Synthesis of Carbon Microspheres from Inedible Crystallized Date Palm Molasses: Influence of Temperature and Reaction Time
Abstract
:1. Introduction
2. Materials and Methods
2.1. Raw Material and Chemicals
2.2. Hydrothermal Carbonization
2.3. Characterizations
2.4. Adsorption Study
3. Results and Discussions
3.1. Product Yield
3.2. CMs Formation
4. Conclusions
Author Contributions
Funding
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Hu, B.; Wang, K.; Wu, L.; Yu, S.H.; Antonietti, M.; Titirici, M.M. Engineering carbon materials from the hydrothermal carbonization process of biomass. Adv. Mater. 2010, 22, 813–828. [Google Scholar] [CrossRef] [PubMed]
- Abbas, A.; Mariana, L.T.; Phan, A.N. Biomass-waste derived graphene quantum dots and their applications. Carbon 2018, 140, 77–99. [Google Scholar] [CrossRef] [Green Version]
- Titirici, M.M.; Antonietti, M.; Baccile, N. Hydrothermal carbon from biomass: A comparison of the local structure from poly-to monosaccharides and pentoses/hexoses. Green Chem. 2008, 10, 1204–1212. [Google Scholar] [CrossRef] [Green Version]
- Sevilla, M.; Fuertes, A.B. The production of carbon materials by hydrothermal carbonization of cellulose. Carbon 2009, 47, 2281–2289. [Google Scholar] [CrossRef] [Green Version]
- Cai, H.; Lin, X.; Qin, Y.; Luo, X. Hydrothermal synthesis of carbon microsphere from glucose at low temperature and its adsorption property of uranium (VI). J. Radioanal. Nucl. Chem. 2017, 311, 695–706. [Google Scholar] [CrossRef]
- Qi, X.; Liu, N.; Lian, Y. Carbonaceous microspheres prepared by hydrothermal carbonization of glucose for direct use in catalytic dehydration of fructose. RSC Adv. 2015, 5, 17526–17531. [Google Scholar] [CrossRef]
