Waste-Coffee-Derived Activated Carbon as Efficient Adsorbent for Water Treatment
Abstract
:1. Introduction
2. Materials and Methods
2.1. Activated Carbon Synthesis
2.2. Materials Characterization
2.3. Adsorption Experiments
3. Results and Discussion
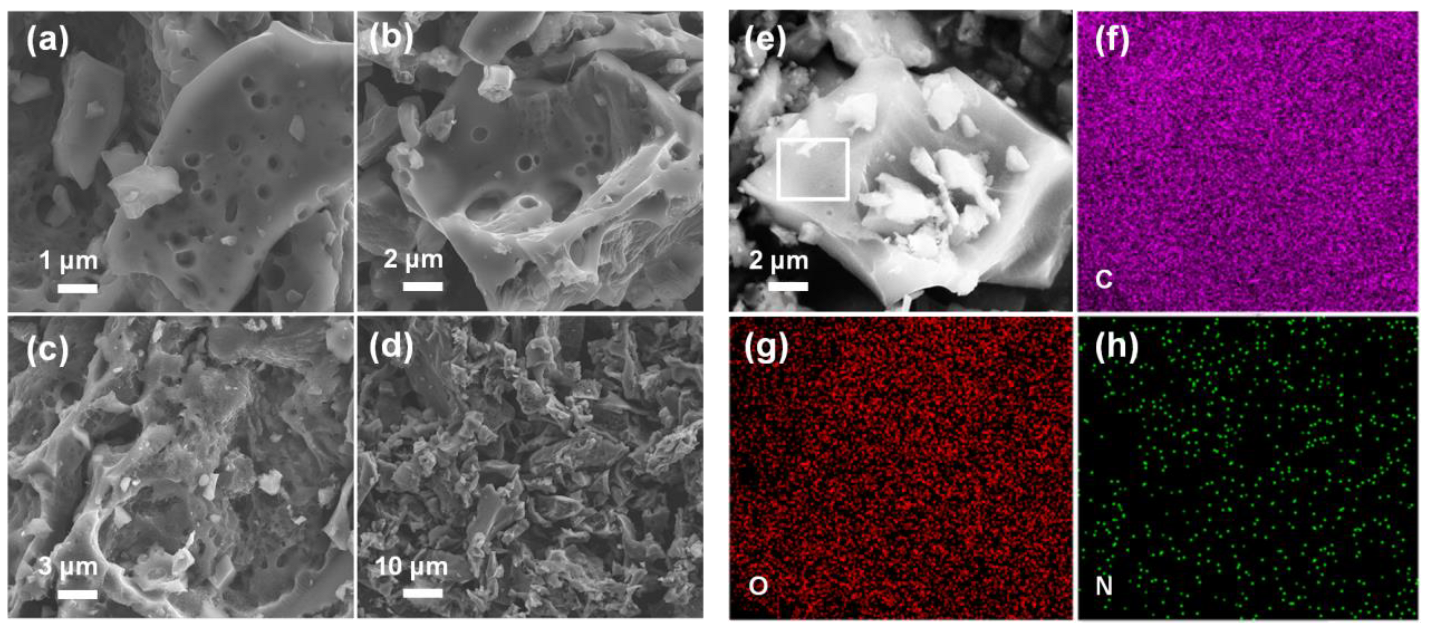
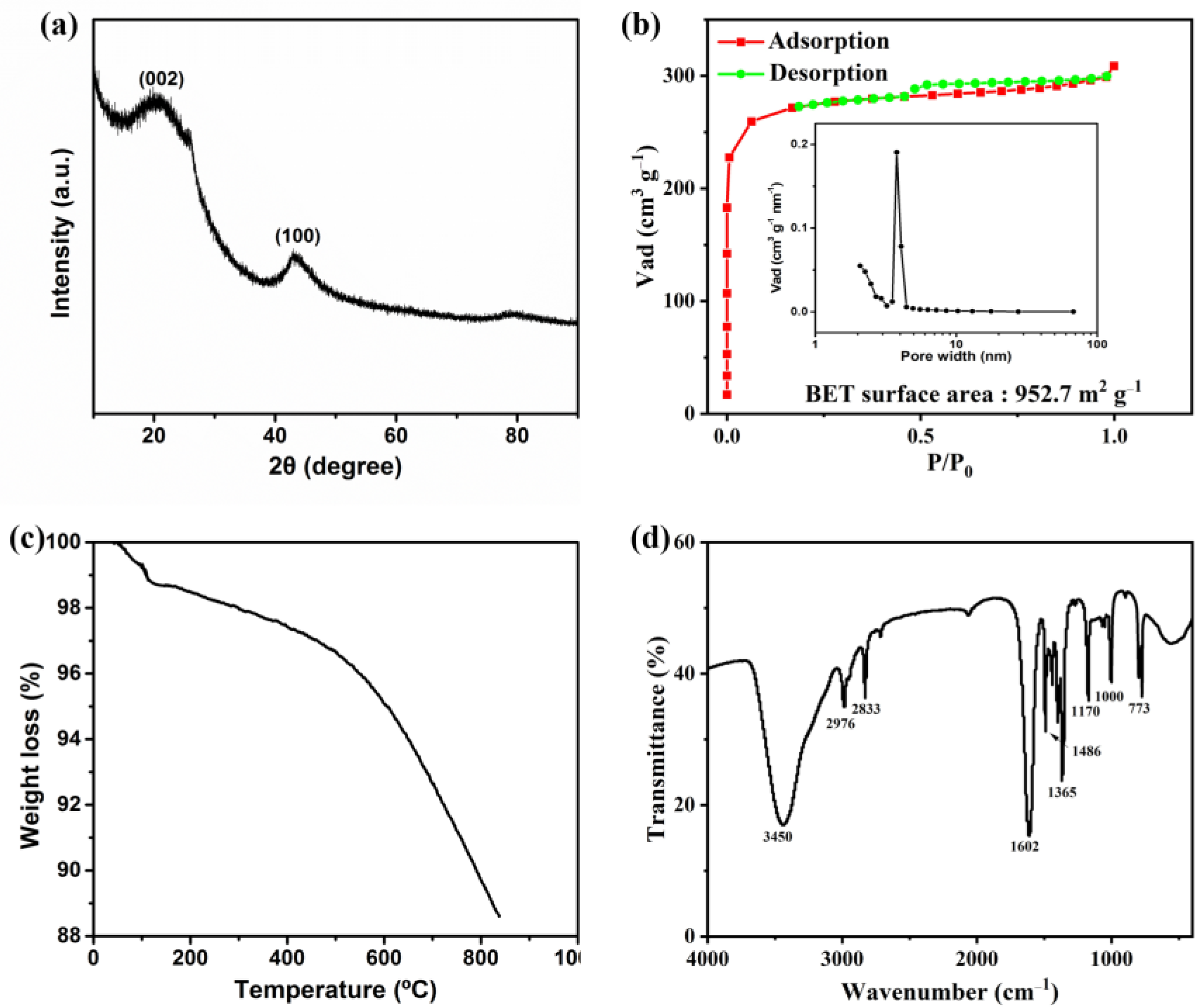
3.1. Physical Properties of The Activated Carbon
3.2. Adsorption Properties Investigation
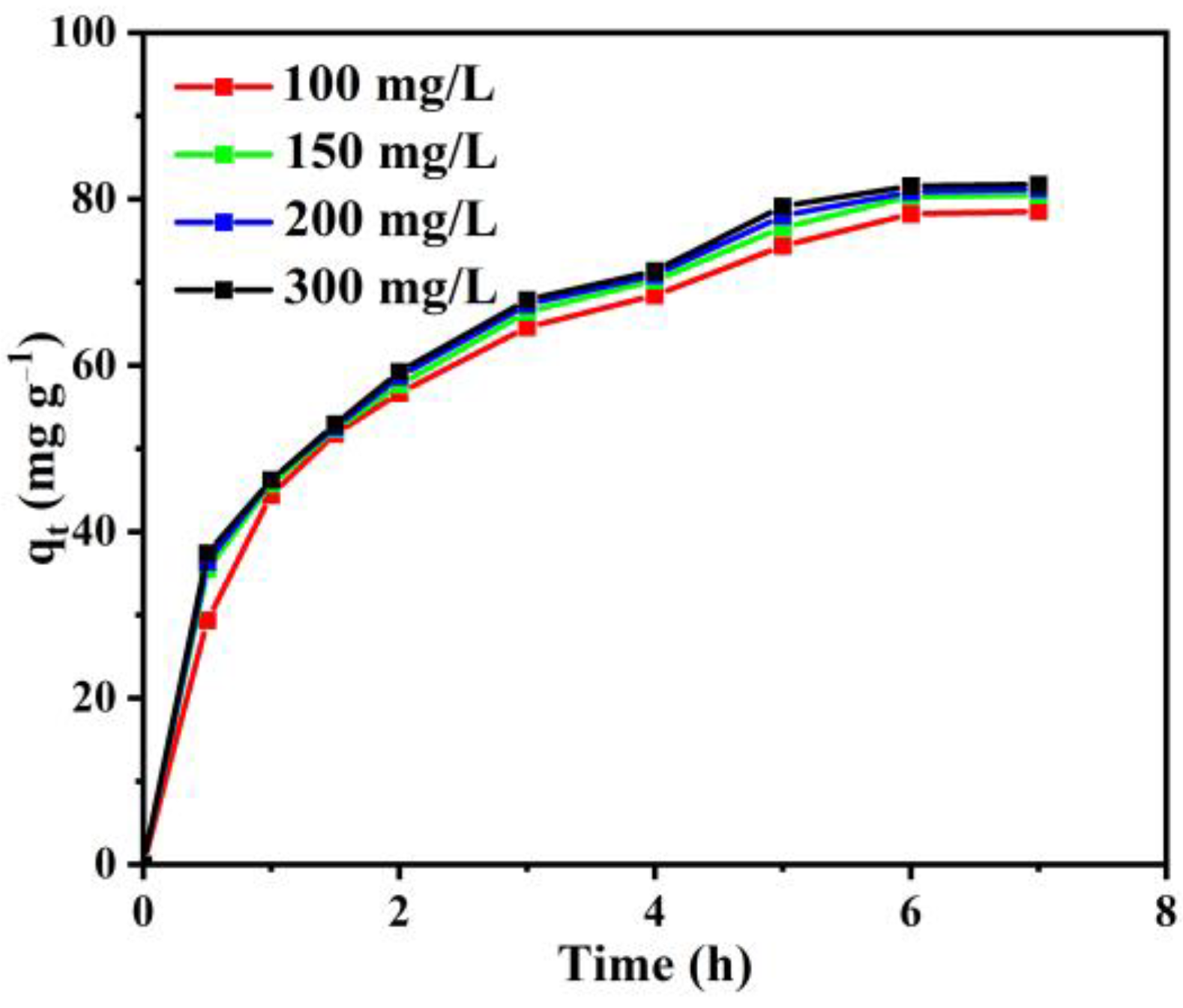
3.2.1. The Effect of Initial RB Concentration
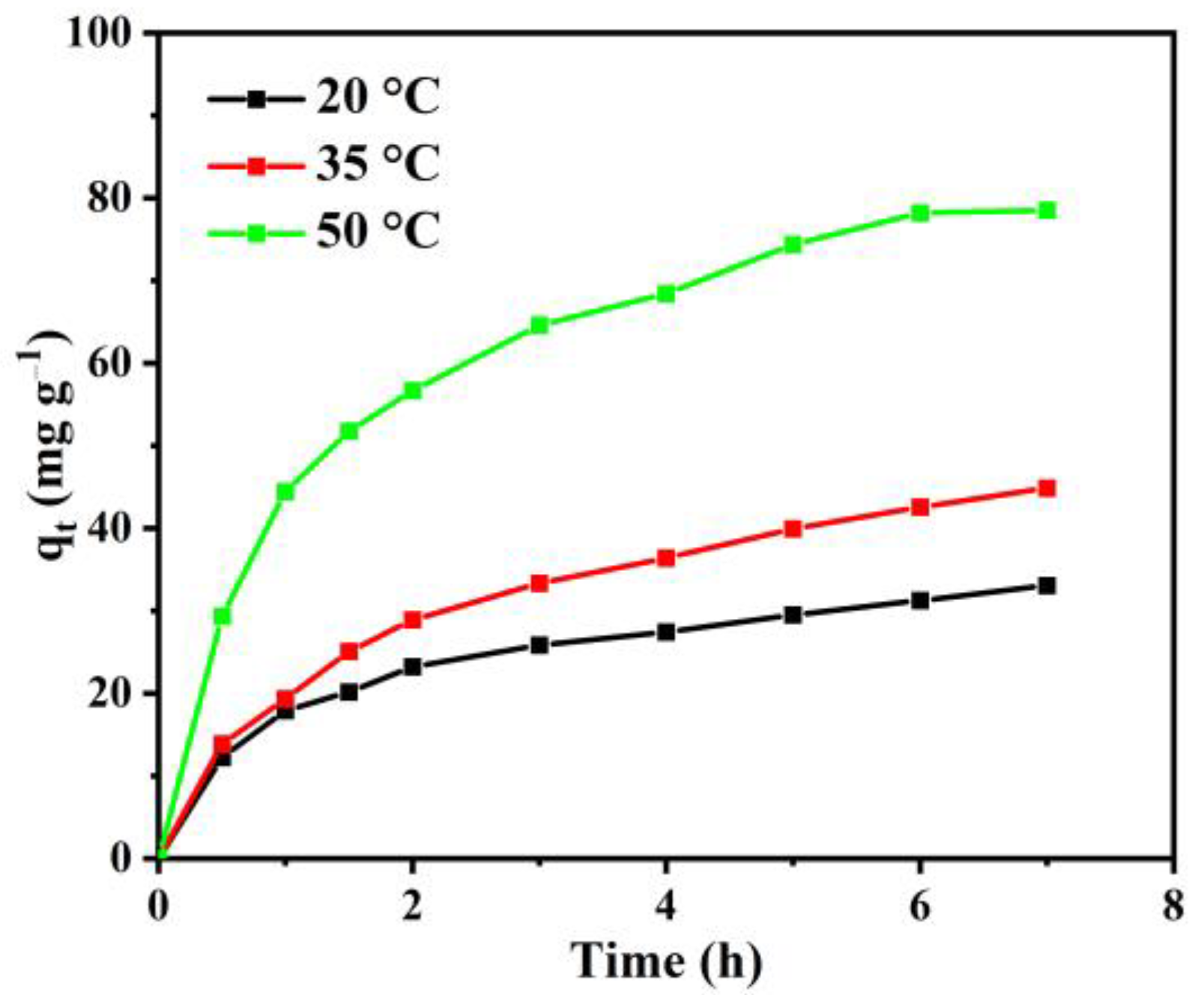
3.2.2. The Effect of Temperature
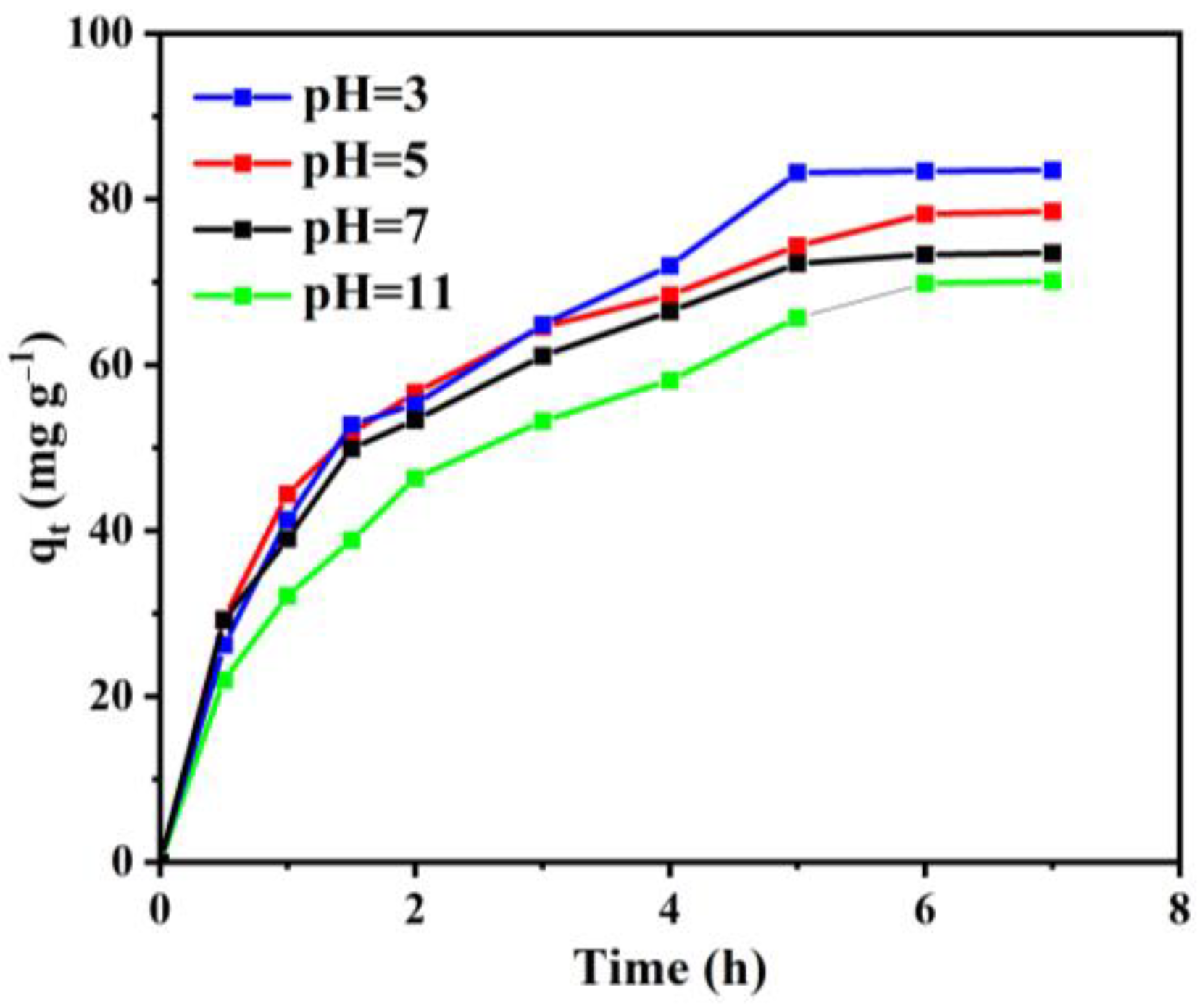
3.2.3. The Effect of Solution PH
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Gagrani, A.; Zhou, J.; Tsuzuki, T. Solvent free mechanochemical synthesis of MnO2 for the efficient degradation of Rhodamine-B. Ceram. Int. 2018, 44, 4694–4698. [Google Scholar] [CrossRef]
- Blanchard, R.; Mekonnen, T.H. Synchronous pyrolysis and activation of poly (ethylene terephthalate) for the generation of activated carbon for dye contaminated wastewater treatment. J. Environ. Chem. Eng. 2022, 10, 108810. [Google Scholar] [CrossRef]
- Baloo, L.; Isa, M.H.; Sapari, N.B.; Jagaba, A.H.; Wei, L.J.; Yavari, S.; Razali, R.; Vasu, R. Adsorptive removal of methylene blue and acid orange 10 dyes from aqueous solutions using oil palm wastes-derived activated carbons. Alex. Eng. J. 2021, 60, 5611–5629. [Google Scholar] [CrossRef]
