Influence of Sandblasting and Chemical Etching on Titanium 99.2–Dental Porcelain Bond Strength
Abstract
:1. Introduction
2. Materials and Methods
2.1. Samples Preparation
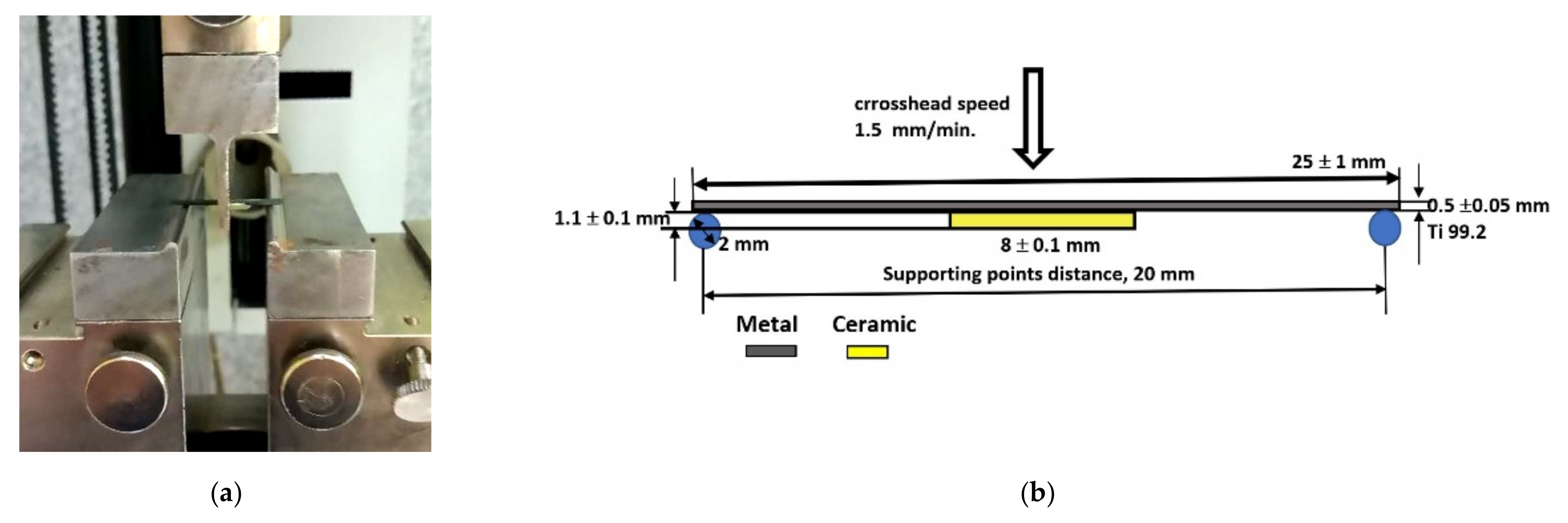
2.2. Microstructural Analysis and Mechanical Testing
2.3. Bond Strenght Estimation and Statistical Analysis
- k—coefficient depending on the thickness of the base metal and Young’s modulus,
- k = 4.6 (E = 113 GPa, [41]);
- Ffail—metal–ceramic bond breaking force
- K—the factor that differentiates the value of the statistic for the test,
- L—number associated with the number of compared groups pairs
- SK—intra-group variance calculated in the analysis of variance.