- Demir-Cakan, R.; Baccile, N.; Antonietti, M.; Titirici, M.-M. Carboxylate-rich carbonaceous materials via one-step hydrothermal carbonization of glucose in the presence of acrylic acid. Chem. Mater. 2009, 21, 484–490. [Google Scholar] [CrossRef]
- Li, M.; Li, W.; Liu, S. Hydrothermal synthesis, characterization, and KOH activation of carbon spheres from glucose. Carbohydr. Res. 2011, 8, 999–1004. [Google Scholar] [CrossRef]
- Sanchez-Sanchez, A.; Braghiroli, F.L.; Izquierdo, M.T.; Parmentier, J.; Celzard, A.; Fierro, V. Synthesis and properties of carbon microspheres based on tannin–sucrose mixtures treated in hydrothermal conditions. Ind. Crops Prod. 2020, 154, 112564. [Google Scholar] [CrossRef]
- Sulistya, E.; Hui-Hui, L.; Attenborough, N.K.; Pourshahrestani, S.; Kadri, N.A.; Zeimaran, E.; Razak, N.A.B.A.; Amini Horri, B.; Salamatinia, B. Hydrothermal synthesis of carbon microspheres from sucrose with citric acid as a catalyst: Physicochemical and structural properties. J. Taibah Univ. Sci. 2020, 14, 1042–1050. [Google Scholar] [CrossRef]
- Bedin, K.C.; Cazetta, A.L.; Souza, I.P.; Pezoti, O.; Souza, L.S.; Souza, P.S.; Yokoyama, J.T.; Almeida, V.C. Porosity enhancement of spherical activated carbon: Influence and optimization of hydrothermal synthesis conditions using response surface methodology. J. Environ. Chem. Eng. 2018, 6, 991–999. [Google Scholar] [CrossRef]
- Zhao, H.; Lu, X.; Wang, Y.; Sun, B.; Wu, X.; Lu, H. Effects of additives on sucrose-derived activated carbon microspheres synthesized by hydrothermal carbonization. J. Mater. Sci. 2017, 52, 10787–10799. [Google Scholar] [CrossRef]
