Resistivity Testing of Palladium Dilution Limits in CoPd Alloys for Hydrogen Storage
Abstract
:1. Introduction
2. Experimental
3. Results and Discussion
4. Conclusions
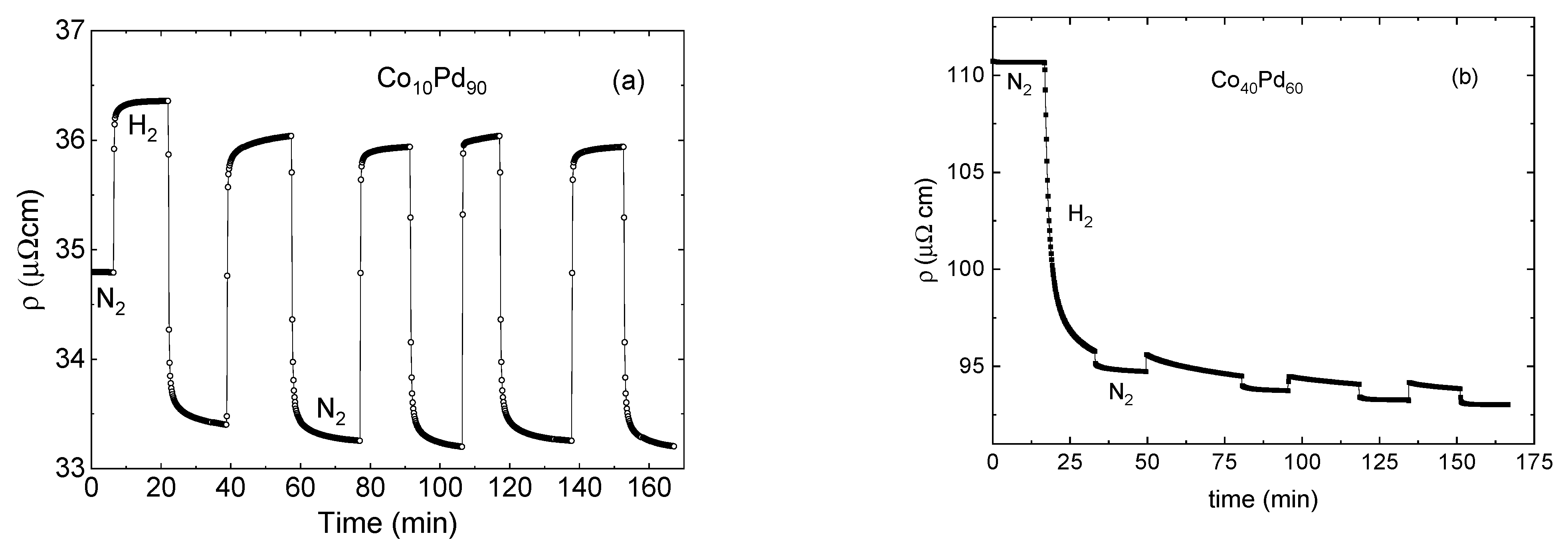
- Thin films (below 15 nm thick) of Pd and CoPd alloys are mechanically stable under repeated hydrogenation and dehydrogenation cycles, which allowed us to perform reliable and reproducible resistivity measurements.
