; Zou, Y.; Riedel, R.; Cheng, L. Effect of PSO and TiB2 content on the high temperature adhesion strength of SiBCNO ceramic. Ceram. Int. 2019, 45, 9515–9521. [Google Scholar] [CrossRef]
- Wang, X.; Wang, J.; Wang, H. Performance and structural evolution of high-temperature organic adhesive for joining Al2O3 ceramics. Int. J. Adhes. Adhes. 2013, 45, 1–6. [Google Scholar] [CrossRef]
- Wang, X.; Wang, J.; Wang, H. Synthesis of a novel preceramic polymer (V-PMS) and its performance in heat-resistant organic adhesives for joining SiC ceramic. J. Eur. Ceram. Soc. 2012, 32, 3415–3422. [Google Scholar] [CrossRef]
- Jeong, D.-H.; Septiadi, A.; Fitriani, P.; Yoon, D.-H. Joining of SiC f/SiC using polycarbosilane and polysilazane preceramic mixtures. Ceram. Int. 2018, 44, 10443–10450. [Google Scholar] [CrossRef]
- Zhao, C.-C.; Lin, R.-L.; Dai, M.-M.; Xu, X.; Zhu, L.-L.; Xue, J.-X.; Zhai, J.-H.; Ma, H.-B.; Guo, W.-M.; Lin, H.-T.; et al. Facile joining of SiC ceramics with screen-printed polycarbosilane without pressure. J. Eur. Ceram. Soc. 2021, 41, 2157–2161. [Google Scholar] [CrossRef]
- Chen, Y.; Cao, Y.; Wang, Y. Electrical property of joints made of polymer-derived SiAlCN ceramic via adhesive joining. Ceram. Int. 2021, 47, 3649–3656. [Google Scholar] [CrossRef]
- Liew, L.; Zhang, W.; An, L.; Shah, S.; Luo, R.; Liu, Y.; Cross, T.; Dunn, M.L.; Bright, V.; Daily, J.W. Ceramic MEMS. Am. Ceram. Soc. Bull. 2001, 80, 25. [Google Scholar]
- Kong, J.; Maute, K.; Frangopol, D.; Liew, L.-A.; Saravanan, R.; Raj, R. A real time human–machine interface for an ultrahigh temperature MEMS sensor–igniter. Sens. Actuators A Phys. 2003, 105, 23–30. [Google Scholar] [CrossRef]