- Shi, J.; Tian, X.D.; Li, X.; Liu, Y.Q.; Sun, H.Z. Micro/mesopore carbon spheres derived from sucrose for use in high performance supercapacitors. New Carbon Mater. 2021, 36, 1149–1155. [Google Scholar] [CrossRef]
- Zhang, M.; Yang, H.; Liu, Y.; Sun, X.; Zhang, D.; Xue, D. Hydrophobic precipitation of carbonaceous spheres from fructose by a hydrothermal process. Carbon 2012, 50, 2155–2161. [Google Scholar] [CrossRef]
- Jung, D.; Duman, G.; Zimmermann, M.; Kruse, A.; Yanik, J. Hydrothermal carbonization of fructose—Effect of salts and reactor stirring on the growth and formation of carbon spheres. Biomass Conv Bioref. 2021. [Google Scholar] [CrossRef]
- Ryu, J.; Suh, Y.W.; Suh, D.J.; Ahn, D.J. Hydrothermal preparation of carbon microspheres from mono-saccharides and phenolic compounds. Carbon 2010, 48, 1990–1998. [Google Scholar] [CrossRef]
- Sun, X.; Li, Y. Colloidal carbon spheres and their core/shell structures with noble-metal nanoparticles. Angew. Chem. Int. Ed. Engl. 2004, 116, 607–611. [Google Scholar] [CrossRef]
- O’sullivan, A.C. Cellulose: The structure slowly unravels. Cellulose 1997, 4, 173–207. [Google Scholar] [CrossRef]
- Falco, C.; Baccile, N.; Titirici, M.M. Morphological and structural differences between glucose, cellulose and lignocellulosic biomass derived hydrothermal carbons. Green Chem. 2011, 13, 3273–3281. [Google Scholar] [CrossRef] [Green Version]
- Wang, T.; Zhai, Y.; Zhu, Y.; Li, C.; Zeng, G. A review of the hydrothermal carbonization of biomass waste for hydrochar formation: Process conditions, fundamentals, and physicochemical properties. Renew. Sustain. Energy Rev. 2018, 90, 223–247. [Google Scholar] [CrossRef]