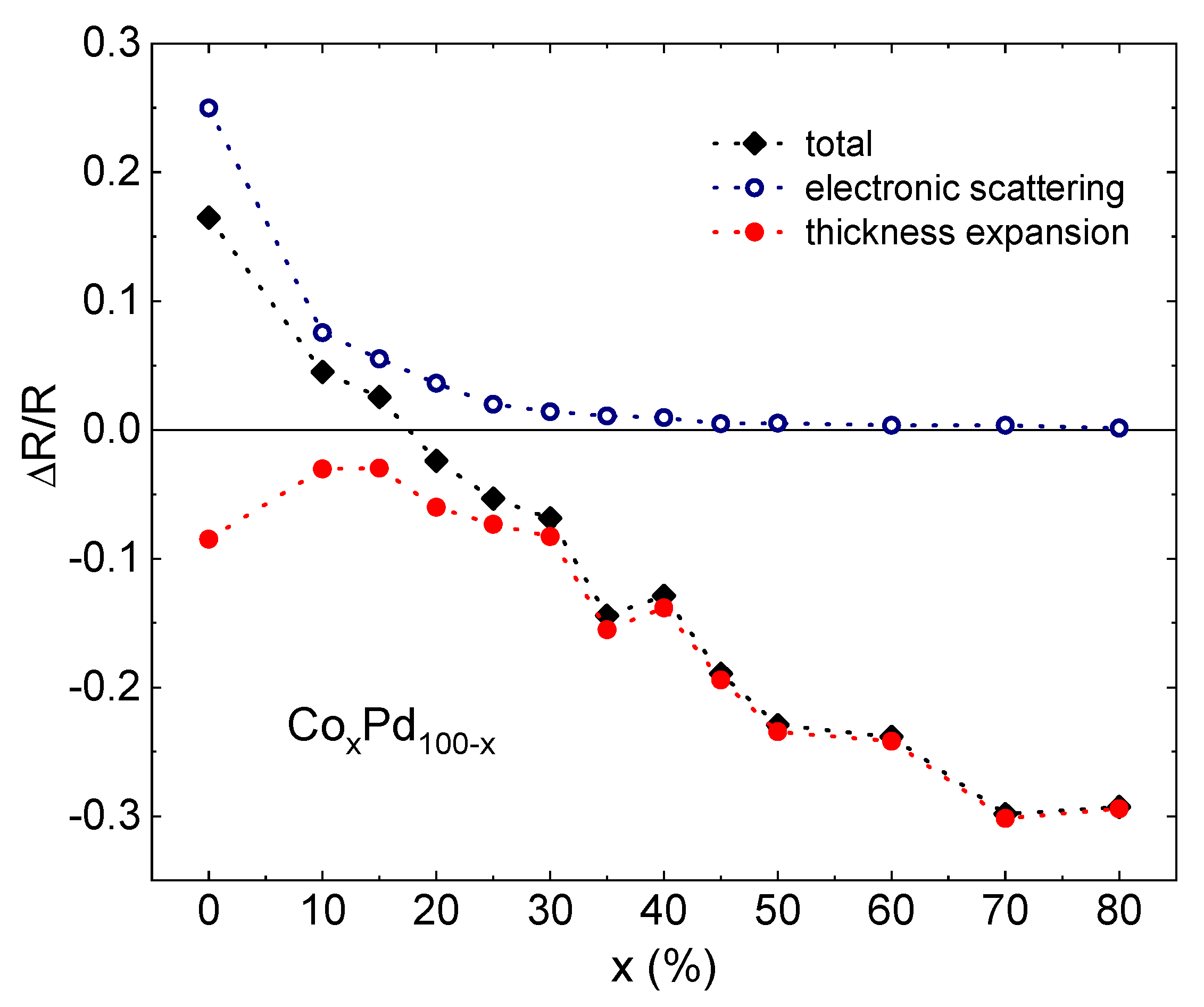
- The change in the resistivity between the hydrogen-free and the hydrogenated states is a superposition of the enhanced hydride scattering and reduction in the resistance due to the thickness expansion.
- The response of low resistivity Pd-rich films to hydrogenation is dominated by the hydride scattering term.