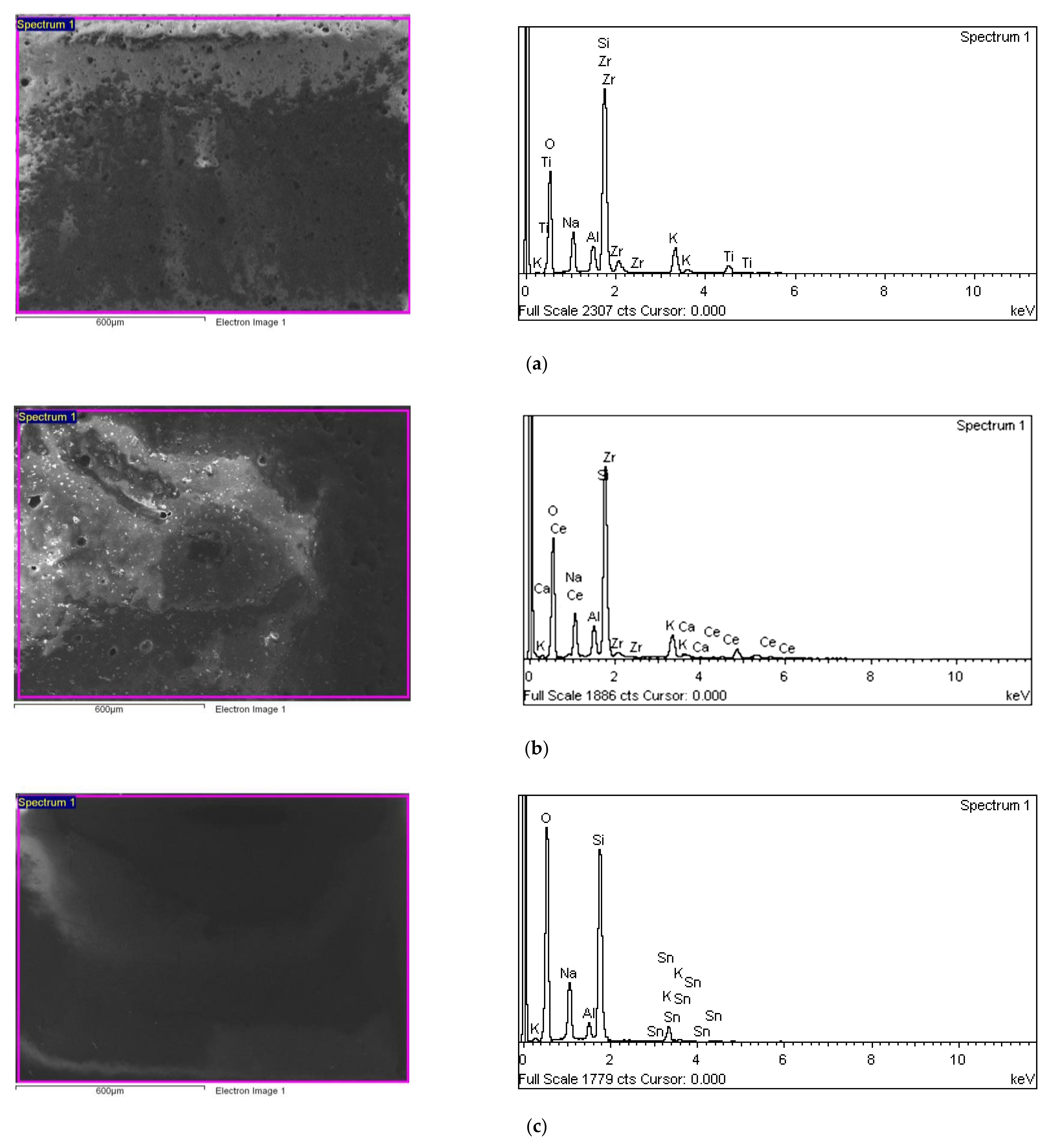
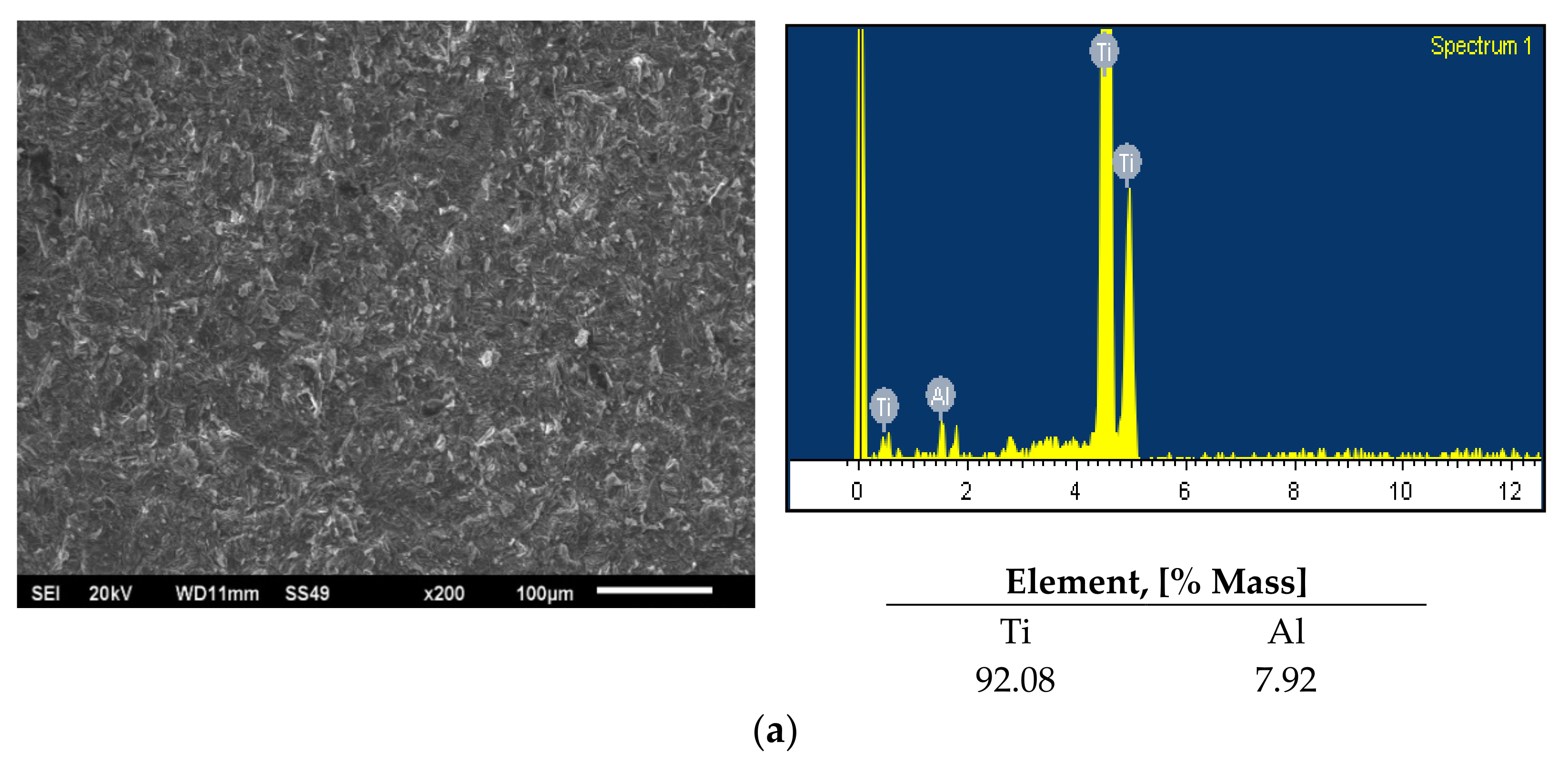
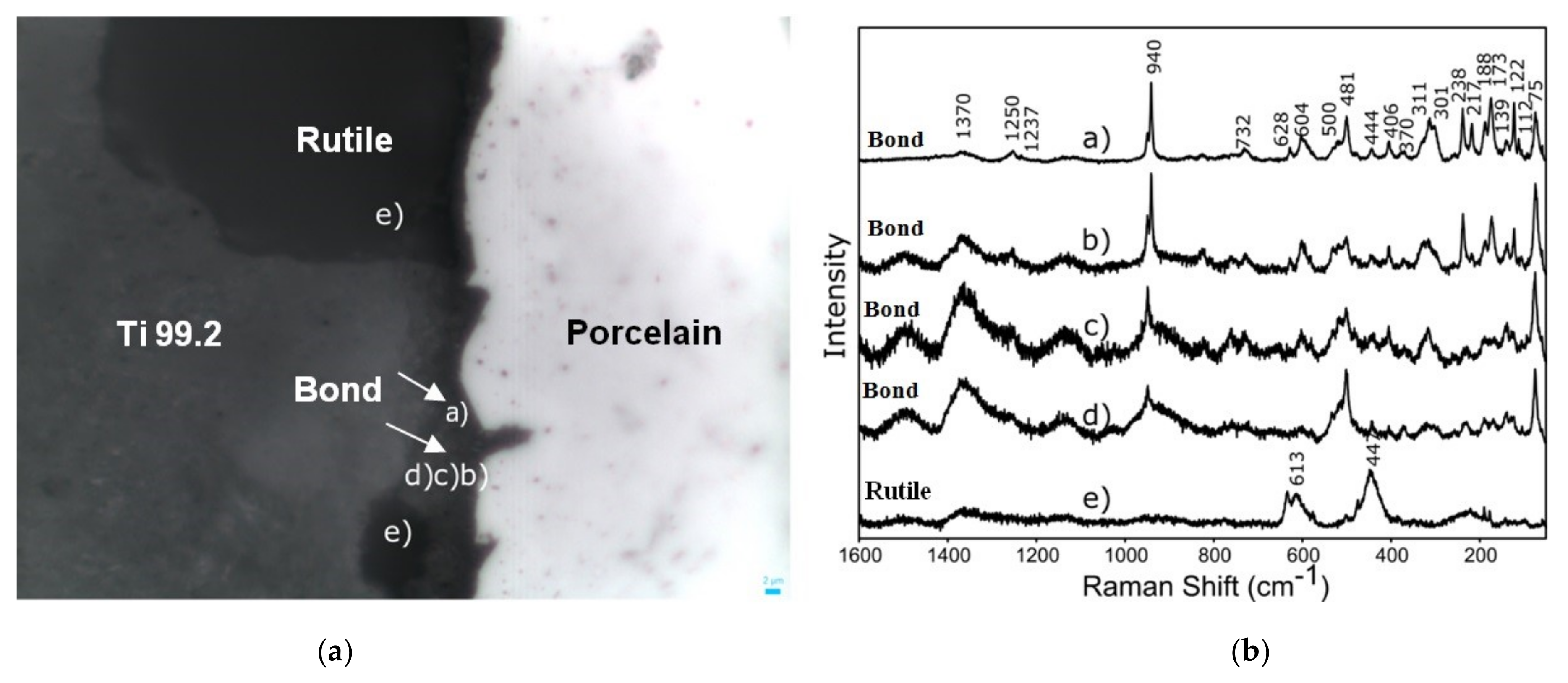
3. Results and Discussion
- -
- surface roughness (by using blasting, unevenness is created on the metal surface, into which the porcelain penetrates, causing mechanical micro fixations) [32];
- -
- compressive stresses caused by shrinkage of metal and porcelain (thermal expansion of porcelain should be slightly smaller than metal, in which case favorable compressive stresses will occur) [29];
- -
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ananth, H.; Kundapur, V.; Mohammed, H.S.; Anand, M.; Amarnath, G.S.; Mankar, S. A Review on Biomaterials in Dental Implantology. Int. J. Biomed. Sci. IJBS 2015, 11, 113–120. [Google Scholar]
- Hey, J.; Beuer, F.; Bensel, T.; Boeckler, A.F. Metal–ceramic-fixed dental prosthesis with CAD/CAM-fabricated substructures: 6-year clinical results. Clin. Oral Investig. 2012, 17, 1447–1451. [Google Scholar] [CrossRef]
- Hey, J.; Beuer, F.; Bensel, T.; Boeckler, A.F. Single crowns with CAD/CAM-fabricated copings from titanium: 6-year clinical results. J. Prosthet. Dent. 2014, 112, 150–154. [Google Scholar] [CrossRef]
- Zhou, Y.; Li, N.; Wang, H.; Yan, J.; Liu, W.; Xu, S. Effects of the rare earth element lanthanum on the metal-ceramic bond strength of dental casting Co-Cr alloys. J. Prosthet. Dent. 2019, 121, 848–857. [Google Scholar] [CrossRef] [PubMed]
- Knosp, H.; Holliday, R.J.; Corti, C.W. Gold in dentistry: Alloys, uses and performance. Gold Bull. 2003, 36, 93–102. [Google Scholar] [CrossRef] [Green Version]
- Wu, J.; Zhou, J.; Zhao, W.; Gao, B. Evaluation of the bond strength of a low-fusing porcelain to cast Ti–24Nb–4Zr–7.9Sn alloy. Mater. Sci. Eng. C 2013, 33, 140–144. [Google Scholar] [CrossRef] [PubMed]
- Takemoto, S.; Hattori, M.; Yoshinari, M.; Kawada, E.; Asami, K.; Oda, Y. Corrosion mechanism of Ti–Cr alloys in solution containing fluoride. Dent. Mater. 2009, 25, 467–472. [Google Scholar] [CrossRef] [Green Version]