- Jagaba, A.H.; Kutty, S.R.M.; Baloo, L.; Hayder, G.; Birniwa, A.H.; Taha, A.T.B.; Mnzool, M.; Lawal, M.I. Waste derived biocomposite for simultaneous biosorption of organic matter and nutrients from green straw biorefinery effluent in continuous mode activated sludge systems. Processes 2022, 10, 2262. [Google Scholar] [CrossRef]
- Lee, L.Z.; Zainia, M.A.A. One-step ZnCl2/FeCl3 composites preparation of magnetic activated carbon for effective adsorption of rhodamine B dye. Toxin Rev. 2022, 41, 64–81. [Google Scholar] [CrossRef]
- Sayed, M.; Pal, H. Supramolecularly assisted modulations in chromophoric properties and their possible applications: An overview. J. Mater. Chem. C 2016, 4, 2685–2706. [Google Scholar] [CrossRef]
- Yusaf, A.; Usman, M.; Mansha, A.; Saeed, M.; Ahmad, M.; Siddiq, M. Micellar-enhanced ultrafiltration (MEUF) for removal of rhodamine B (RhB) from aqueous system. J. Dispers. Sci. Technol. 2022, 43, 336–348. [Google Scholar] [CrossRef]
- Alakhras, F.; Ouachtak, H.; Alhajri, E.; Rehman, R.; Al-Mazaideh, G.; Anastopoulos, I.; Lima, E.C. Adsorptive removal of cationic rhodamine B dye from aqueous solutions using chitosan-derived schiff base. Sep. Sci. Technol. 2022, 57, 542–554. [Google Scholar] [CrossRef]
- Steplin, P.S.S.; Ganesh, K.A.; Sarala, L.; Rajaram, R.; Sathiyan, A.; Princy, M.J.; Sharmila, L.I. Photocatalytic degradation of rhodamine B using zinc oxide activated charcoal polyaniline nanocomposite and its survival assessment using aquatic animal model. ACS Sustain. Chem. Eng. 2017, 6, 258–267. [Google Scholar] [CrossRef]
- Bar, N.; Chowdhury, P. A brief review on advances in rhodamine B based chromic materials and their prospects. ACS Appl. Electron. Mater. 2022, 4, 3749–3771. [Google Scholar] [CrossRef]