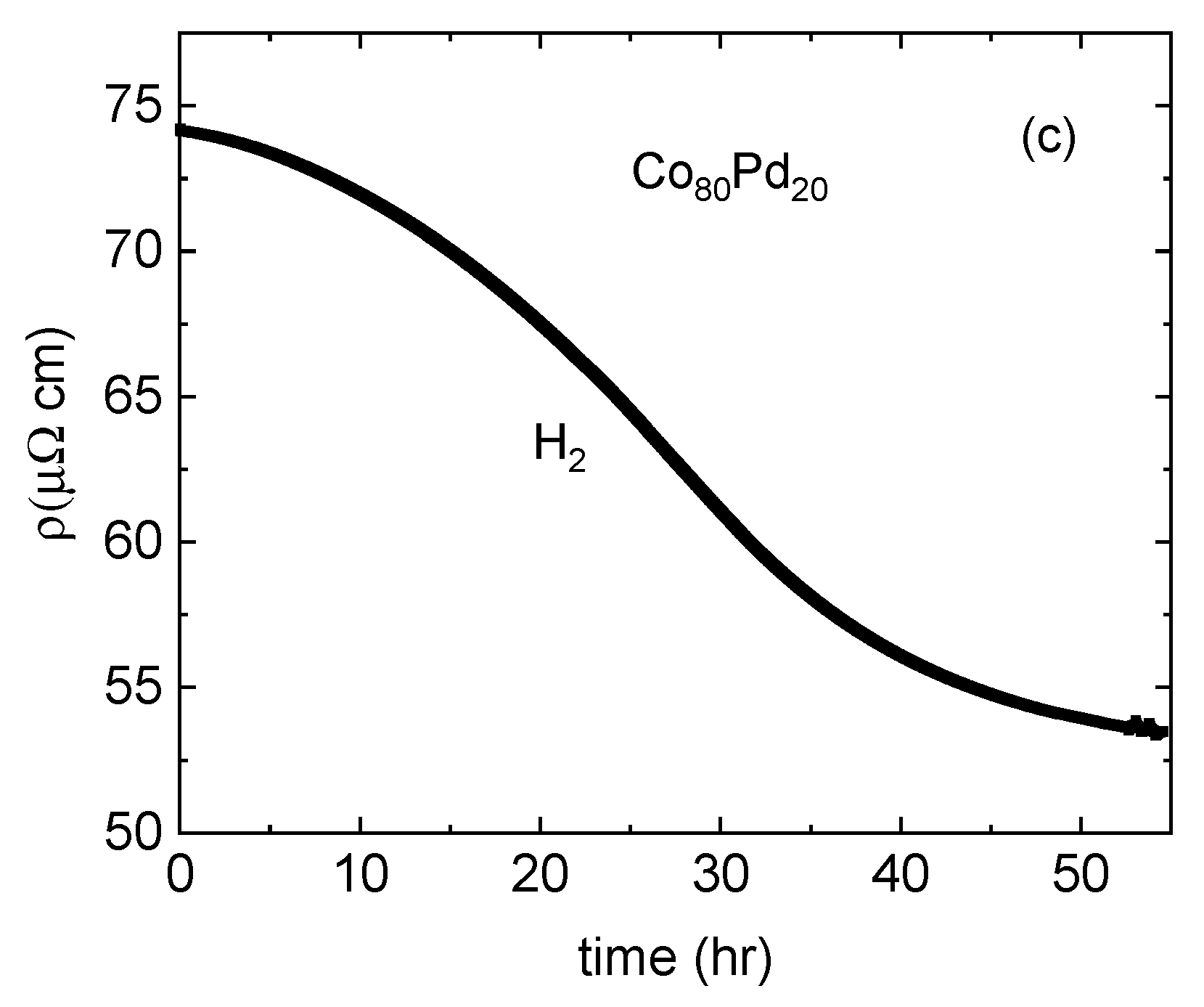
- The reduction in resistivity due to the lattice expansion is dominant in high resistivity Pd-poor alloys.
- Evidence of significant hydrogen absorption was found in CoPd alloys diluted to just 20% Pd.