- Tamac, E.; Kumbuloglu, T.O.; Toksavul, S.; Toman, M.; Sarikanat, M. Effects of sandblasting and silicoating on bond strength between titanium and porcelain. Niger. J. Clin. Pract. 2018, 21, 1177–1181. [Google Scholar] [PubMed]
- Dolgov, N.A.; Dikova, T.; Dzhendov, D.; Pavlova, D.; Simov, M. Mechanical properties of dental Co-Cr alloys fabricated via Casting and Selective Laser Melting. Mater. Sci. Nonequilib. Phase Transform 2016, 3, 3–7. [Google Scholar]
- Tschernitschek, H.; Borchers, L.; Geurtsen, W. Nonalloyed titanium as a bioinert metal—A review. J. Prosthet. Dent. 2006, 96, 12. [Google Scholar] [CrossRef]
- Novaes, A.B., Jr.; De Souza, S.L.S.; De Barros, R.R.M.; Pereira, K.K.Y.; Iezzi, G.; Piattelli, A. Influence of implant surfaces on osseointegration. Braz. Dent. J. 2010, 21, 471–481. [Google Scholar] [CrossRef] [PubMed]
- Jivraj, S.; Chee, W. Rationale for dental implants. Br. Dent. J. 2006, 200, 661–665. [Google Scholar] [CrossRef] [PubMed]
- Javed, F.; Romanos, G.E. The role of primary stability for successful immediate loading of dental implants. A literature review. J. Dent. 2010, 38, 612–620. [Google Scholar] [CrossRef]
- Kimura, H.; Horng, C.-J.; Okazaki, M.; Takahashi, J. Oxidation Effects on Porcelain-Titanium Interface Reactions and Bond Strength. Dent. Mater. J. 1990, 9, 91–99. [Google Scholar] [CrossRef]
- Papadopoulos, T.D.; Spyropoulos, K.D. The effect of a ceramic coating on the cpTi–porcelain bond strength. Dent. Mater. 2009, 25, 247–253. [Google Scholar] [CrossRef]
- Kurup, A.; Dhatrak, P.; Khasnis, N. Surface modification techniques of titanium and titanium alloys for biomedical dental applications: A review. Mater. Today Proc. 2020, 39, 84–90. [Google Scholar] [CrossRef]
- Wang, R.R.; Fung, K.K. Oxidation behavior of surface-modified titanium for titanium-ceramic restorations. J. Prosthet. Dent. 1997, 77, 423–434. [Google Scholar] [CrossRef]
- Al Hussaini, I.; Al Wazzan, K.A. Effect of surface treatment on bond strength of low-fusing porcelain to commercially pure titanium. J. Prosthet. Dent. 2005, 94, 350–356. [Google Scholar] [CrossRef]