- Cheng, Y.Y.; Tsai, T.H. Pharmacokinetics and biodistribution of the illegal food colorant rhodamine B in rats. J. Agric. Food Chem. 2017, 65, 1078–1085. [Google Scholar] [CrossRef] [PubMed]
- Peng, M.; Kaczmarek, A.M.; Hecke, K.V. Ratiometric thermometers based on rhodamine B and fluorescein dye-incorporated (nano) cyclodextrin metal–organic frameworks. ACS Appl. Mater. Interfaces 2022, 14, 14367–14379. [Google Scholar] [CrossRef] [PubMed]
- Jakimińska, A.; Pawlicki, M.; Macyk, W. Photocatalytic transformation of rhodamine B to rhodamine-110–The mechanism revisited. J. Photochem. Photobiol. A 2022, 433, 114176. [Google Scholar] [CrossRef]
- Baldisserri, C.; Ortelli, S.; Blosi, M.; Costa, A.L. Pilot-plant study for the photocatalytic/electrochemical degradation of rhodamine B. J. Environ. Chem. Eng. 2018, 6, 1794–1804. [Google Scholar] [CrossRef]
- Yazdani, E.B.; Mehrizad, A. Sonochemical preparation and photocatalytic application of Ag-ZnS-MWCNTs composite for the degradation of rhodamine B under visible light: Experimental design and kinetics modelling. J. Mol. Liq. 2018, 255, 102–112. [Google Scholar] [CrossRef]
- Zhou, X.; Jia, K.; He, X.; Wei, S.; Wang, P.; Liu, X. Microemulsion self-assembling of novel amphiphilic block co-polyarylene ether nitriles and photosensitizer ZnPc towards hybrid superparticles for photocatalytic degradation of rhodamine B. Mater. Chem. Phys. 2018, 207, 212–220. [Google Scholar] [CrossRef]