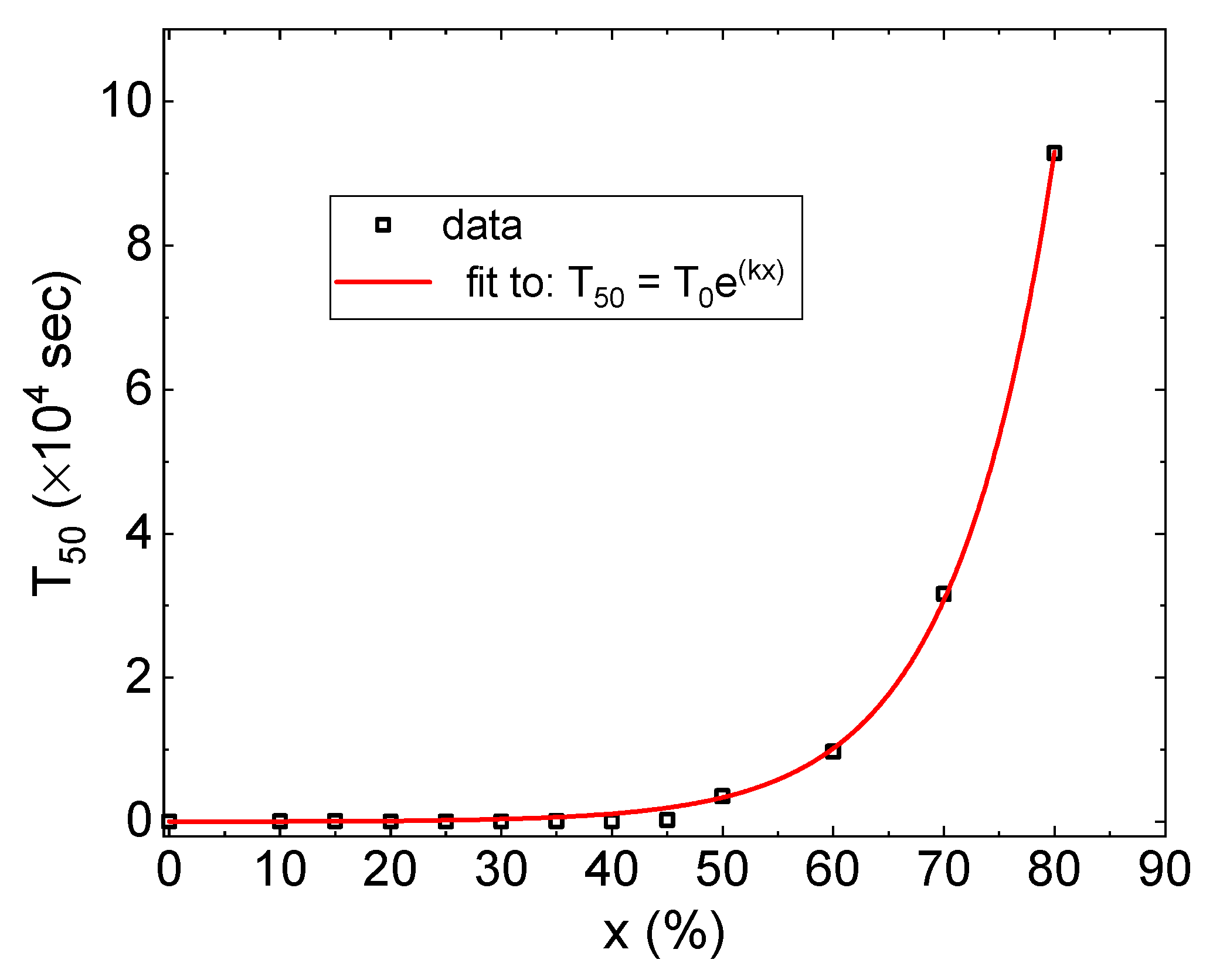
- The time scale of lattice expansion due to hydrogen absorption increases exponentially with the Co concentration.
- Resistivity measurements can be used for the qualitative high-throughput screening of Pd-based alloys over the entire concentration range.
