Nd3+, Yb3+:YF3 Optical Temperature Nanosensors Operating in the Biological Windows
Abstract
:1. Introduction
2. Materials and Methods
3. Results and Discussion
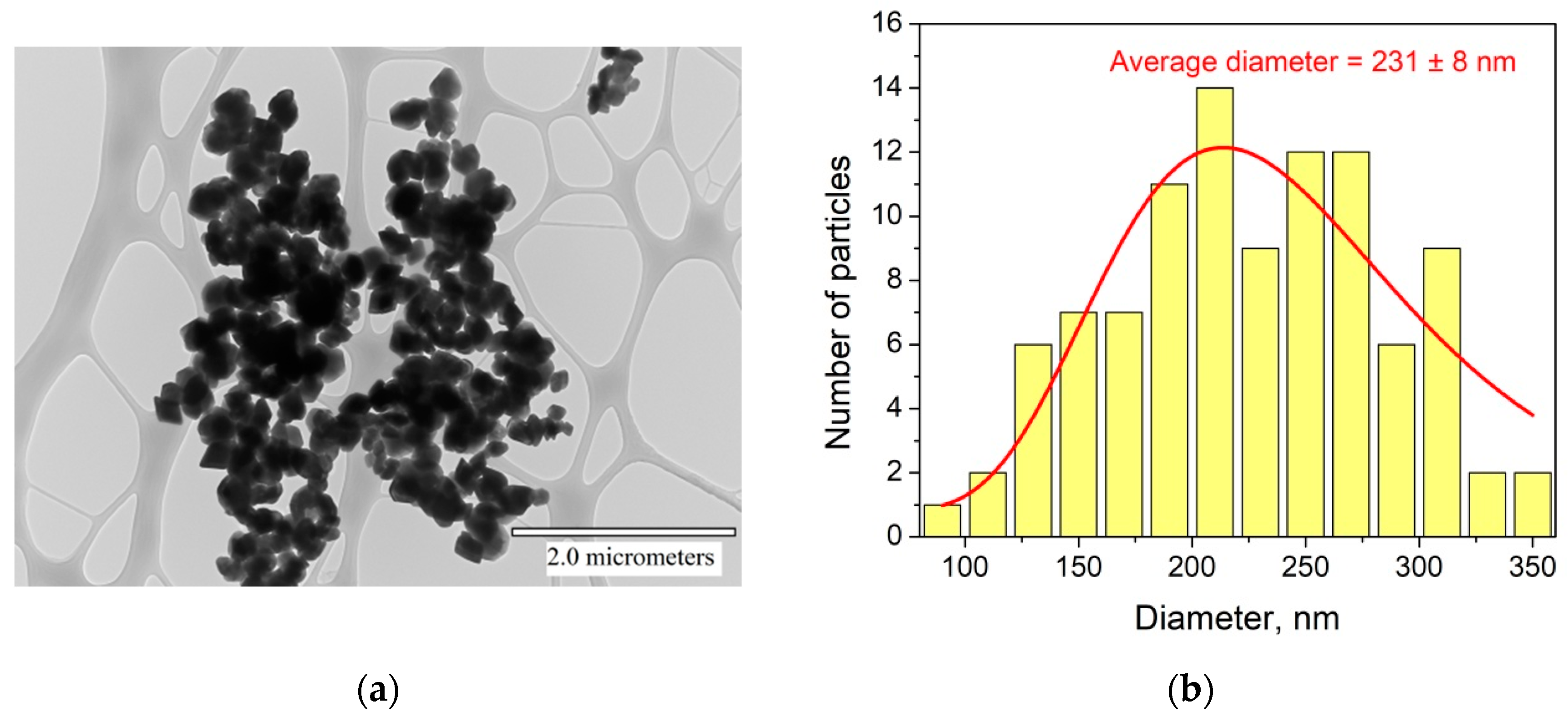