- Wang, M.; Han, J.; Guo, P.; Sun, M.; Zhang, Y.; Tong, Z.; You, M.; Lv, C. Hydrothermal synthesis of B-doped Bi2MoO6 and its high photocatalytic performance for the degradation of Rhodamine B. J. Phys. Chem. Solids 2018, 113, 86–93. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhou, J.; Cai, W.; Zhou, J.; Li, Z. Enhanced photocatalytic performance and degradation pathway of rhodamine B over hierarchical double-shelled zinc nickel oxide hollow sphere heterojunction. Appl. Surf. Sci. 2018, 430, 549–560. [Google Scholar] [CrossRef]
- Yilmaz, E.; Soylak, M. Use of vegetable oil in supported liquid membrane for the transport of Rhodamine B, A novel and simple deep eutectic solvent based liquid phase microextraction method for rhodamine B in cosmetic products and water samples prior to its spectrophotometric determination. Spectrochim. Acta Part A 2018, 202, 81–86. [Google Scholar]
- Jinisha, R.; Gandhimathi, R.; Ramesh, S.T.; Nidheesh, P.V.; Velmathi, S. Removal of rhodamine B dye from aqueous solution by electro-fenton process using iron-doped mesoporous silica as a heterogeneous catalyst. Chemosphere 2018, 200, 446–454. [Google Scholar] [CrossRef]
- Araújo, D.; Sáez, C.; Martínez-Huitle, C.A.; Cañizares, P.; Rodrigo, M.A. Influence of mediated processes on the removal of rhodamine with conductive-diamond electrochemical oxidation. Appl. Catal. B 2015, 166–167, 454–459. [Google Scholar] [CrossRef]
- Saravanana, S.; Carolin, C.F.; Kumar, P.S.; Chitra, B.; Rangasamy, G. Biodegradation of textile dye rhodamine-B by brevundimonas diminuta and screening of their breakdown metabolites. Chemosphere 2022, 308, 136266. [Google Scholar] [CrossRef] [PubMed]