- Taghizadeh-Alisaraei, A.; Motevali, A.; Ghobadian, B. Ethanol production from date wastes: Adapted technologies, challenges, and global potential. Renew. Energy 2019, 143, 1094–1110. [Google Scholar] [CrossRef]
- El-Sharnouby, G.A.; Aleid, S.M.; Al-Otaibi, M.M. Liquid sugar extraction from date palm (Phoenix dactylifera L.) fruits. Int. J. Food Process. Technol. 2014, 5, 1–5. [Google Scholar]
- Siddeeg, A.; Zeng, X.A.; Ammar, A.F.; Han, Z. Sugar profile, volatile compounds, composition and antioxidant activity of Sukkari date palm fruit. J. Food Sci. Technol. 2019, 56, 754–762. [Google Scholar] [CrossRef] [PubMed]
- Al-Awadi, A.S.; El-Harbawi, M.; Algarawi, A.; Alalawi, A.; Alrashed, M.M.; Yin, C.Y. Synthesis of carbon microspheres via hydrothermal carbonization of Sabal palms (Sabal palmetto) biomass for adsorption of methylene blue. Biomass Conv. Bioref. 2022. [Google Scholar] [CrossRef]
- Duy Nguyen, H.; Nguyen Tran, H.; Chao, H.P.; Lin, C.C. Activated carbons derived from teak sawdust-hydrochars for efficient removal of methylene blue, copper, and cadmium from aqueous solution. Water 2019, 11, 2581. [Google Scholar] [CrossRef] [Green Version]
- Yaah, V.B.; Zbair, M.; Botelho de Oliveira, S.; Ojala, S. Hydrochar-derived adsorbent for the removal of diclofenac from aqueous solution. Nanotechnol. Environ. Eng. 2021, 6, 3. [Google Scholar] [CrossRef]
- Yao, F.; Wu, Q.; Lei, Y.; Guo, W.; Xu, Y. Thermal decomposition kinetics of natural fibers: Activation energy with dynamic thermogravimetric analysis. Polym. Degrad. Stab. 2008, 93, 90–98. [Google Scholar] [CrossRef]
- Li, M.; Li, W.; Liu, S. Control of the morphology and chemical properties of carbon spheres prepared from glucose by a hydrothermal method. J. Mater. Res. 2012, 27, 1117–1123. [Google Scholar] [CrossRef]