- Ramakrishnaiah, R.; Alkheraif, A.A.; Divakar, D.D.; Matinlinna, J.P.; Vallittu, P.K. The Effect of Hydrofluoric Acid Etching Duration on the Surface Micromorphology, Roughness, and Wettability of Dental Ceramics. Int. J. Mol. Sci. 2016, 17, 822. [Google Scholar] [CrossRef] [Green Version]
- Kim, J.-T.; Cho, S.-A. The effects of laser etching on shear bond strength at the titanium ceramic interface. J. Prosthet. Dent. 2009, 101, 101–106. [Google Scholar] [CrossRef]
- Egoshi, T.; Taira, Y.; Soeno, K.; Sawase, T. Effects of sandblasting, H2SO4/HCl etching, and phosphate primer application on bond strength of veneering resin composite to commercially pure titanium grade 4. Dent. Mater. J. 2013, 32, 219–227. [Google Scholar] [CrossRef] [Green Version]
- Gökçe, B.; Ozpinar, B.; Dündar, M.; Cömlekoglu, E.; Sen, B.H.; Güngör, M.A. Bond Strengths of All-Ceramics: Acid vs. Laser Etching. Oper. Dent. 2007, 32, 173–178. [Google Scholar] [CrossRef] [Green Version]
- Vignesh, S.N.; Bhuminathan, M.; Santhosh, S. Comparative evaluation of the three different surface treatments—Conventional, laser and Nano technology methods in enhancing the surface characteristics of commercially pure titanium discs and their effects on cell adhesion: An in vitro study. J. Pharm. Bioallied Sci. 2015, 7, 89–91. [Google Scholar] [CrossRef]
- Akin, H.; Tugut, F.; Topcuoglu, S.; Kirmali, O. Effects of Sandblasting and Laser Irradiation on Shear Bond Strength of Low-fusing Porcelain to Titanium. J. Adhes. Dent. 2013, 15, 55–63. [Google Scholar] [CrossRef] [PubMed]
- Saygin, A.G.; Özdemir, A.K.; Görler, O. Influence of Various Laser Surface Modifications on SBS of Titanium and Zirconium Oxide Substructures. Cumhur. Sci. J. 2017, 38, 245. [Google Scholar] [CrossRef]
- Dundar, B.; Guzel, K.G. An analysis of the shear strength of the bond between enamel and porcelain laminate veneers with different etching systems: Acid and Er, Cr: YSGG laser separately and combined. Lasers Med. Sci. 2010, 26, 777–782. [Google Scholar] [CrossRef]