- Yu, Y.; Liu, Y. A large-pressure-range, large-sensing-distance wireless passive pressure sensor based on polymer infiltration pyrolysis-enhanced polymer-derived ceramic films. Meas. Sci. Technol. 2020, 31, 075103. [Google Scholar] [CrossRef]
- Shao, G.; Jiang, J.; Jiang, M.; Su, J.; Liu, W.; Wang, H.; Xu, H.; Lit, H.; Zhang, R. Polymer-derived SiBCN ceramic pressure sensor with excellent sensing performance. J. Adv. Ceram. 2020, 9, 374–379. [Google Scholar] [CrossRef]
- Hu, L.-H.; Raj, R. Semiconductive Behavior of Polymer-Derived SiCN Ceramics for Hydrogen Sensing. J. Am. Ceram. Soc. 2015, 98, 1052–1055. [Google Scholar] [CrossRef]
- Luo, C.; Jiao, T.; Tang, Y.; Kong, J. Excellent Electromagnetic Wave Absorption of Iron-Containing SiBCN Ceramics at 1158 K High-Temperature. Adv. Eng. Mater. 2018, 20, 1701168. [Google Scholar] [CrossRef]



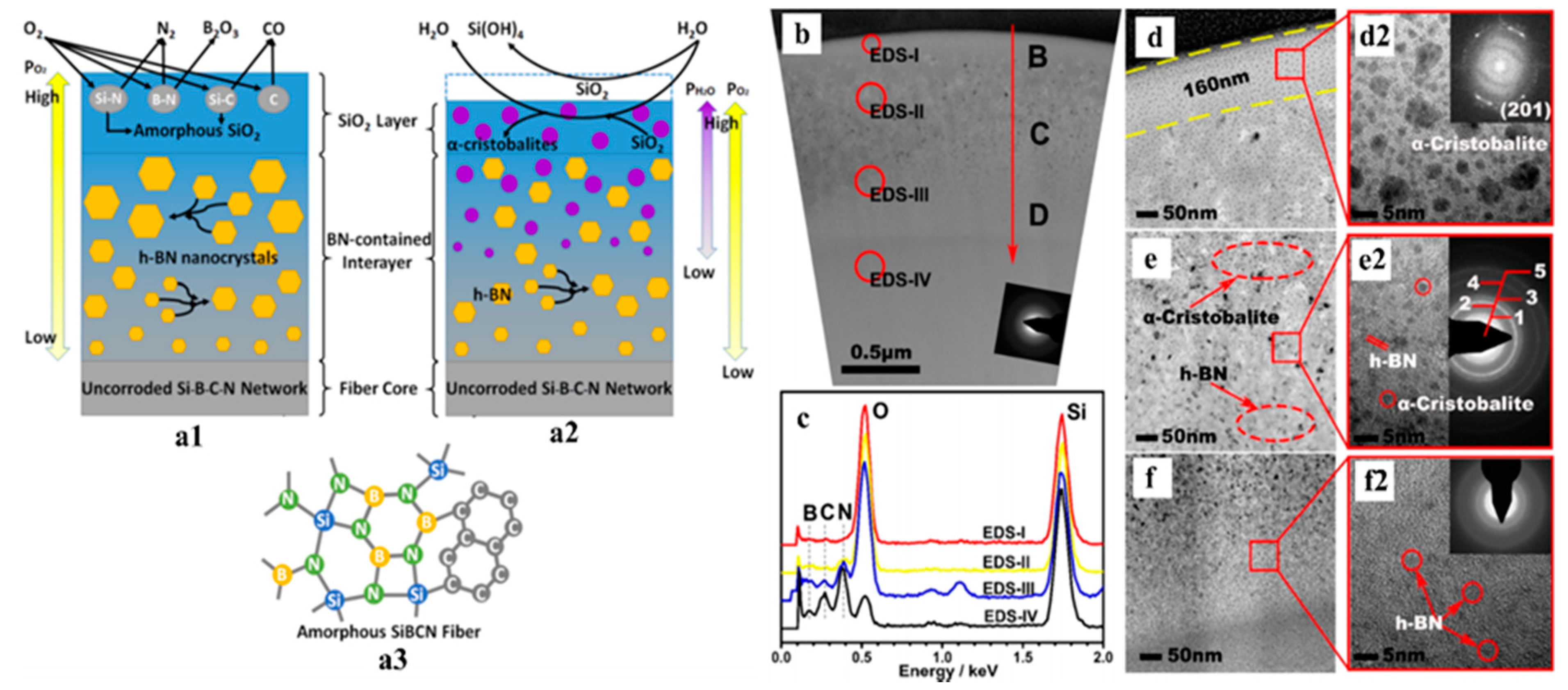
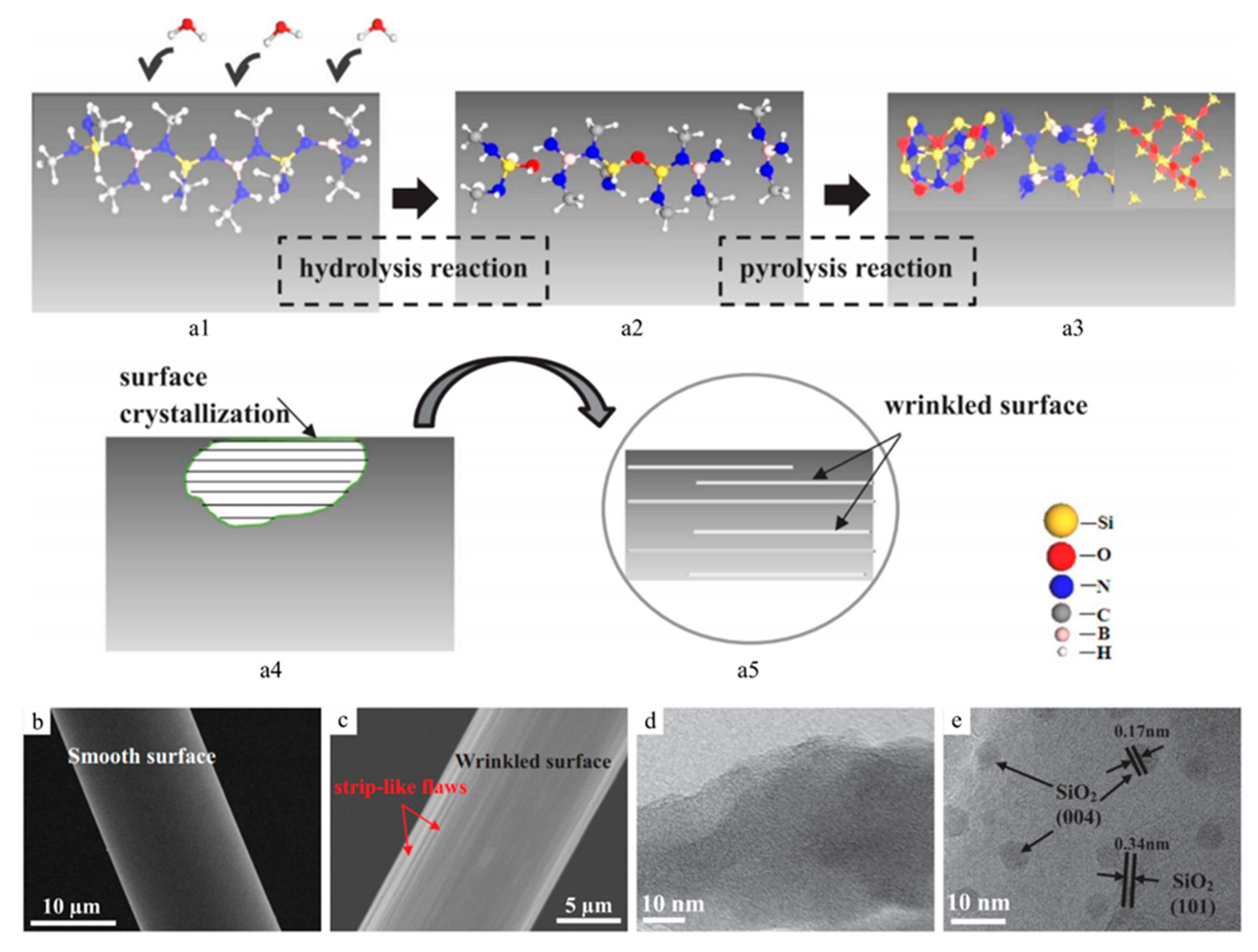
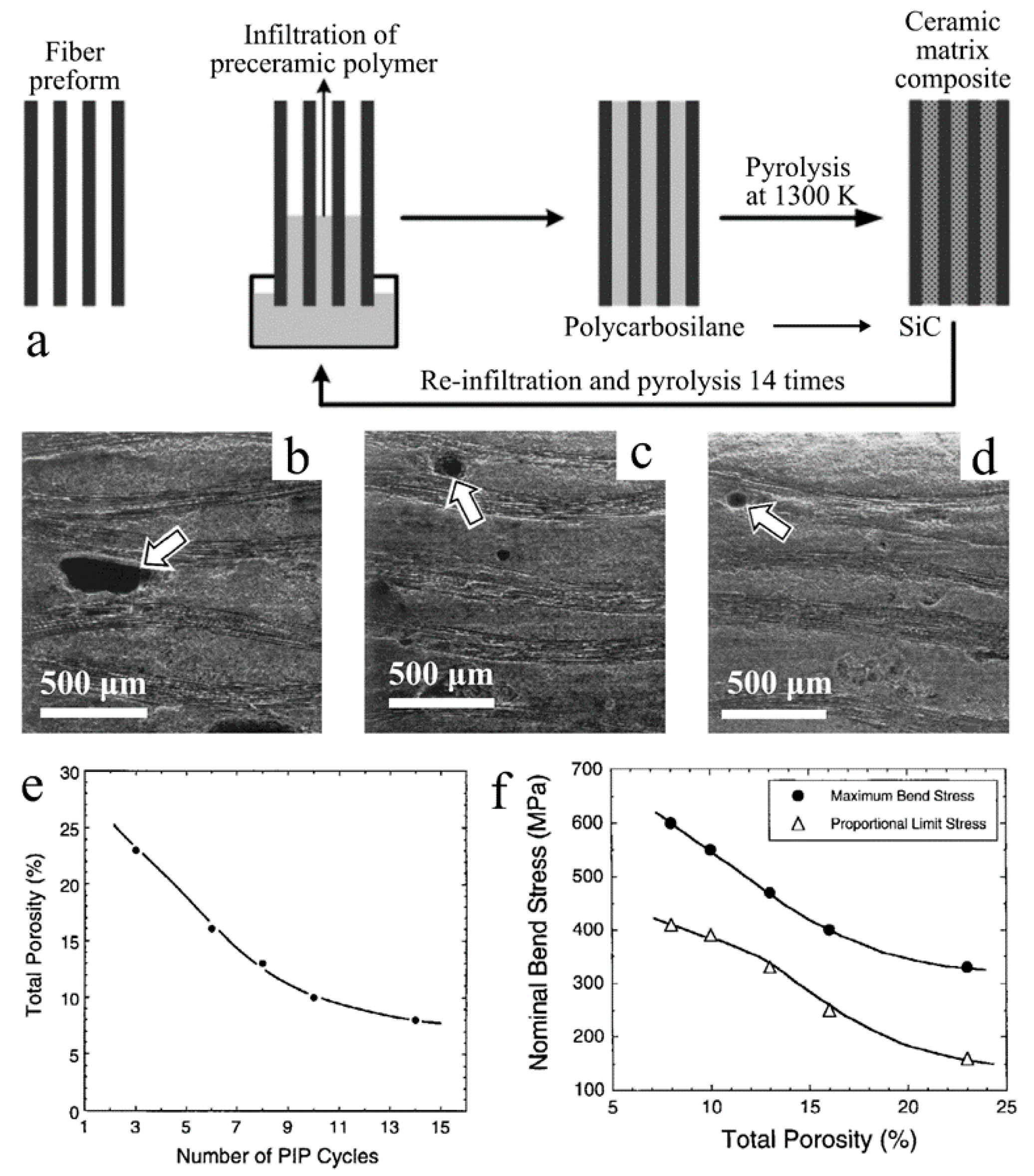