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Brandon, N.P.; Kurban, Z. Clean energy and the hydrogen economy. Phil. Trans. R. Soc. A 2017, 375, 20160400. [Google Scholar] [CrossRef] [PubMed]
- Gielen, D.; Taibi, E.; Miranda, R. Hydrogen: A Renewable Energy Perspective; International Renewable Energy Agency (IRENA): Abu Dhabi, United Arab Emirates, 2019; ISBN 978-92-9260-151-5. [Google Scholar]
- Rönnebro, E.C.E.; Majzoub, E.H. Recent advances in metal hydrides for clean energy applications. MRS Bull. 2013, 38, 452. [Google Scholar] [CrossRef] [Green Version]
- Abe, J.O.; Popoola, A.P.I.; Ajenifuja, E.; Popoola, O.M. Hydrogen energy, economy and storage: Review and recommendation. Int. J. Hydrog. Energy 2019, 44, 15072. [Google Scholar] [CrossRef]
- Hirscher, M.; Yartys, V.A.; Baricco, M.; von Colbe, J.B.; Blanchard, D.; Bowman, R.C., Jr.; Broom, D.P.; Buckley, C.E.; Chang, F.; Chen, P. Materials for hydrogen-based energy storage: Past, recent progress and future outlook. J. Alloy. Compd. 2020, 827, 153548. [Google Scholar] [CrossRef]
- Lai, Q.; Sun, Y.; Wang, T.; Modi, P.; Cazorla, C.; Demirci, U.; Fernandez, J.; Leardini, F.; Aguey-Zinsou, K. How to Design Hydrogen Storage Materials? Fundamentals, Synthesis, and Storage Tanks. Adv. Sustainable Syst. 2019, 3, 1900043. [Google Scholar] [CrossRef]
- Adams, B.D.; Chen, A. The role of palladium in a hydrogen economy. Mater. Today 2011, 14, 282. [Google Scholar] [CrossRef]
- Dekura, S.; Kobayashi, H.; Kusada, K.; Kitagawa, H. Hydrogen in Palladium and Storage Properties of Related Nanomaterials: Size, Shape, Alloying, and Metal-Organic Framework Coating Effects. Chem. Phys. Chem. 2019, 20, 1158. [Google Scholar] [CrossRef] [Green Version]
- Grochala, W.; Edwards, P.P. Thermal decomposition of the non-interstitial hydrides for the storage and production of hydrogen. Chem. Rev. 2004, 104, 1283. [Google Scholar] [CrossRef]
- Sakamoto, Y.; Chen, F.L.; Ura, M.; Flanagan, T.B. Thermodynamic Properties for Solution of Hydrogen in Palladium-Based Binary Alloys. Ber. Bunsenges. Phys. Chem. 1995, 99, 807. [Google Scholar] [CrossRef]
- Griessen, R.; Driessen, A. Heat of formation and band structure of binary and ternary metal hydrides. Phys. Rev. B 1984, 30, 4372. [Google Scholar] [CrossRef] [Green Version]
- Lewis, F.A.; Kandsamy, K.; McNicholl, R.A.; Tong, X.Q. Hydrogen pressure-hydrogen content relationship of palladium and palladium alloy-hydrogen systems. Int. J. Hydrog. Energy 1995, 20, 369. [Google Scholar] [CrossRef]
- Yoshihara, M.; McLellan, R.B. The diffusivity of hydrogen in palladium-based solid solutions. Acta Metall. 1982, 30, 1605. [Google Scholar] [CrossRef]
- Maestas, S.; Flanagan, T.B. Diffusion of hydrogen in gold-palladium alloys. J. Phys. Chem. 1973, 77, 850. [Google Scholar] [CrossRef]
- Züchner, H. Investigation of the diffusion of hydrogen in Pd- and Pd/Ag alloys by means of a current pulse method. Z. Naturforsch. A 1970, 25, 1490. [Google Scholar] [CrossRef] [Green Version]
- McLellan, R.B.; Kirchheim, R. The thermodynamics of palladium-based solid solutions containing a noble-metal substitutional solute and hydrogen. J. Phys. Chem. Solids 1981, 42, 157. [Google Scholar] [CrossRef]
- Barlag, H.; Opara, L.; Züchner, H. Hydrogen diffusion in palladium based f.c.c. alloys. J. Alloy. Compd. 2002, 330, 434. [Google Scholar] [CrossRef]