3.1. Characterization of Nd3+, Yb3+:YF3 Phosphors
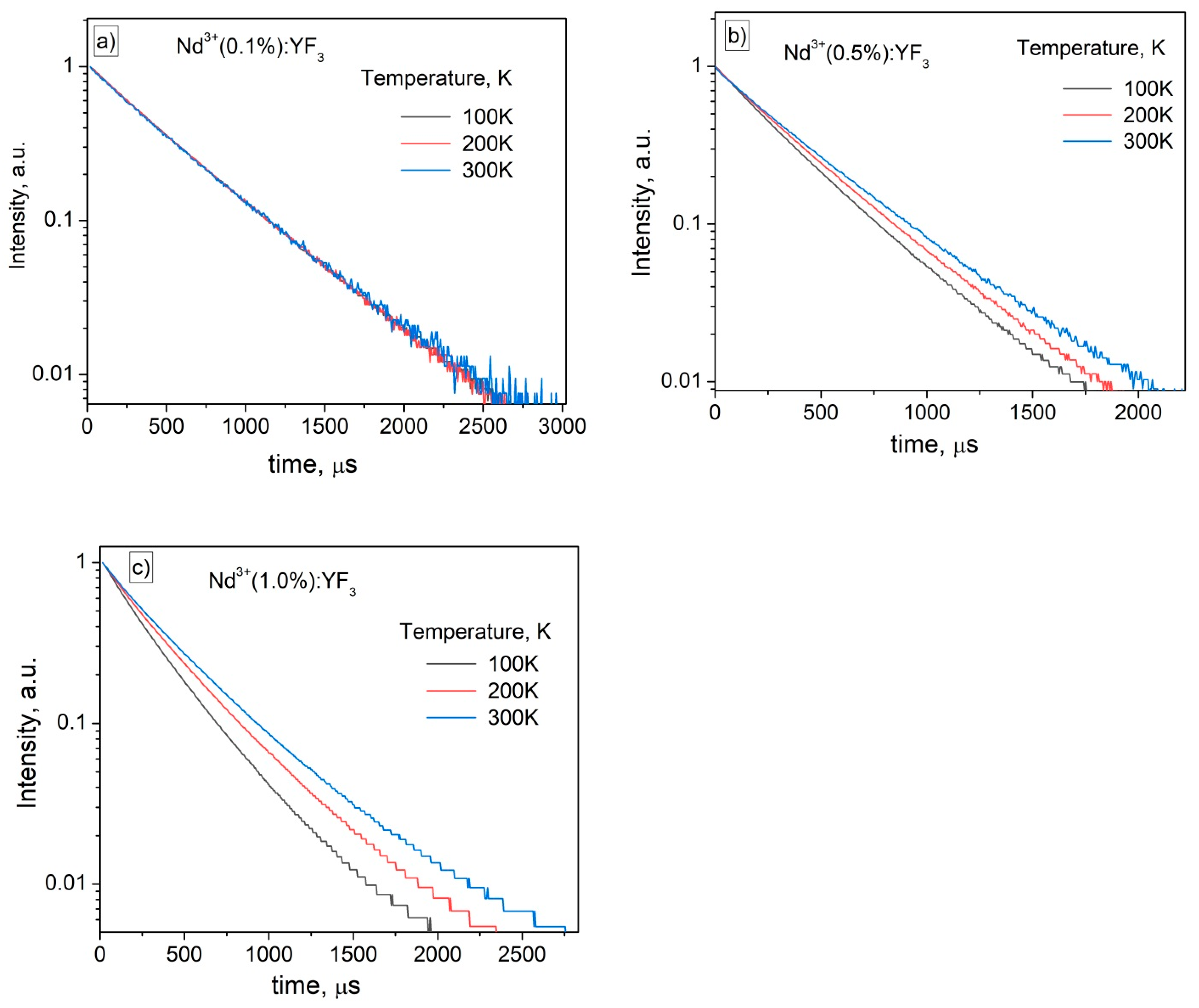