- Nazir, M.A.; Najam, T.; Bashir, M.S.; Javed, M.S.; Bashir, M.A.; Imran, M.; Azhar, U.; Shah, S.S.A.; ur Rehman, A. Kinetics, isothermal and mechanistic insight into the adsorption of eosin yellow and malachite green from water via tri-metallic layered double hydroxide nanosheets. Korean J. Chem. Eng. 2022, 39, 216–226. [Google Scholar] [CrossRef]
- Nazira, M.A.; Khana, N.A.; Cheng, C.; Shah, S.S.A.; Najamd, T.; Arshadf, M.; Shariff, A.; Akhtare, S.; ur Rehmana, A. Surface induced growth of ZIF-67 at Co-layered double hydroxide: Removal of methylene blue and methyl orange from water. Appl. Clay Sci. 2020, 190, 105564. [Google Scholar] [CrossRef]
- Liu, J.; Liu, X.; Sun, Y.; Sun, C.; Liu, H.; Stevens, L.A.; Li, K.; Snape, C.E. High density and super ultra-microporous-activated carbon macrospheres with high volumetric capacity for CO2 capture. Adv. Sustain. Syst. 2018, 2, 1700115. [Google Scholar] [CrossRef] [Green Version]
- Valkila, J. Do fair trade pricing policies reduce inequalities in coffee production and trade. Dev. Policy Rev. 2014, 32, 475–493. [Google Scholar] [CrossRef]
- Hua, H.J.; Liu, J.Y.; Xu, Z.H.; Zhang, L.Y.; Cheng, B.; Hoc, W. Hierarchical porous Ni/Co-LDH hollow dodecahedron with excellent adsorption property for Congo red and Cr(VI) ions. Appl. Surf. Sci. 2019, 478, 981. [Google Scholar] [CrossRef]
- Guo, Y.; Zhao, J.; Zhang, H.; Yang, S.; Qi, J.; Wang, Z.; Xu, H. Use of rice husk-based porous carbon for adsorption of rhodamine B from aqueous solutions. Dye. Pigm. 2005, 66, 123–128. [Google Scholar] [CrossRef]