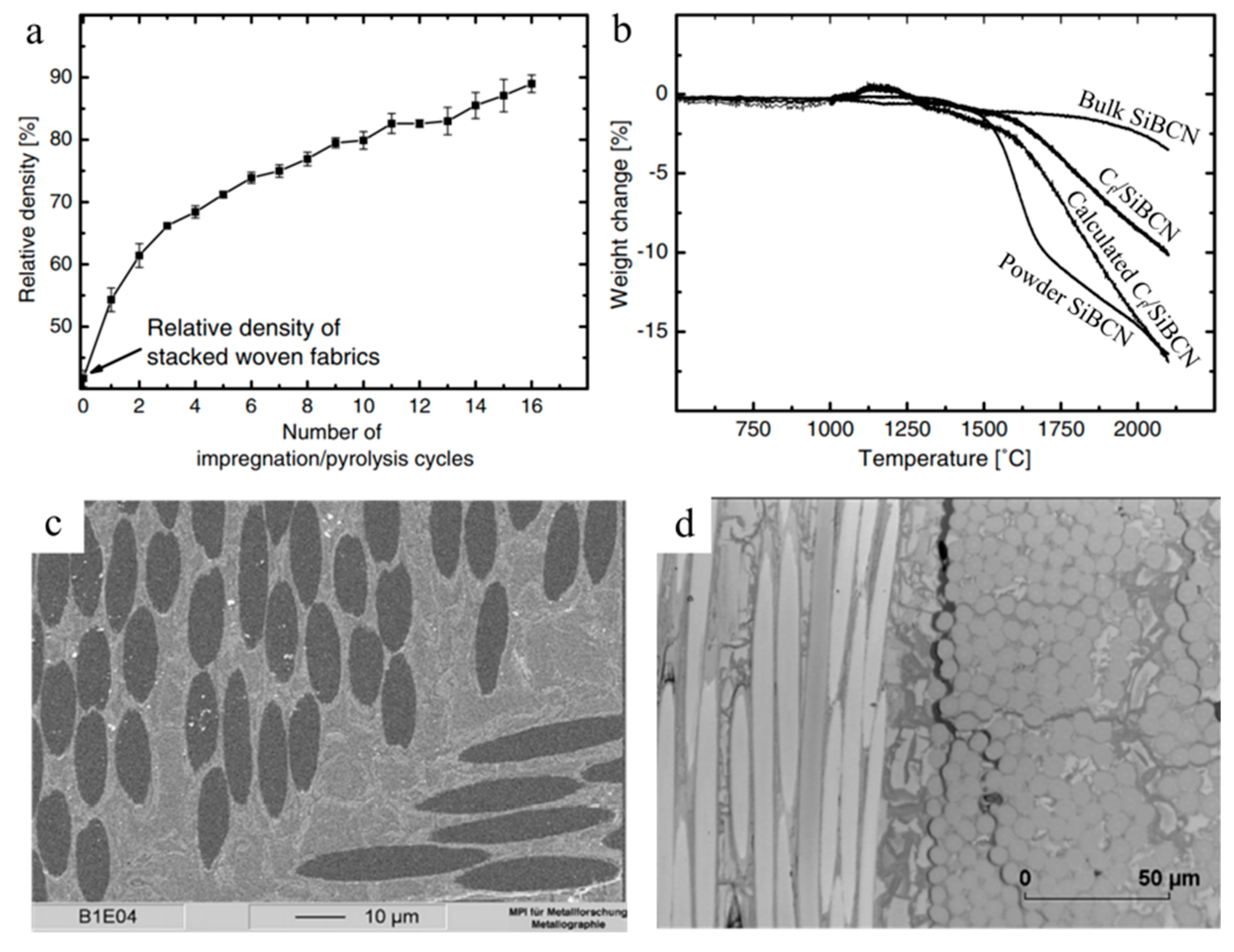


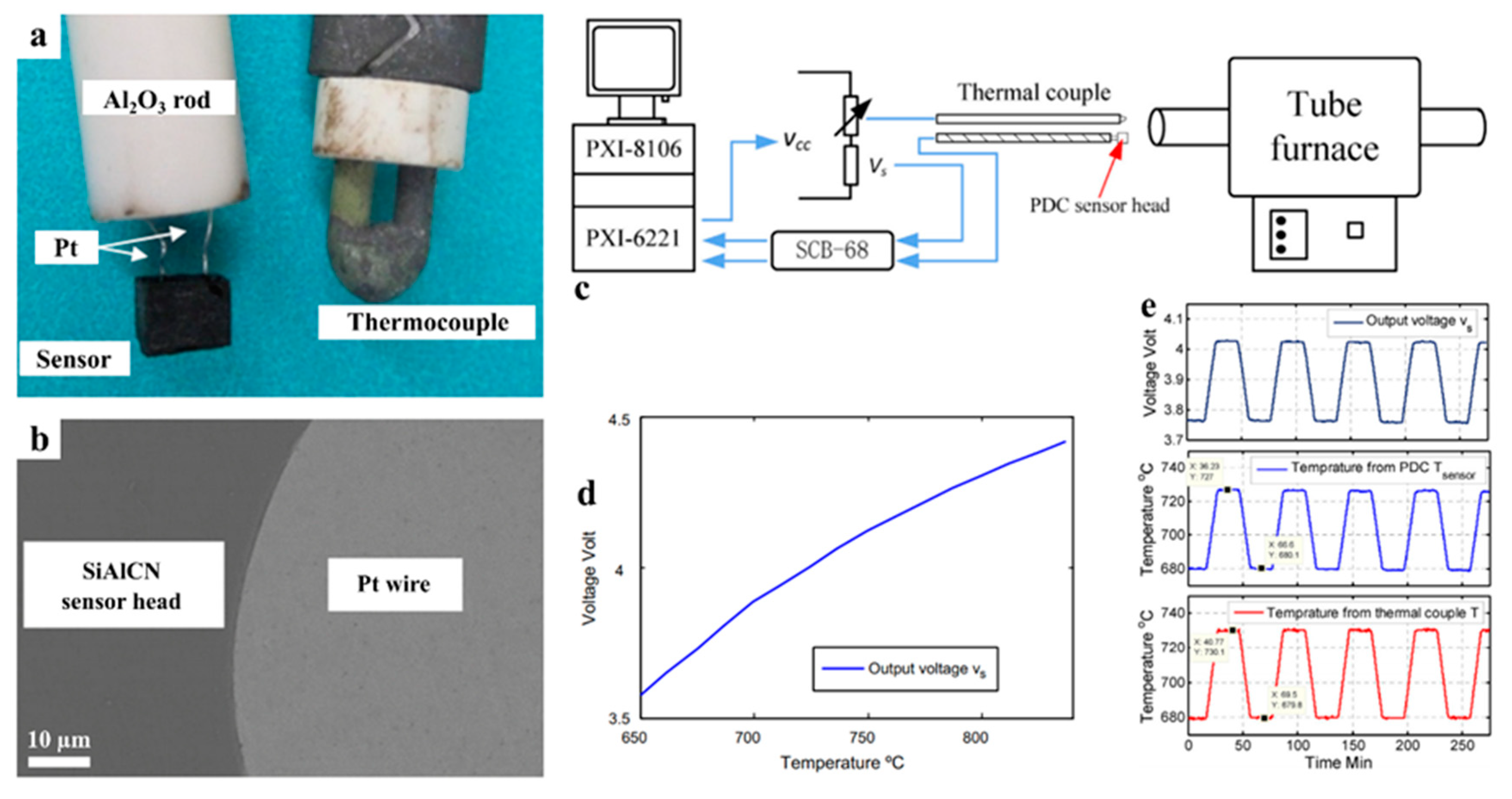
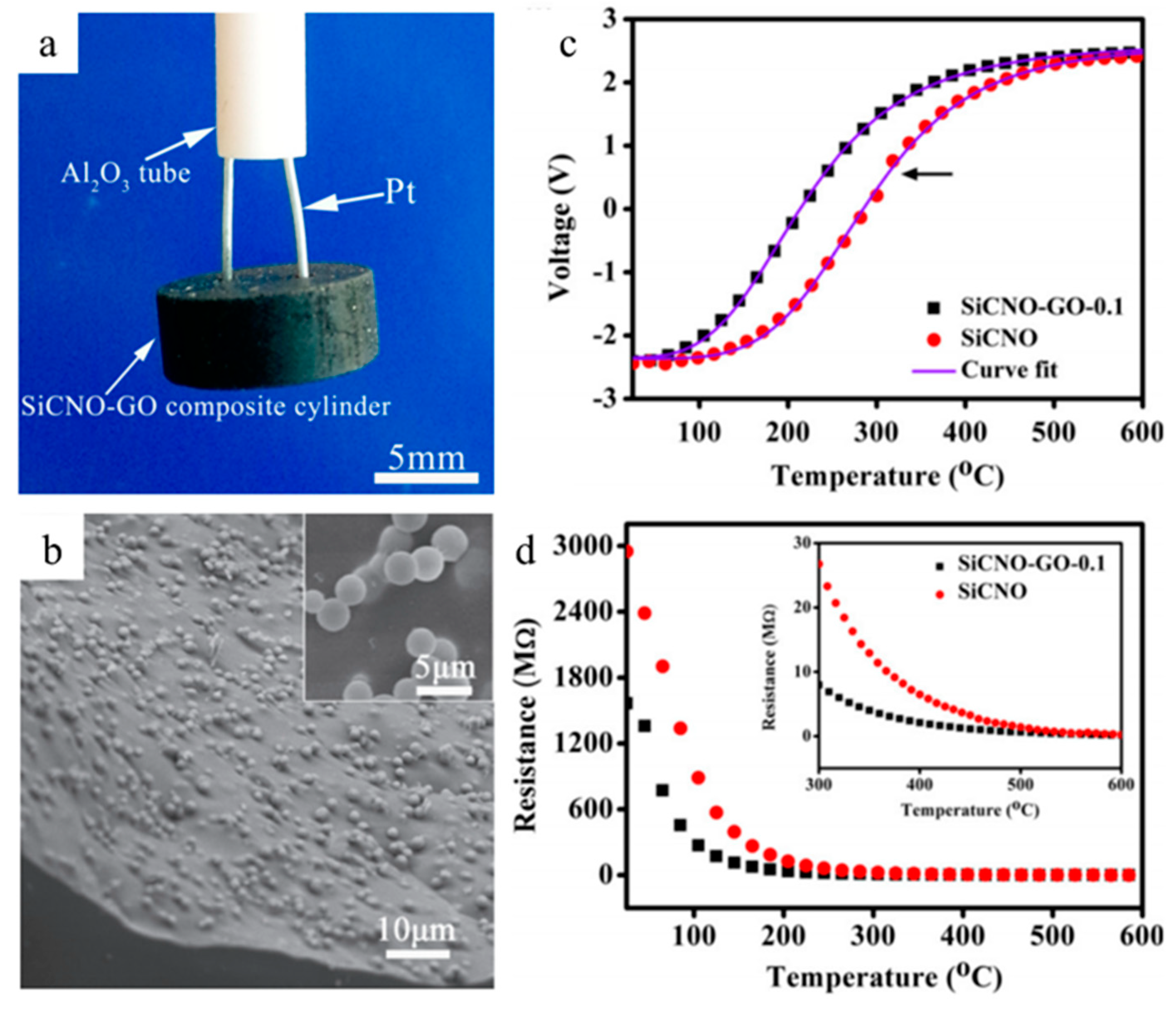
| Application | PDC | Filler | Precursor | Remarkable Properties | Thermal Stability (°C) | Reference |
|---|---|---|---|---|---|---|
| Fiber | SiCN | - | Polysilazane | D = 100 μm | 1400 | [66] |
| SiBCN | - | Polyborosilazane | σT = 1.03 GPa | 1600 (Tp) | [67] | |
| SiBN | - | Polyborosilazane | σT = 0.91 GPa | 1400 (Tp) | [43] | |
| SiBCN | - | Polyborosilazane | D = 12 μm | 1400 | [42] | |
| SiOC | Pb | Polycarbosilane | WCA = 135° | 700 | [68] | |
| SiOC | TiO2 | Polyhydromethyl-siloxane | WCA = 130° | 1300 | [69] | |
| SiOC | HfO2 | Tetraethoxysilane + dimethyldiethoxyl-silane | σT = 1.5 GPa | 1000 (Tp) | [70] | |
| SiOC | HfO2 | Tetraethoxysilane + dimethyldiethoxyl-silane | σT = 930 MPa E = 155 GPa | 1500 | [23] | |