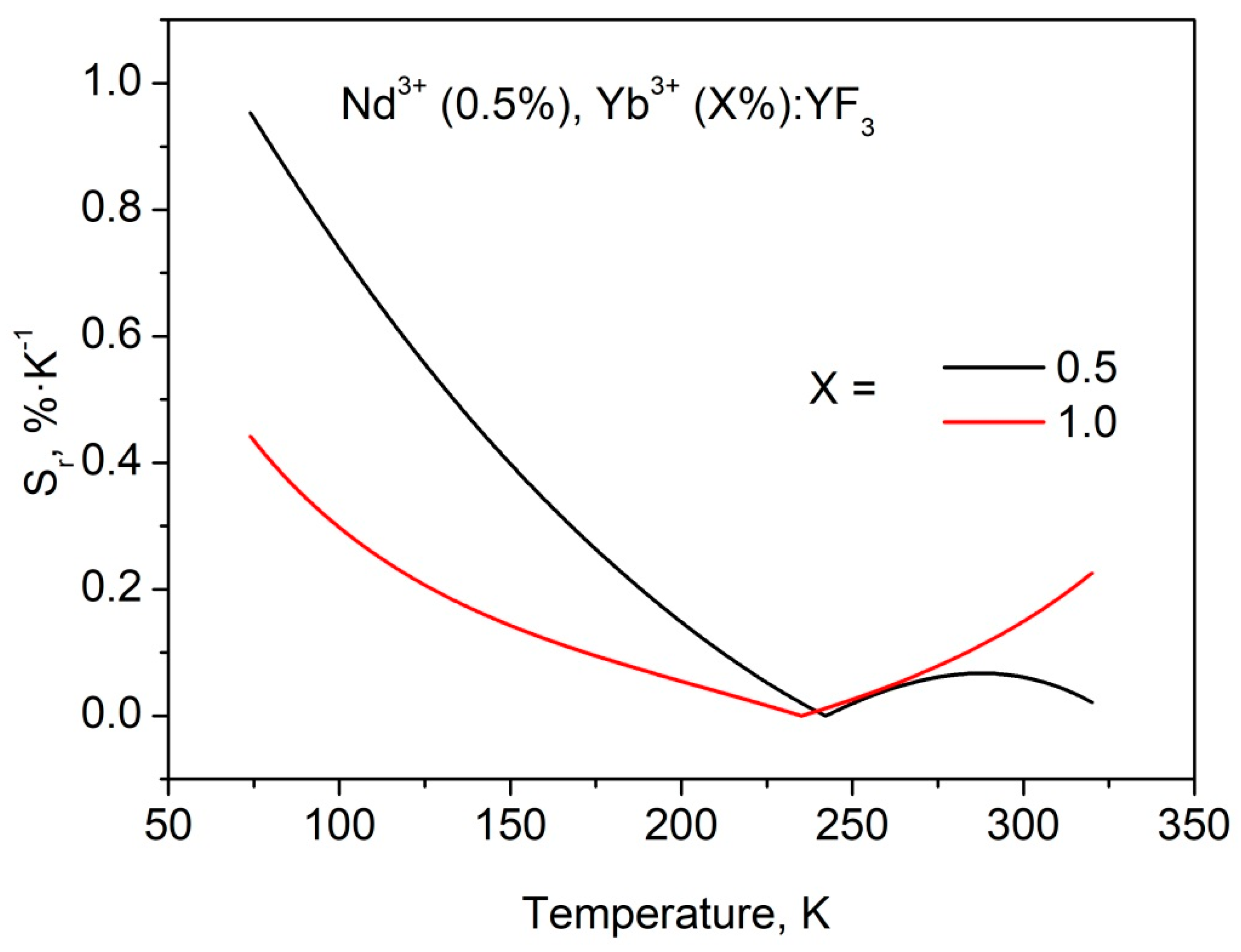
3.2. Temperature Dependent Spectral-Kinetic Characterization of Single-Doped