- Hoekman, S.K.; Broch, A.; Robbins, C. Hydrothermal carbonization (HTC) of lignocellulosic biomass. Energy Fuels 2011, 25, 1802–1810. [Google Scholar] [CrossRef]
- Lucian, M.; Volpe, M.; Gao, L.; Piro, G.; Goldfarb, J.L.; Fiori, L. Impact of hydrothermal carbonization conditions on the formation of hydrochars and secondary chars from the organic fraction of municipal solid waste. Fuel 2018, 233, 257–268. [Google Scholar] [CrossRef]
- Basso, D.; Weiss-hortala, E.; Patuzzi, F.; Castello, D.; Baratieri, M.; Fiori, L. Hydrothermal carbonization of off-specification compost: A byproduct of the organic municipal solid waste treatment. Bioresour. Technol. 2015, 182, 217–224. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kalderis, D.; Kotti, M.S.; Méndez, A.; Gascó, G. Characterization of hydrochars produced by hydrothermal carbonization of rice husk. Solid Earth 2014, 5, 477–483. [Google Scholar] [CrossRef] [Green Version]
- Lin, Y.; Xu, H.; Gao, Y.; Zhang, X. Preparation and characterization of hydrochar-derived activated carbon from glucose by hydrothermal carbonization. Biomass Conv. Bioref. 2021. [Google Scholar] [CrossRef]
- Sevilla, M.; Fuertes, A.B. Chemical and structural properties of carbonaceous products obtained by hydrothermal carbonization of saccharides. Chem. Eur. J. 2009, 15, 4195–4203. [Google Scholar] [CrossRef]
- Elaigwu, S.E.; Greenway, G.M. Chemical, structural and energy properties of hydrochars from microwave-assisted hydrothermal carbonization of glucose. Int. J. Ind. Chem. 2016, 7, 449–456. [Google Scholar] [CrossRef] [Green Version]