- Chaiyabutr, Y.; McGowan, S.; Phillips, K.M.; Kois, J.C.; Giordano, R.A. The effect of hydrofluoric acid surface treatment and bond strength of a zirconia veneering ceramic. J. Prosthet. Dent. 2008, 100, 194–202. [Google Scholar] [CrossRef]
- Bhandari, K.S.; Moldi, A.I.; Nagral, S.; Deshpandey, S.; Kulkarni, P. Effect of sandblasting on fracture load of titanium ceramic crowns. J. Indian Prosthodont. Soc. 2015, 15, 224–228. [Google Scholar] [CrossRef] [PubMed]
- Golebiowski, M.; Wolowiec, E.; Klimek, L. Airborne-particle abrasion parameters on the quality of titanium-ceramic bonds. J. Prosthet. Dent. 2015, 113, 453–459. [Google Scholar] [CrossRef] [Green Version]
- Walczak, M. Influence of Selected Technological Procedures on the Service Life of Metal-Ceramic Systems Used in Dental Prosthetics; Habilitation Monograph; Lublin University of Technology: Lublin, Poland, 2014; p. 187. [Google Scholar]
- Papadopoulos, T.; Tsetsekou, A.; Eliades, G. Effect of aluminum oxide sandblasting on cast commercially pure titanium surfaces. Eur. J. Prosthodont. Restor. Dent. 1999, 7, 15–21. [Google Scholar] [PubMed]
- Kou, Z.; Yi, Q.; Zhi, X.Z. The effect of different size of aluminum oxide for sandblasting on bonding strength of porcelain to metal. Zhonghua Kou Qiang Yi Xue Za Zhi. 1994, 29, 229–231. [Google Scholar]
- Cai, Z.; Bunce, N.; E Nunn, M.; Okabe, T. Porcelain adherence to dental cast CP titanium: Effects of surface modifications. Biomaterials 2001, 22, 979–986. [Google Scholar] [CrossRef]
- Akyil, M.S.; Yilmaz, A.; Karaalioğlu, O.F.; Duymuş, Z.Y.; Akyıl, M. Shear Bond Strength of Repair Composite Resin to an Acid-Etched and a Laser-Irradiated Feldspathic Ceramic Surface. Photomed. Laser Surg. 2010, 28, 539–545. [Google Scholar] [CrossRef]
- Guilherme, N.; Wadhwani, C.; Zheng, C.; Chung, K.-H. Effect of surface treatments on titanium alloy bonding to lithium disilicate glass-ceramics. J. Prosthet. Dent. 2016, 116, 797–802. [Google Scholar] [CrossRef]