- Baba, K.; Miyagawa, U.; Watanabe, K.; Sakamoto, Y.; Flanagan, T.B. Electrical resistivity changes due to interstitial hydrogen in palladium-rich substitutional alloys. J. Mater. Sci. 1990, 25, 3910. [Google Scholar] [CrossRef]
- Peters, T.A.; Kaleta, T.; Stange, M.; Bredesen, R. Development of thin binary and ternary Pd-based alloy membranes for use in hydrogen production. J. Membr. Sci. 2011, 383, 124. [Google Scholar] [CrossRef]
- Bosko, M.L.; Fontana, A.D.; Tarditi, A.; Cornaglia, L. Advances in hydrogen selective membranes based on palladium ternary alloys. Int. J. Hydrog. Energy 2021, 46, 15572. [Google Scholar] [CrossRef]
- Green, M.L.; Takeuchi, I.; Hattrick-Simpers, J.R. Applications of high throughput (combinatorial) methodologies to electronic, magnetic, optical, and energy-related materials. J. Appl. Phys. 2013, 113, 231101. [Google Scholar] [CrossRef] [Green Version]
- Baldi, A.; Dam, B. Thin film metal hydrides for hydrogen storage applications. J. Mater. Chem. 2011, 21, 4021. [Google Scholar] [CrossRef]
- Vajo, J.J.; Mertens, F.; Ahn, C.C.; Bowman, R.C.; Fultz, B. Altering Hydrogen Storage Properties by Hydride Destabilization through Alloy Formation: LiH and MgH2 Destabilized with Si. J. Phys. Chem. B 2004, 108, 13977. [Google Scholar] [CrossRef]
- Bogdanovic, B.; Schwickardi, M. Ti-doped alkali metal aluminium hydrides as potential novel reversible hydrogen storage materials. J. Alloys Compd. 1997, 1, 253–254. [Google Scholar] [CrossRef]
- Chen, J.; Kuriyama, N.; Xu, Q.; Takeshita, H.T.; Sakai, T. Reversible Hydrogen Storage via Titanium-Catalyzed LiAlH4 and Li3AlH6. J. Phys. Chem. B 2001, 105, 11214. [Google Scholar] [CrossRef]
- Fukai, Y. The Metal-Hydrogen System: Basic Bulk Properties; Springer Series in Materials Science; Springer: Berlin/Heidelberg, Germany, 2005; Volume 21. [Google Scholar]
- Callori, S.J.; Rehm, C.; Causer, G.L.; Kostylev, M.; Klose, F. Hydrogen Absorption in Metal Thin Films and Heterostructures Investigated in Situ with Neutron and X-ray Scattering. Metals 2016, 6, 125. [Google Scholar] [CrossRef] [Green Version]
- Ludwig, A.; Cao, J.; Savan, A.; Ehmann, M. High-throughput characterization of hydrogen storage materials using thin films on micromachined Si substrates. J. Alloys Compd. 2007, 446, 516. [Google Scholar] [CrossRef]
- Gremaud, R.; Broedersz, C.P.; Borsa, D.M.; Borgschulte, A.; Mauron, P.; Schreuders, H.; Rector, J.H.; Dam, B.; Griessen, R. Hydrogenography: An Optical Combinatorial Method to Find New Light-Weight Hydrogen-Storage Materials. Adv. Mater. 2007, 19, 2813. [Google Scholar] [CrossRef]
- Manchester, F.D. Phase Diagrams of Binary Hydrogen Alloys; ASM International: Materials Park, OH, USA, 2000. [Google Scholar]
- Hübert, T.; Boon-Brett, L.; Black, G.; Banach, U. Hydrogen sensors—A review. Sens. Actuators B Chem. 2011, 157, 329. [Google Scholar] [CrossRef]
- Mirzaei, A.; Yousefi, H.R.; Falsafi, F.; Bonyani, M.; Lee, J.-H.; Kim, J.-H.; Kim, H.W.; Kim, S.S. An overview on how Pd on resistive-based nanomaterial gas sensors can enhance response toward hydrogen gas. Int. J. Hydrog. Energy 2019, 44, 20552. [Google Scholar] [CrossRef]
- Favier, F.; Walter, E.C.; Zach, M.P.; Benter, T.; Penner, R.M. Hydrogen Sensors and Switches from Electrodeposited Palladium Mesowire Arrays. Science 2001, 293, 2227. [Google Scholar] [CrossRef]
- Wagner, S.; Hamm, M.; Pundt, A. Huge hydrogen-induced resistive switching in percolating palladium thin films. Scr. Mater. 2013, 69, 756. [Google Scholar] [CrossRef]