- Lua, A.C.; Yang, T. Effect of activation temperature on the textural and chemical properties of potassium hydroxide activated carbon prepared from pistachio-nut shell. J. Colloid Interface Sci. 2004, 274, 594–601. [Google Scholar] [CrossRef]
- Duy Nguyen, H.; Tran, H.N.; Chao, H.-P.; Lin, C.-C. Effect of nitric acid oxidation on the surface of hydrochars to sorb methylene blue: An adsorption mechanism comparison. Adsorp. Sci. Technol. 2019, 37, 607–622. [Google Scholar] [CrossRef] [Green Version]
- Naderi, M.; Vesali-Naseh, M. Hydrochar-derived fuels from waste walnut shell through hydrothermal carbonization: Characterization and effect of processing parameters. Biomass Convers. Biorefin. 2021, 11, 1443–1451. [Google Scholar] [CrossRef]
- Ahmed Khan, T.; Kim, H.J.; Gupta, A.; Jamari, S.S.; Jose, R. Synthesis and characterization of carbon microspheres from rubber wood by hydrothermal carbonization. J. Chem. Technol. Biotechnol. 2019, 94, 1374–1383. [Google Scholar] [CrossRef]
- Zhang, Y.F.; Dai, J.M.; Guo, H.; Shi, S.; Yan, Z.F.; Hou, W.S. A comparative study of carbon microsphere preparation by the hydrothermal carbonization of waste cotton fibers, viscose fibers and Avicel. New Carbon Mater. 2020, 35, 286–294. [Google Scholar] [CrossRef]
- Lv, B.W.; Xu, H.; Guo, J.Z.; Bai, L.Q.; Li, B. Efficient adsorption of methylene blue on carboxylate-rich hydrochar prepared by one-step hydrothermal carbonization of bamboo and acrylic acid with ammonium persulphate. J. Hazard. Mater. 2022, 421, 126741. [Google Scholar] [CrossRef] [PubMed]
- Ferrentino, R.; Ceccato, R.; Marchetti, V.; Andreottola, G.; Fiori, L. Sewage sludge hydrochar: An option for removal of methylene blue from wastewater. Appl. Sci. 2020, 10, 3445. [Google Scholar] [CrossRef]