- Antanasova, M.; Kocjan, A.; Kovač, J.; Žužek, B.; Jevnikar, P. Influence of thermo-mechanical cycling on porcelain bonding to cobalt–chromium and titanium dental alloys fabricated by casting, milling, and selective laser melting. J. Prosthodont. Res. 2018, 62, 184–194. [Google Scholar] [CrossRef] [PubMed]
- Yılmaz, K.; Gonuldas, F.; Ozturk, C. The effect of repeated firings on the color change of dental ceramics using different glazing methods. J. Adv. Prosthodont. 2014, 6, 427–433. [Google Scholar] [CrossRef] [Green Version]
- Yang, J.; Kelly, J.R.; Bailey, O.; Fischman, G. Porcelain-titanium bonding with a newly introduced, commercially available system. J. Prosthet. Dent. 2016, 116, 98–101. [Google Scholar] [CrossRef] [PubMed]
- García-Sanz, V.; Paredes-Gallardo, V.; Mendoza-Yero, O.; Leal, M.C.; Albaladejo, A.; Montiel-Company, J.M.; Bellot-Arcís, C. The effects of lasers on bond strength to ceramic materials: A systematic review and meta-analysis. PLoS ONE 2018, 13, e0190736. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Papia, E.; Arnoldsson, P.; Baudinova, A.; Jimbo, R.; Von Steyern, P.V. Cast, milled and EBM-manufactured titanium, differences in porcelain shear bond strength. Dent. Mater. J. 2018, 37, 214–221. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sawada, T.; Schille, C.; Schweizer, E.; Geis-Gerstorfer, J.; Takemoto, S. Bond strength of commercial veneering porcelain to experimental cast Ti-Cr alloy. Dent. Mater. J. 2020, 39, 825–833. [Google Scholar] [CrossRef]
- ISO. Metal Ceramic Dental Systems; ISO 9693:1999; International Organization for Standardization: Geneva, Switzerland, 2019. [Google Scholar]
- Duceratin Kiss Directions for Use GB—DeguDent/Distributed by: Dentsply International Inc. Prosthetics Division. 2017. Available online: https://www.dentsplysirona.com/content/dam/dentsply/pim/manufacturer/Prosthetics/Fixed/Ceramics/Veneering_Porcelain/Duceram_Kiss/Ansichts-PDF_DFU_Duceram%20Kiss_multi_2017_09.pdf (accessed on 14 October 2021).