| SiBOC | - | Polyborosiloxane | D = 1 μm | 800 | [11] | |
| SiCN | - | Polysilazane | D < 100 nm | 1100 (Tp) | [71] | |
| Matrix | SiC | - | Polycarbosilane | σB = 600 MPa | 1027 (Tp) | [44] |
| SiC | ZrC | Polycarbosilane | ρ = 2.13 g/cm3 porosity = 15% | 1700 | [72] | |
| SiC | ZrC | Polycarbosilane | Porosity = 0.9% | 1950 | [73] | |
| SiBCN | - | Oligovinylsilazane + tris(methyldihydridosilylethylene)borane | σB = 225 MPa | 1500 | [50] | |
| SiOC | ZrO2 | Polymethylsilsesquioxane | ρ = 2.3–3.5 g/cm3 | 1400 | [4] | |
| Coating/Membrane | SiO2 | Al | Polyhydridomethylsiloxane | IE = 0.2 in 8–14 μm | 800 | [74] |
| SiOC | Y2O3 | Polysiloxane | CTE = 4.43–7.26 × 10−6 | 1400 | [75] | |
| SiCN | - | Polysilylcarbodiimide | E = 105–117 GPa Hardness = 10–11 GPa | 1400 | [60] | |
| SiOC | ZrB2 | Polycarbosilane | SET = 72 dB at Ka | 1000 | [76] | |