- Ronix, A.; Pezoti, O.; Souza, L.S.; Souza, I.P.; Bedin, K.C.; Souza, P.S.; Silva, T.L.; Melo, S.A.; Cazetta, A.L.; Almeida, V.C. Hydrothermal carbonization of coffee husk: Optimization of experimental parameters and adsorption of methylene blue dye. J. Environ. Chem. Eng. 2017, 5, 4841–4849. [Google Scholar] [CrossRef]
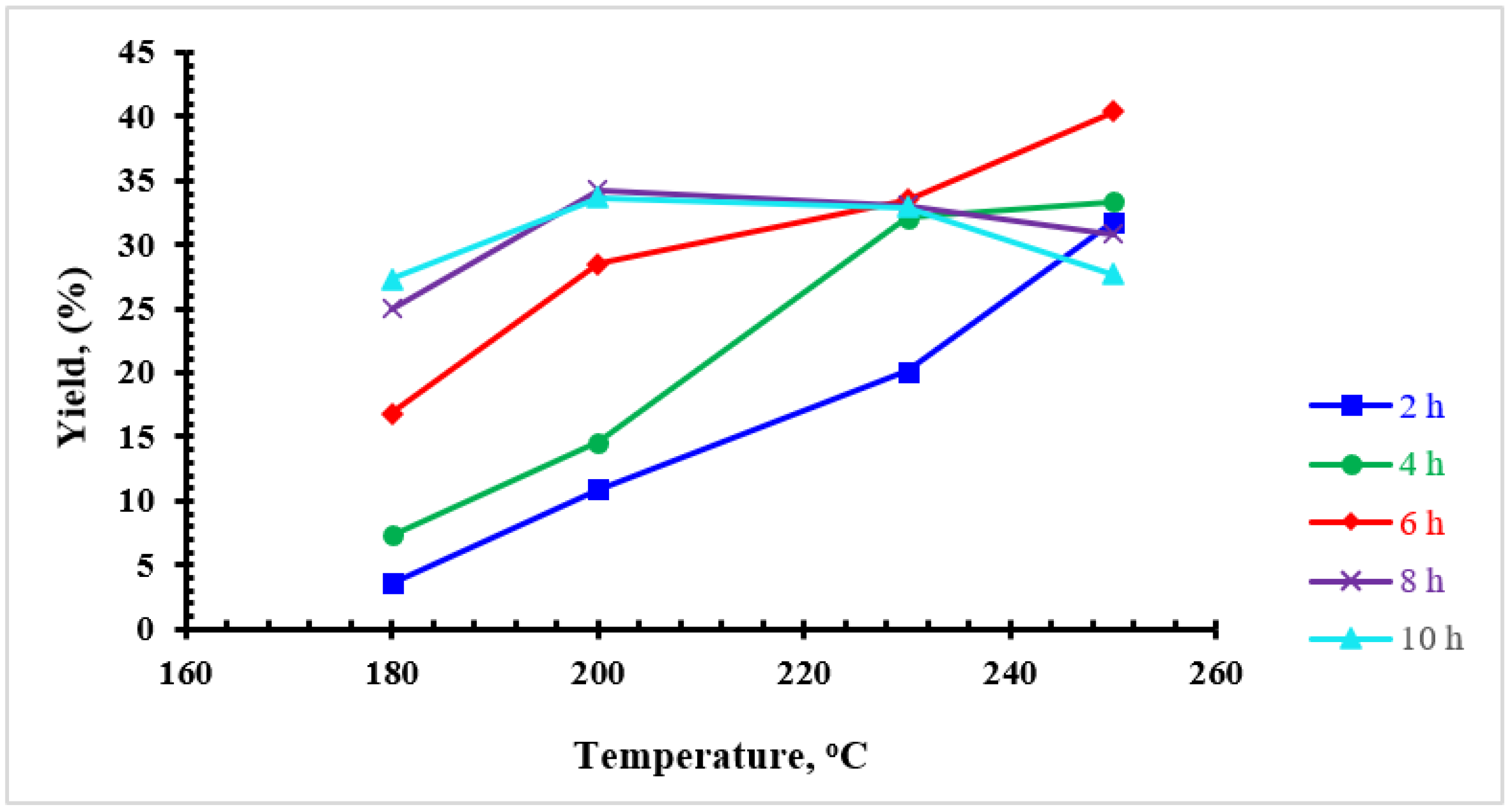
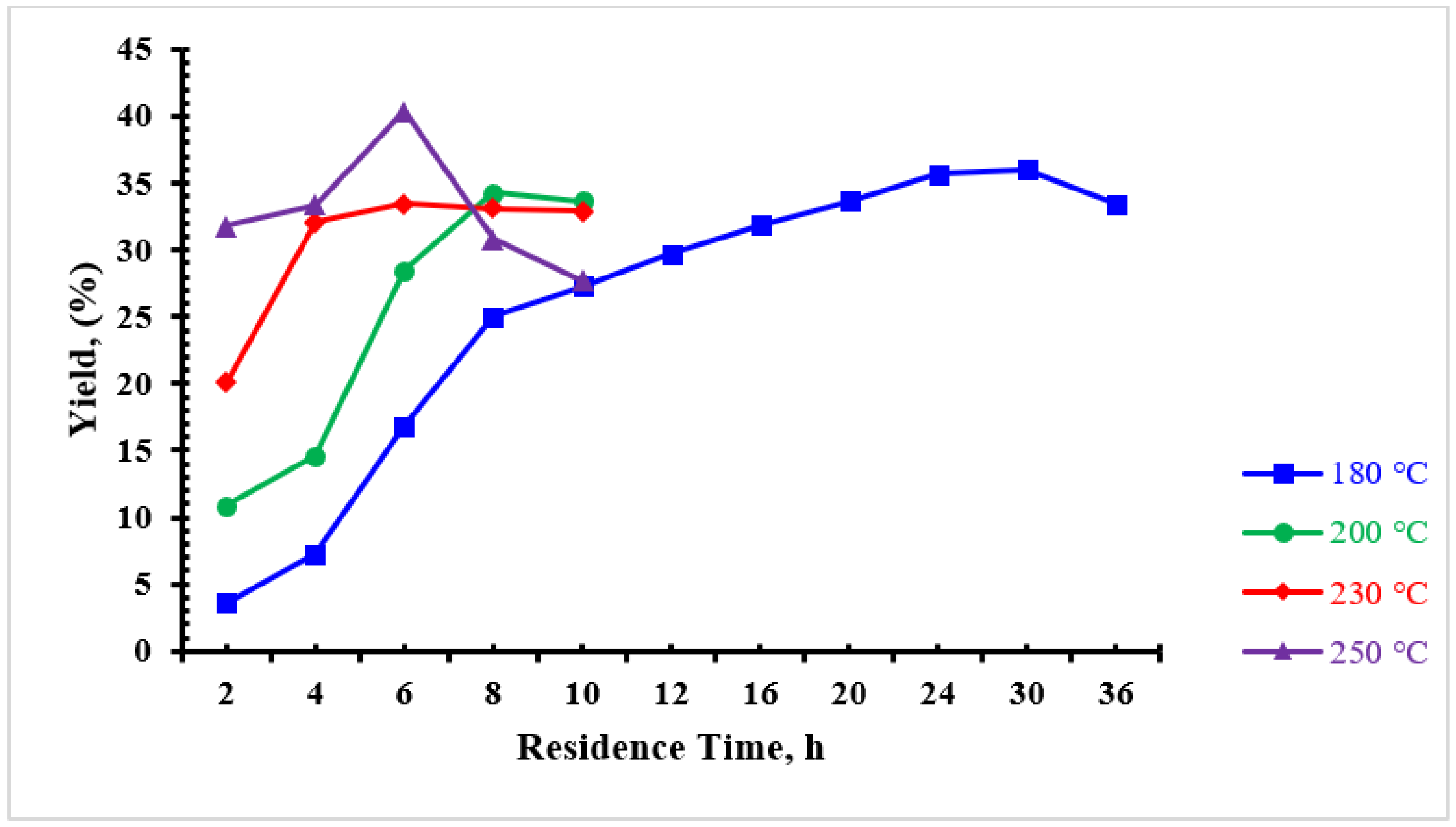


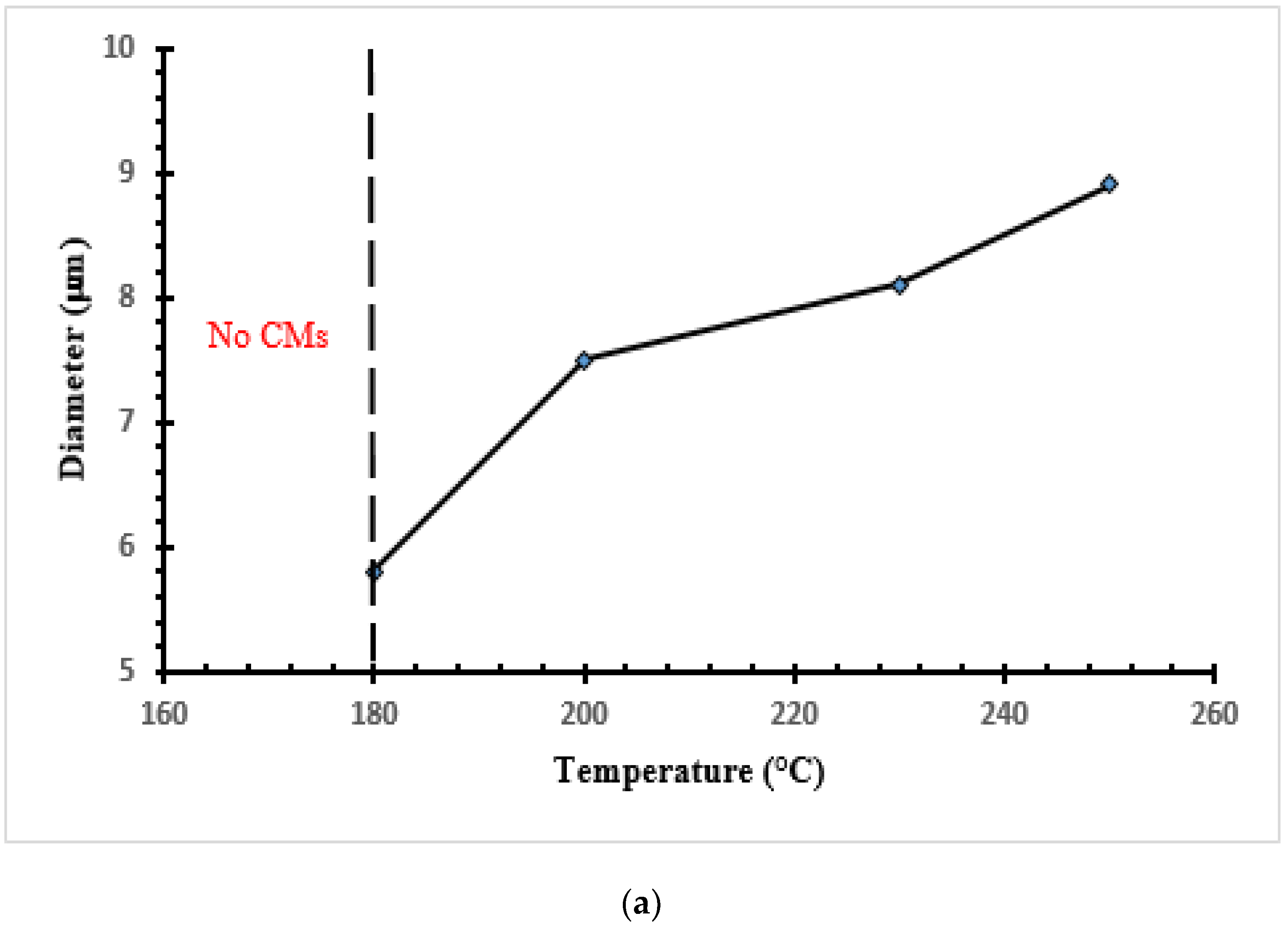
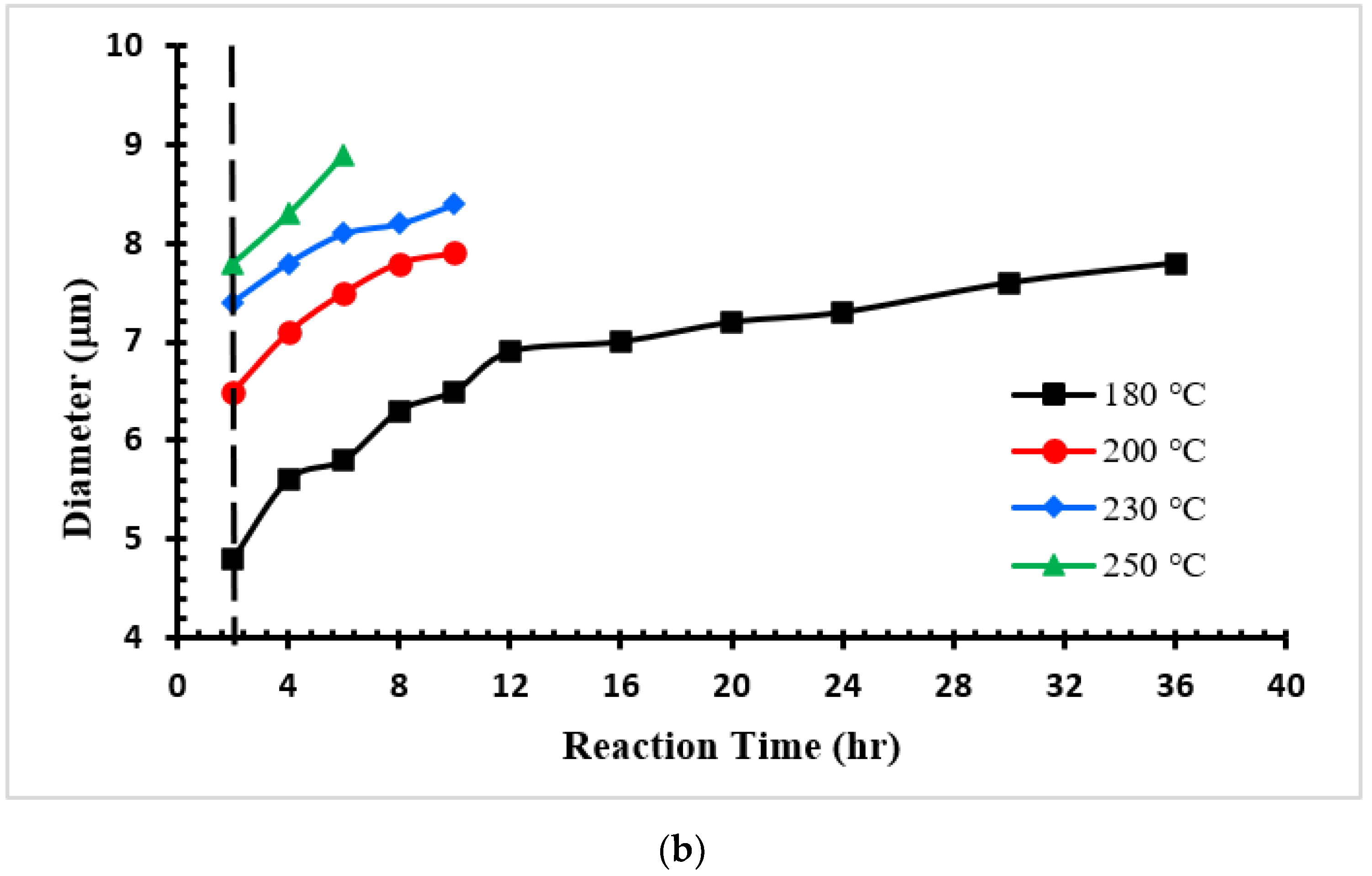
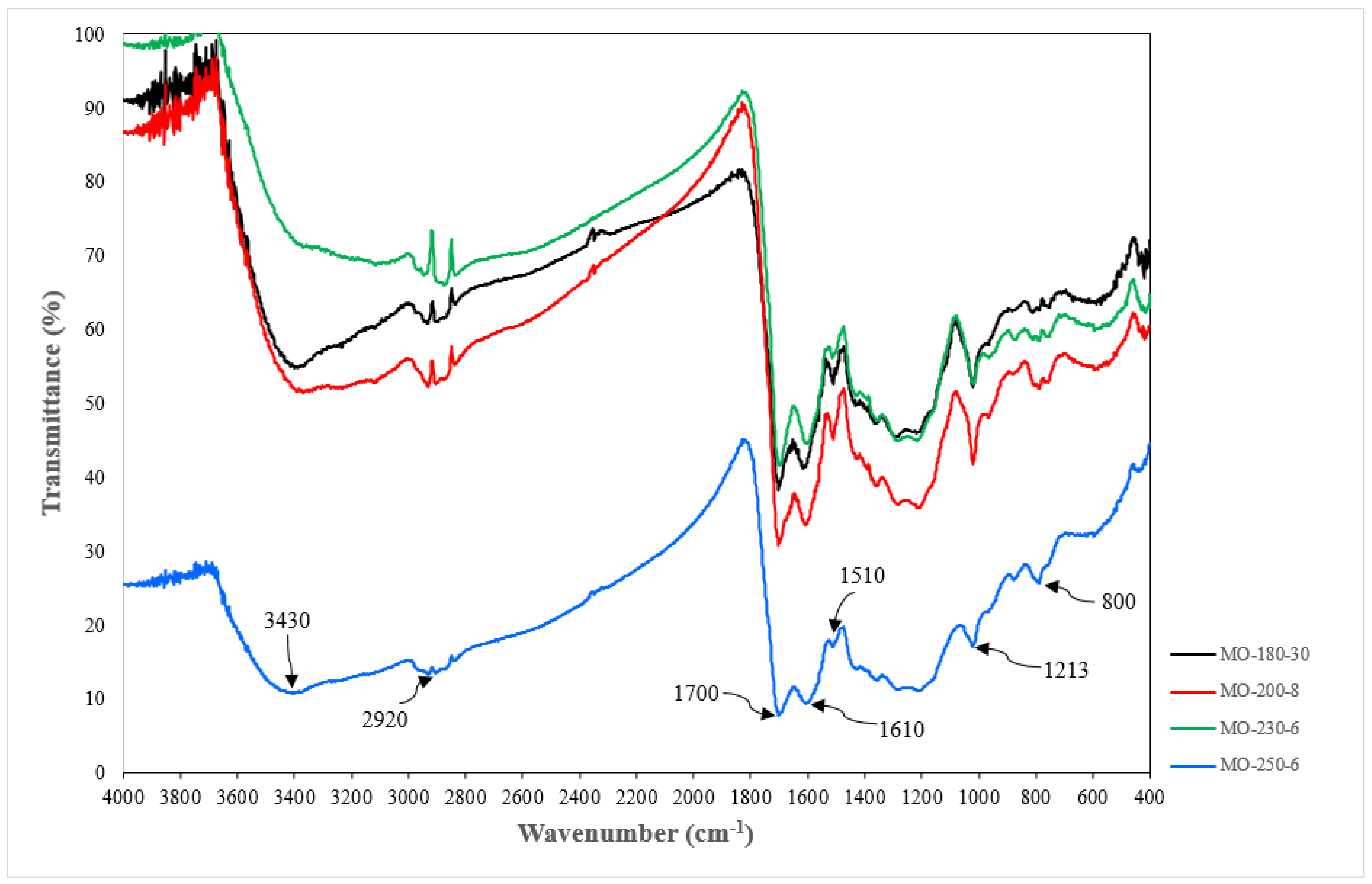
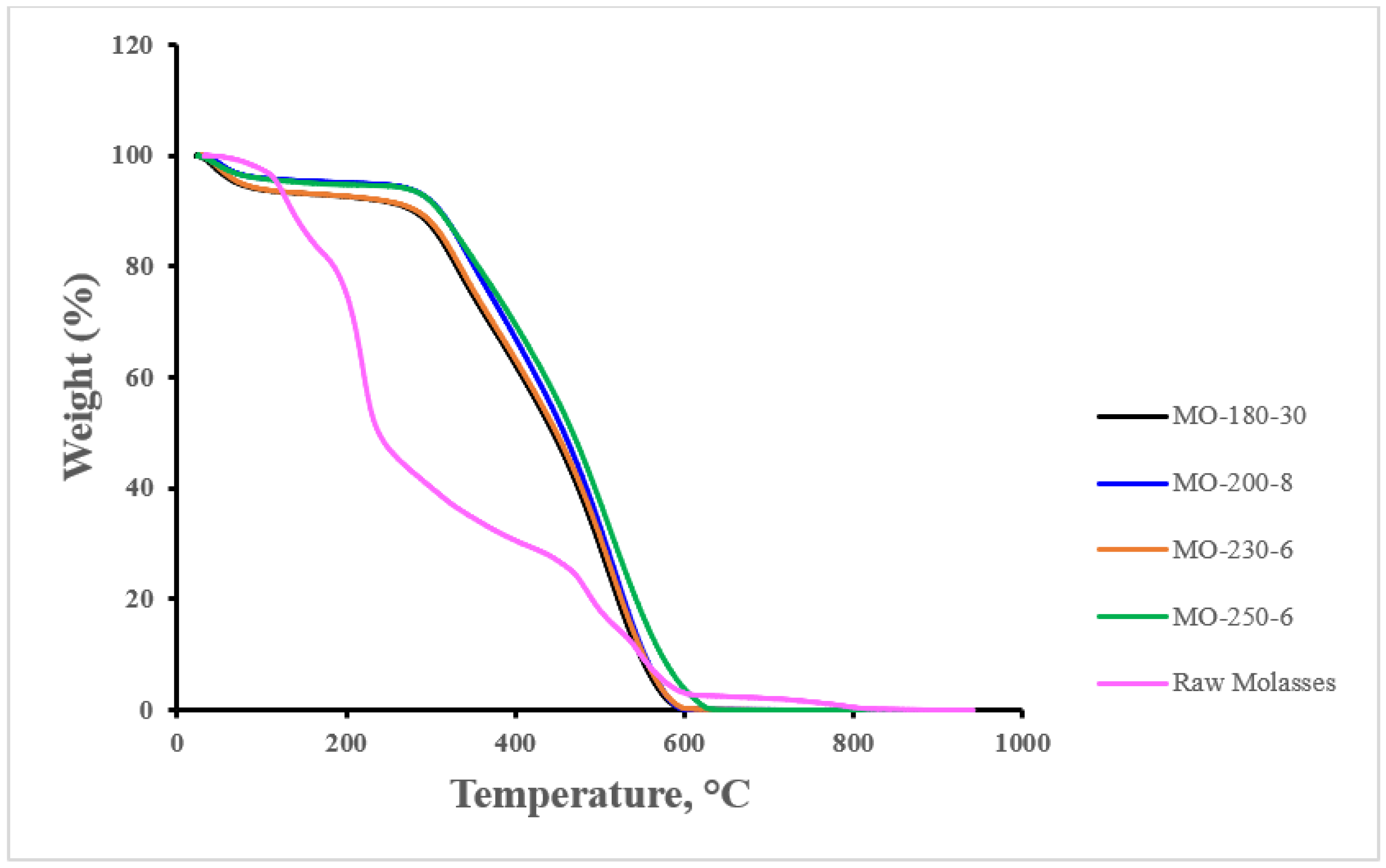
| Sample | Yield (%) | Particle Diameter (μm) |
|---|---|---|
| MO-180-2 | 3.6 | 4.8 |
| MO-180-4 | 7.3 | 5.6 |
| MO-180-6 | 16.8 | 5.8 |
| MO-180-8 | 25.0 | 6.3 |
| MO-180-10 | 27.3 | 6.5 |
| MO-180-12 | 29.7 | 6.9 |
| MO-180-16 | 31.9 | 7.0 |
| MO-180-20 | 33.7 | 7.2 |
| MO-180-24 | 35.7 | 7.3 |
| MO-180-30 | 36.0 | 7.6 |
| MO-180-36 | 33.5 | 7.8 |
| MO-200-2 | 10.9 | 6.5 |
| MO-200-4 | 14.6 | 7.1 |
| MO-200-6 | 28.5 | 7.5 |
| MO-200-8 | 34.3 | 7.8 |
| MO-200-10 | 33.7 | 7.9 |
| MO-230-2 | 20.1 | 7.4 |
| MO-230-4 | 32.1 | 7.8 |
| MO-230-6 | 33.5 | 8.1 |
| MO-230-8 | 33.1 | 8.2 |
| MO-230-10 | 32.9 | 8.4 |
| MO-250-2 | 31.8 | 7.8 |
| MO-250-4 | 33.4 | 8.3 |
| MO-250-6 | 40.4 | 8.9 |
| MO-250-8 | 30.8 | No CMs |
| MO-250-10 | 27.7 | No CMs |
| CM Sample | BET Surface Area (m2/g) | Pore Volume (cm3/g) | Pore Size (nm) |
|---|---|---|---|
| MO-180-30 | 0.7976 | 0.0029 | 48.70 |
| MO-200-8 | 0.7246 | 0.0033 | 36.66 |
| MO-230-6 | 0.7968 | 0.0023 | 46.31 |
| MO-250-6 | 0.3500 | 0.0020 | 43.34 |
| CM Samples | Chemical Composition | O/C (Atomic) | H/C (Atomic) | ||||