- Sattar, M.; Hayeeye, F.; Chinpa, W.; Sirichote, O. Preparation and characterization of poly (lactic acid)/activated carbon composite bead via phase inversion method and its use as adsorbent for rhodamine B in aqueous solution. J Environ. Chem. Eng. 2017, 5, 3780–3791. [Google Scholar] [CrossRef]
- Cheng, Z.-L.; Li, Y.-X.; Liu, Z. Fabrication of graphene oxide/silicalite-1 composites with hierarchical porous structure and investigation on their adsorption performance for rhodamine B. J. Ind. Eng. Chem. 2017, 55, 234–243. [Google Scholar] [CrossRef]
- Guo, Z.; Zhang, J.; Liu, H. Ultra-high rhodamine B adsorption capacities from an aqueous solution by activated carbon derived from Phragmites australis doped with organic acid by phosphoric acid activation. RSC Adv. 2016, 6, 40818–40827. [Google Scholar] [CrossRef]
- Xie, Q.; Bao, R.; Zheng, A.; Zhang, Y.; Wu, S.; Xie, C.; Zhao, P. Sustainable low-cost green electrodes with high volumetric capacitance for aqueous symmetric supercapacitors with high energy density. ACS Sustain. Chem. Eng. 2016, 4, 1422–1430. [Google Scholar] [CrossRef]
- Espinosa, J.C.; Navalon, S.; Alvaro, M.; Dhakshinamoorthy, A.; Garcia, H. Reduction of C=C double bonds by hydrazine using active carbons as metal-free catalysts. ACS Sustain. Chem. Eng. 2018, 6, 5607–5614. [Google Scholar] [CrossRef]
- Preethi, M.; Viswanathan, C.; Ponpandian, N. A metal-free, dual catalyst for the removal of rhodamine B using novel carbon quantum dots from muskmelon peel under sunlight and ultrasonication: A green way to clean the environment. J. Photochem. Photobiol. A 2022, 426, 113765. [Google Scholar] [CrossRef]