| SiOC | - | Polysiloxane | Conductivity = 11 mW m−1 K−1 | 1000 | [77] | |
| SiBCN | - | Polysilazane + borane dimethylsulfide | Ceramic yield = 65% | 1800 | [10] | |
| Adhesive | SiBCN | Nano Al2O3 | Polyborosilazane + polysiloxane | Bond strength = 6.65 MPa | 1000 | [78] |
| SiBCN | borazine | Polymethylsilane | Bond strength = 7.9 MPa | 1600 | [79] | |
| SiCN | TiB2 | Polysilazane | Bond strength = 8.0 MPa | 800 | [80] | |
| SiBCNO | TiB2 | Polysilazane + polysiloxane | Bond strength = 12.3 MPa | 1000 | [81] | |
| SiOC | B4C, glass powder | Polymethylsilane + polysiloxane | Bond strength = 66.9 MPa | 1000 | [82] | |
| SiOC | - | Polymethylsilane + Polysiloxane | Bond strength = 50.8 MPa | 1200 | [83] | |
| SiC | - | Polycarbosilane | Bond strength = 120 MPa | 1750 | [84] | |
| SiC | - | Polycarbosilane | Bond strength = 105.8 MPa | 1500 | [85] | |
| SiOC | - | Polycarbosilane | Bond strength = 66.9 MPaR = 1.15–5.6 kΩ | 850 | [86] | |