|---|---|---|---|---|---|---|---|
| C (wt.%) | H (wt.%) | N (wt.%) | S (wt.%) | O (wt.%) | |||
| Molasses | 34.53 | 7.95 | 0 | 1.08 | 56.44 | 1.23 | 2.76 |
| MO-180-30 | 67.85 | 4.83 | 0.09 | 0.63 | 26.60 | 0.29 | 0.85 |
| MO-200-8 | 64.87 | 4.72 | 0.04 | 0.59 | 29.78 | 0.34 | 0.87 |
| MO-230-6 | 67.57 | 4.62 | 0.30 | 0.57 | 26.94 | 0.30 | 0.82 |
| MO-250-6 | 68.22 | 4.60 | 0.18 | 0.56 | 26.44 | 0.29 | 0.81 |
| Biomass | BET Surface Area (m2/g) | Adsorption Capacity (mg/g) | Reference |
|---|---|---|---|
| Sabal palm | 14.68–59.77 | 18–25 | [24] |
| Sludge | 31.00–11.85 | 4.79–37.95 | [42] |
| Coffee husk | 2.90–31.22 | 34.85 | [43] |
| Inedible crystallized date molasses | 0.7968–0.3500 | 12 | This study |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
El-Harbawi, M.; Alhawtali, S.; Al-Awadi, A.S.; El Blidi, L.; Alrashed, M.M.; Alzobidi, A.; Yin, C.-Y. Synthesis of Carbon Microspheres from Inedible Crystallized Date Palm Molasses: Influence of Temperature and Reaction Time. Materials 2023, 16, 1672. https://doi.org/10.3390/ma16041672
El-Harbawi M, Alhawtali S, Al-Awadi AS, El Blidi L, Alrashed MM, Alzobidi A, Yin C-Y. Synthesis of Carbon Microspheres from Inedible Crystallized Date Palm Molasses: Influence of Temperature and Reaction Time. Materials. 2023; 16(4):1672. https://doi.org/10.3390/ma16041672
Chicago/Turabian StyleEl-Harbawi, Mohanad, Saeed Alhawtali, Abdulrhman S. Al-Awadi, Lahssen El Blidi, Maher M. Alrashed, Abdulrahman Alzobidi, and Chun-Yang Yin. 2023. "Synthesis of Carbon Microspheres from Inedible Crystallized Date Palm Molasses: Influence of Temperature and Reaction Time" Materials 16, no. 4: 1672. https://doi.org/10.3390/ma16041672