- Eftekhari, S.; Habibi-Yangjeh, A.; Sohrabnezhad, S. Application of AlMCM-41 for competitive adsorption of methylene blue and rhodamine B: Thermodynamic and kinetic studies. J. Hazard. Mater. 2010, 178, 349–355. [Google Scholar] [CrossRef]
- Gupta, V.K. Equilibrium and thermodynamic studies on the adsorption of the dye rhodamine-B onto mustard cake and activated carbon. J. Chem. Eng. Data 2015, 55, 5225–5229. [Google Scholar] [CrossRef]
- Yun, Y.S.; Park, M.H.; Hong, S.J.; Lee, M.E.; Park, Y.W.; Jin, H.J. Najafpour removal of rhodamine B from aqueous solution using palm shell-based activated carbon: Adsorption and kinetic studies. J. Chem. Eng. Data 2010, 55, 5777–5785. [Google Scholar]
- Rodriguez, A.; Garcia, J.; Ovejero, G.; Mestanza, M. Adsorption of anionic and cationic dyes on activated carbon from aqueous solutions: Equilibrium and kinetics. J. Hazard. Mater. 2009, 172, 1311–1320. [Google Scholar] [CrossRef]
- Nazir, M.A.; Najam, T.; Shahzad, K.; Wattoo, M.A.; Hussain, T.; Tufail, M.K.; Shah, S.S.A.; ur Rehman, A. Heterointerface engineering of water stable ZIF-8@ZIF-67: Adsorption of rhodamine B from water. Surf. Interfaces 2022, 34, 102324. [Google Scholar] [CrossRef]
- Saleh, T.A.; Ali, I. Synthesis of polyamide grafted carbon microspheres for removal of rhodamine B dye and heavy metals. J. Environ. Chem. Eng. 2018, 6, 5361–5368. [Google Scholar] [CrossRef]
- Shi, W.N.; Fang, W.-X.; Wang, J.-C.; Qiao, X.; Wang, B.B.; Guo, X.W. pH-controlled mechanism of photocatalytic RhB degradation over g-C3N4 under sunlight irradiation. Photochem. Photobiol. Sci. 2021, 20, 303–313. [Google Scholar] [CrossRef] [PubMed]