- Zhao, M.; Wong, M.H.; Ong, C.W. Achievement of controlled resistive response of nanogapped palladium film to hydrogen. Appl. Phys. Lett. 2015, 107, 033108. [Google Scholar] [CrossRef]
- Nakamura, N.; Ueno, T.; Ogi, H. Hydrogen-gas sensing at low concentrations using extremely narrow gap palladium nanoclusters prepared by resistive spectroscopy. J. Appl. Phys. 2019, 126, 225104. [Google Scholar] [CrossRef]
- Das, S.S.; Kopnov, G.; Gerber, A. Positive versus negative resistance response to hydrogenation in palladium and its alloys. AIP Adv. 2020, 10, 065129. [Google Scholar] [CrossRef]
- Wagner, S.; Kramer, T.; Uchida, H.; Dobron, P.; Cizek, J.; Pundt, A. Mechanical stress and stress release channels in 10–350 nm palladium hydrogen thin films with different micro-structures. Acta Mater. 2016, 114, 116. [Google Scholar] [CrossRef]
- Kudielka-Artner, E.; Argent, B.B. Magnetic and other Properties of some Binary Palladium Alloys. Proc. Phys. Soc. 1962, 80, 1143. [Google Scholar] [CrossRef]
- Das, S.S.; Kopnov, G.; Gerber, A. Kinetics of the lattice response to hydrogen absorption in thin Pd and CoPd films. Molecules 2020, 25, 3597. [Google Scholar] [CrossRef] [PubMed]
- Ishida, K.; Nishizawa, T. The Co-Pd (Cobalt-Palladium) system. J. Phase Equilib. 1991, 12, 83. [Google Scholar] [CrossRef]
- Akamaru, S.; Kimura, A.; Hara, M.; Nishimura, K.; Abe, T. Hydrogenation effect on magnetic properties of Pd-Co alloys. J. Magn. Magn. Mater. 2019, 484, 8. [Google Scholar] [CrossRef]
- Gerber, A.; Kopnov, G.; Karpovski, M. Hall effect spintronics for gas detection. Appl. Phys. Lett. 2017, 111, 143505. [Google Scholar] [CrossRef] [Green Version]
- Das, S.S.; Kopnov, G.; Gerber, A. Detection of hydrogen by the extraordinary Hall effect in CoPd alloys. J. Appl. Phys. 2018, 124, 104502. [Google Scholar] [CrossRef] [Green Version]
- Harumoto, T.; Nakamura, Y.; Shi, J. Correlation among hydrogenation, magnetoelastic coupling, magnetic anisotropy, and magnetoresistance in magnetostrictive, hydrogen-absorbing palladium-cobalt alloy films for hydrogen sensing. Int. J. Hydrog. Energy 2021, 46, 30204. [Google Scholar] [CrossRef]
- Sonwane, C.G.; Wilcox, J.; Ma, Y.H. Achieving optimum hydrogen permeability in PdAg and PdAu alloys. J. Chem. Phys. 2006, 125, 184714. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Flanagan, T.B.; Wang, D. Hydrogen permeation through fcc Pd-Au alloy membranes. J. Phys. Chem. C 2011, 115, 11618. [Google Scholar] [CrossRef]
- Namba, K.; Ogura, S.; Ohno, S.; Di, W.; Kato, K.; Wilde, M.; Pletikosic, I.; Pervan, P.; Milun, M.; Fukutani, K. Acceleration of hydrogen absorption by palladium through surface alloying with gold. Proc. Natl. Acad. Sci. USA 2018, 115, 7896. [Google Scholar] [CrossRef] [Green Version]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Das, S.S.; Kopnov, G.; Gerber, A. Resistivity Testing of Palladium Dilution Limits in CoPd Alloys for Hydrogen Storage. Materials 2022, 15, 111. https://doi.org/10.3390/ma15010111
Das SS, Kopnov G, Gerber A. Resistivity Testing of Palladium Dilution Limits in CoPd Alloys for Hydrogen Storage. Materials. 2022; 15(1):111. https://doi.org/10.3390/ma15010111
Chicago/Turabian StyleDas, Sudhansu Sekhar, Gregory Kopnov, and Alexander Gerber. 2022. "Resistivity Testing of Palladium Dilution Limits in CoPd Alloys for Hydrogen Storage" Materials 15, no. 1: 111. https://doi.org/10.3390/ma15010111