| MEMS/Semiconductor | SiCN | - | Polysilazane | E = 158 GPa | 1500 | [87] |
| SiCN | - | - | E = 80–225 GPa | 1650 | [88] | |
| SiCN | - | Polysilazane | FS = 326 um/s | - | [54] | |
| SiCNO | - | Polysilazane | BG = 2.2 eV | 1300 | [51] | |
| Sensor | SiAlOC | - | Aluminum tri-sec-butoxide | E = 150 GPa GF = 16,000 | 1000 | [62] |
| SiAlCN | - | - | EC = 0–100/Ω/cm | 1400 | [61] | |
| SiCNO | - | Polymethylsilsesquioxane | GF = 600–1700 | 1500 | [52] | |
| SiCN | - | Polysilazane | R = 60 kΩ | 1400 | [53] | |
| SiCN | - | Polysilazane | Sensitivity = 290 kHz kPa−1 | 800 | [89] | |
| SiBCN | - | Polyborosilazane | GF = 5500 | 1000 | [90] | |
| SiAlCN | - | Polysilzane + aluminum-tri-sec-butoxide | c1 = −3235 c2 = −3.6 | 830 | [63] | |
| SiCN | - | Polysilazane | BG = 0.08 eV | 1000 | [91] | |
| SiCNO | GO | Polyvinylsilazane | EC = 1.39 × 10−7 | 600 | [64] | |
| Wave absorb | SiBCN | - | Polyborosilazane | RL = −12.62 dB at 3.2 GHz | 1400 | [92] |
| SiC | Fe3Si, CNTs | Polysilylacetylene + ferric acetylacetonate | RL = −40 dB at 10.36 GHz | 800 | [65] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ren, Z.; Mujib, S.B.; Singh, G. High-Temperature Properties and Applications of Si-Based Polymer-Derived Ceramics: A Review. Materials 2021, 14, 614. https://doi.org/10.3390/ma14030614
Ren Z, Mujib SB, Singh G. High-Temperature Properties and Applications of Si-Based Polymer-Derived Ceramics: A Review. Materials. 2021; 14(3):614. https://doi.org/10.3390/ma14030614
Chicago/Turabian StyleRen, Zhongkan, Shakir Bin Mujib, and Gurpreet Singh. 2021. "High-Temperature Properties and Applications of Si-Based Polymer-Derived Ceramics: A Review" Materials 14, no. 3: 614. https://doi.org/10.3390/ma14030614