| Sample | Isotherm Models | |||||
|---|---|---|---|---|---|---|
| Langmuir | Freundlich | |||||
| Qm (mg g−1) | KL (L mg−1) | R2 | KF ((mg g−1) (L/mg)1/n) | 1 /n | R2 | |
| Activated Carbon | 83.3 | 0.86 | 0.999 | 74.3 | 0.018 | 0.975 |
| Kinetic Models | Pseudo-First Order | Pseudo-Second Order | |||||
|---|---|---|---|---|---|---|---|
| Parameters | Qe,exp (mg g−1) | Qe,cal (mg g−1) | K1 (min−1) | R2 | Qe,cal (mg g−1) | K2 (mg min−1) | R2 |
| 50°C | 80.5 | 60.3 | 0.0083 | 0.974 | 88.0 | 1.7 × 10−4 | 0.997 |
| 35°C | 44.9 | 36.6 | 0.0065 | 0.998 | 51.0 | 2.2 × 10−4 | 0.994 |
| 20°C | 33.1 | 22.2 | 0.0061 | 0.984 | 34.5 | 4.9 × 10−4 | 0.998 |
| pH = 5 | 70.1 | 64.1 | 0.0081 | 0.956 | 81.2 | 1.3 × 10−4 | 0.990 |
| pH = 7 | 83.5 | 66.7 | 0.0073 | 0.984 | 94.6 | 9.8 × 10−5 | 0.991 |
| pH = 11 | 73.5 | 54.6 | 0.0086 | 0.993 | 82.6 | 1.7 × 10−4 | 0.994 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, H.-M.; Lau, W.-M.; Zhou, D. Waste-Coffee-Derived Activated Carbon as Efficient Adsorbent for Water Treatment. Materials 2022, 15, 8684. https://doi.org/10.3390/ma15238684
Chen H-M, Lau W-M, Zhou D. Waste-Coffee-Derived Activated Carbon as Efficient Adsorbent for Water Treatment. Materials. 2022; 15(23):8684. https://doi.org/10.3390/ma15238684
Chicago/Turabian StyleChen, Hong-Ming, Woon-Ming Lau, and Dan Zhou. 2022. "Waste-Coffee-Derived Activated Carbon as Efficient Adsorbent for Water Treatment" Materials 15, no. 23: 8684. https://doi.org/10.3390/ma15238684