- Lubas, M. The impact of an innovative sandblasting medium on the titanium–dental porcelain joint. Ceram. Mater. 2019, 3, 276–285. [Google Scholar]
- Lubas, M.; Jasinski, J.J.; Jeleń, P.; Sitarz, M. Effect of ZrO2 sol-gel coating on the Ti 99.2—Porcelain bond strength investigated with mechanical testing and Raman spectroscopy. J. Mol. Struct. 2018, 1168, 316–321. [Google Scholar] [CrossRef]
- Chakmakchi, M.; Eliades, G.; Zinelis, S. Bonding agents of low fusing cpTi porcelains: Elemental and morphological characterization. J. Prosthodont. Res. 2009, 53, 166–171. [Google Scholar] [CrossRef]
- Guo, L.; Shi, Y.; Guo, L.; Zhang, Q.; Tian, J.; Zhu, Y.; Guo, T. Preparation and characterization of a titanium bonding porcelain. Mater. Sci. Eng. C 2012, 32, 1531–1535. [Google Scholar] [CrossRef]
- Troia, M.G.; Henriques, G.E.; Mesquita, M.F.; Fragoso, W.S. The effect of surface modifications on titanium to enable titanium–porcelain bonding. Dent. Mater. 2008, 24, 28–33. [Google Scholar] [CrossRef]
- Elsaka, S.E.; Swain, M. Effect of surface treatments on adhesion of low-fusing porcelain to titanium as determined by strain energy release rate. Dent. Mater. 2011, 27, 1213–1220. [Google Scholar] [CrossRef]
- Banaszek, K.; Pietnicki, K.; Klimek, L. The influence of parameters of abrasive jet machining processing on the number of stubble elements stuck in nickel-chrome alloy surface. Mater. Eng. 2011, 32, 312–315. [Google Scholar]
- Craig, R.; Powers, M.; Wataha, J. Dental Materials; Elsevier Urban & Partner Editors: Wroclaw, Poland, 2000; p. 324. [Google Scholar]
- Kula, Z.; Kołodziejczyk, Ł.; Szymanowski, H. Influence of the intermediate layer on the metal-ceramics bond strength. Eng. Biomater. 2019, 152, 21–28. [Google Scholar]
- Pacheco de Castro Henriques, B. Bond Strength Enhancement of Metal-Ceramic Dental Restorations by FGM Design. Ph.D. Thesis, Universidade do Minho, Braga, Portugal, 2012. [Google Scholar]



| Element, [% Mass] | |||||
|---|---|---|---|---|---|
| O | N | C | H | Fe | Ti |
| 0.25 | 0.03 | 0.08 | 0.015 | 0.30 | Balance |
| Lp. | Surface Treatment Type | Surface Treatment Parameters |
|---|---|---|
| Sample Set 1 | ||
| 1. | Al2O3 reference sample | 1. Ultrasonic cleaning (room temp.)—5 min |
| 2. Sandblasting Al2O3—1 min (±5 s) | ||
| 3.Ultrasonic cleaning (room temp.)—5 min | ||
| Sample Set 2 | ||
| 2. | Al2O3/H3PO4 | 1. Ultrasonic cleaning (room temp.)—5 min |
| 2. Sandblasting Al2O3—1 min (±5 s) | ||
| 3. Ultrasonic cleaning (room temp.)—5 min | ||
| 4. Etching 40% H3PO4—1 min | ||
| 5. Ultrasonic cleaning (room temp.)—5 min | ||
| 3. | Al2O3/HCl | 1. Ultrasonic cleaning (room temp.)—5 min |
| 2. Sandblasting Al2O3—1 min (±5 s) | ||
| 3. Ultrasonic cleaning (room temp.)—5 min | ||
| 4. Etching 35% HCl—1 min | ||
| 5.Ultrasonic cleaning (room temp.)—5 min | ||
| Sample Set 3 | ||
| 4. | Al2O3/NaOH + 10% CuSO4 + 5H2O/H3PO4 | 1. Ultrasonic cleaning (room temp.)—5 min |
| 2. Sandblasting Al2O3—1 min (±5 s) | ||
| 3. Ultrasonic cleaning (room temp.)—5 min | ||
| 4. Etching in 50% NaOH + 10% CuSO4 + 5H2O—10 min | ||
| 5. Ultrasonic cleaning (room temp.)—5 min | ||
| 6. Etching in 40% H3PO4 acid—1 min | ||
| 7. Ultrasonic cleaning (room temp.)—5 min | ||
| 5. | Al2O3/NaOH + 10% CuSO4 + 5H2O/HCl | 1. Ultrasonic cleaning (room temp.)—5 min |
| 2. Sandblasting Al2O3—1 min (±5 s) | ||
| 3. Ultrasonic cleaning (room temp.)—5 min | ||
| 4. Etching in 50% NaOH + 10% CuSO4 + 5H2O—10 min | ||
| 5. Ultrasonic cleaning (room temp.)—5 min | ||
| 6. Etching in 35% HCl—1 min | ||
| 7. Ultrasonic cleaning (room temp.)—5 min | ||
| Surface Treatment Method | Fracture Type | Fmax Fracture Force [N] | Standard Deviation SD | Standard Error of the Mean Value Sx | τ—Bending Strength Mean Value [MPa] | Standard Deviation SD | Standard Error of the Mean Value Sx |
|---|---|---|---|---|---|---|---|
| Sample Set 1 | |||||||
| Al2O3 reference sample | Adhesive | 6.54 | 1.22 | 0.55 | 30.08 | 5.79 | 2.50 |
| Sample Set 2 | |||||||
| Al2O3/H3PO4 | Cohesive | 8.15 | 1.26 | 0.56 | 39.16 | 2.06 | 0.92 |
| Al2O3/HCl | Cohesive | 9.17 | 1.11 | 0.50 | 42.17 | 5.10 | 0.28 |
| Sample Set 3 | |||||||
| Al2O3/NaOH + CuSO4 + 5H2O/H3PO4 | Adhesive/Cohesive | 8.92 | 1.17 | 0.52 | 40.31 | 3.76 | 1.68 |
| Al2O3/NaOH + CuSO4 + 5H2O/HCl | Adhesive/Cohesive | 10.59 | 1.39 | 0.62 | 48.77 | 2.88 | 1.29 |
| Source of Variation | Sum of Squares of Deviations | Degrees of Freedom | Variance | Test F |
|---|---|---|---|---|
| Between groups | ||||
| Inside groups | 20 |
| Paired Samples Combination * | Differences between Means | L | ||
|---|---|---|---|---|
| 1—2 | 9.08 | 0.632 | 5.49 | + |
| 1—3 | 12.09 | 0.632 | 5.49 | + |
| 1—4 | 10.23 | 0.632 | 5.49 | + |
| 1—5 | 18.69 | 0.632 | 5.49 | + |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lubas, M.; Jasinski, J.J.; Zawada, A.; Przerada, I. Influence of Sandblasting and Chemical Etching on Titanium 99.2–Dental Porcelain Bond Strength. Materials 2022, 15, 116. https://doi.org/10.3390/ma15010116
Lubas M, Jasinski JJ, Zawada A, Przerada I. Influence of Sandblasting and Chemical Etching on Titanium 99.2–Dental Porcelain Bond Strength. Materials. 2022; 15(1):116. https://doi.org/10.3390/ma15010116
Chicago/Turabian StyleLubas, Malgorzata, Jaroslaw Jan Jasinski, Anna Zawada, and Iwona Przerada. 2022. "Influence of Sandblasting and Chemical Etching on Titanium 99.2–Dental Porcelain Bond Strength" Materials 15, no. 1: 116. https://doi.org/10.3390/ma15010116