Nd3+:YF3 Nanoparticles and Microparticles
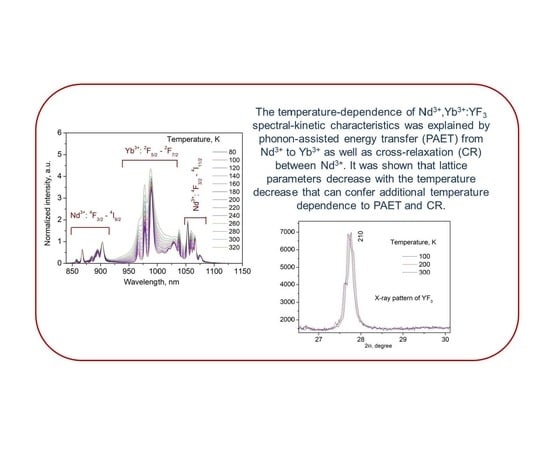
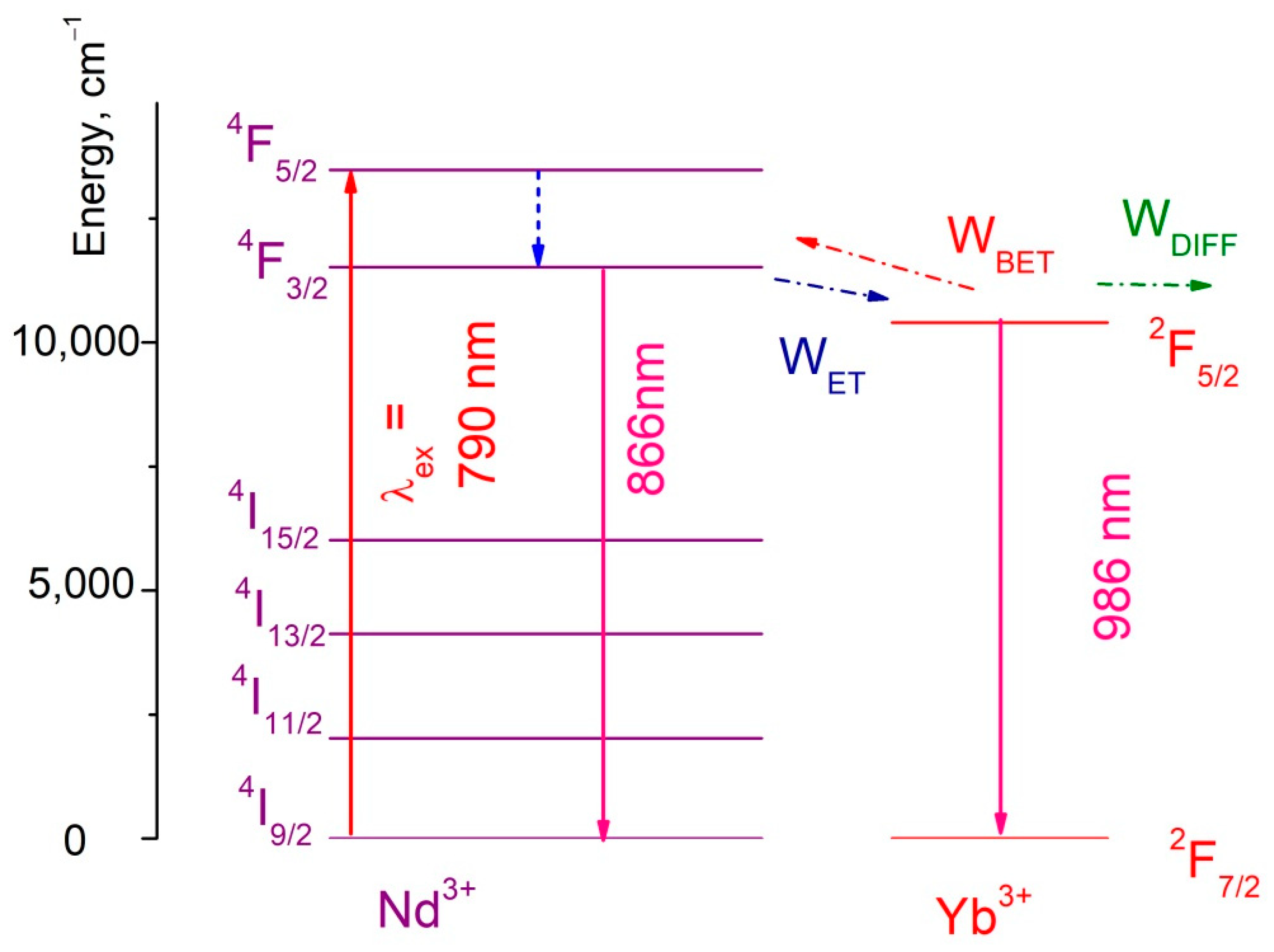
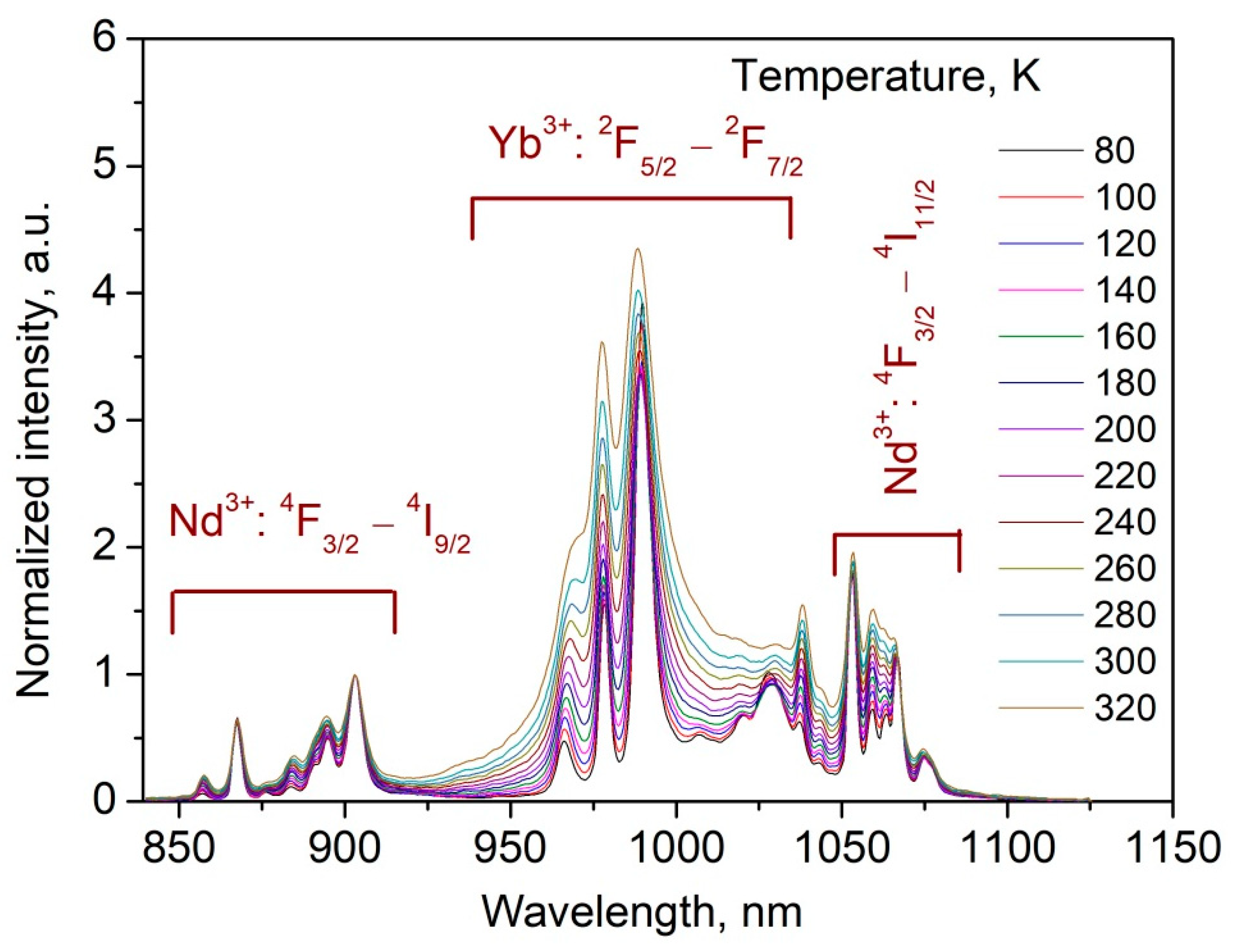
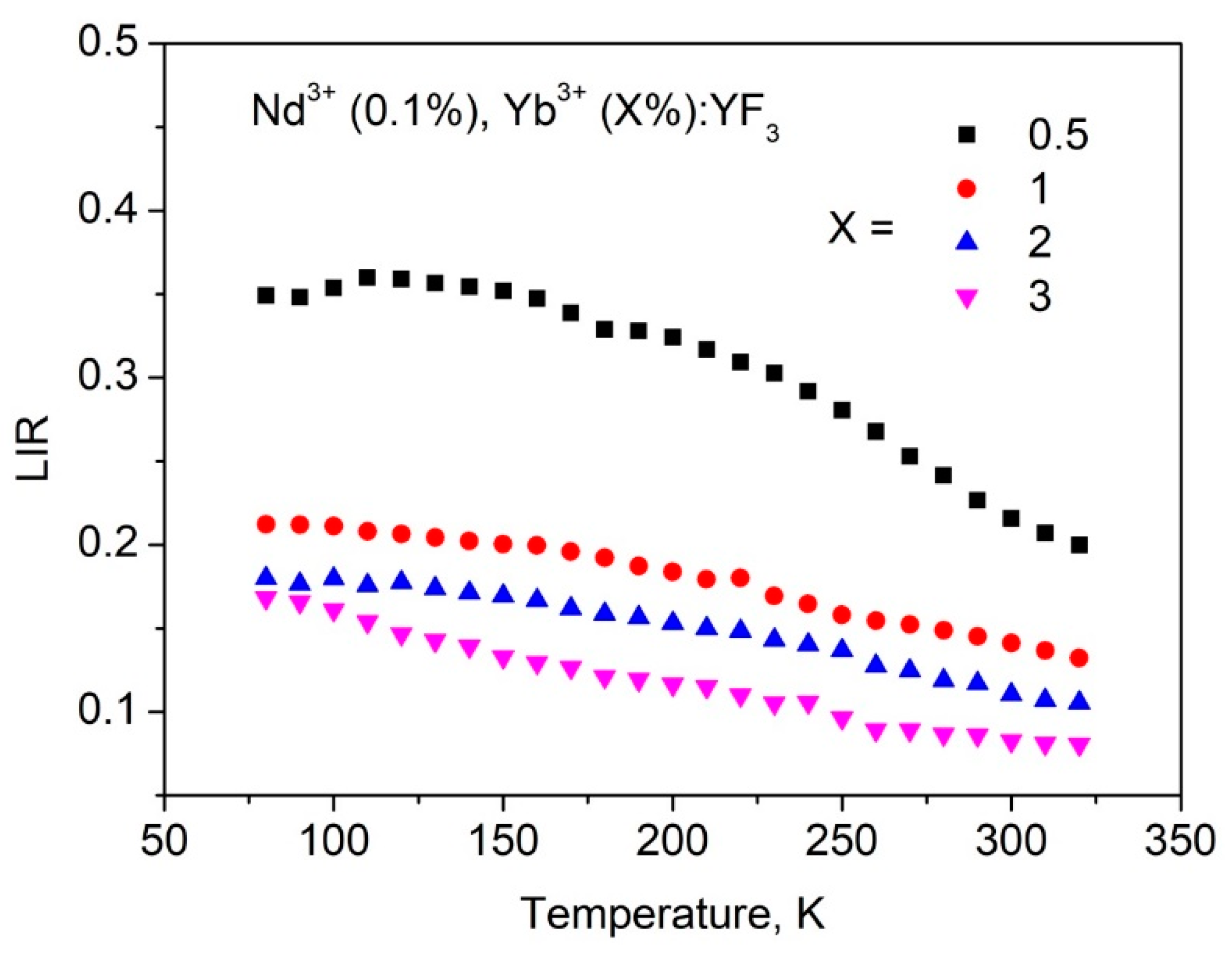
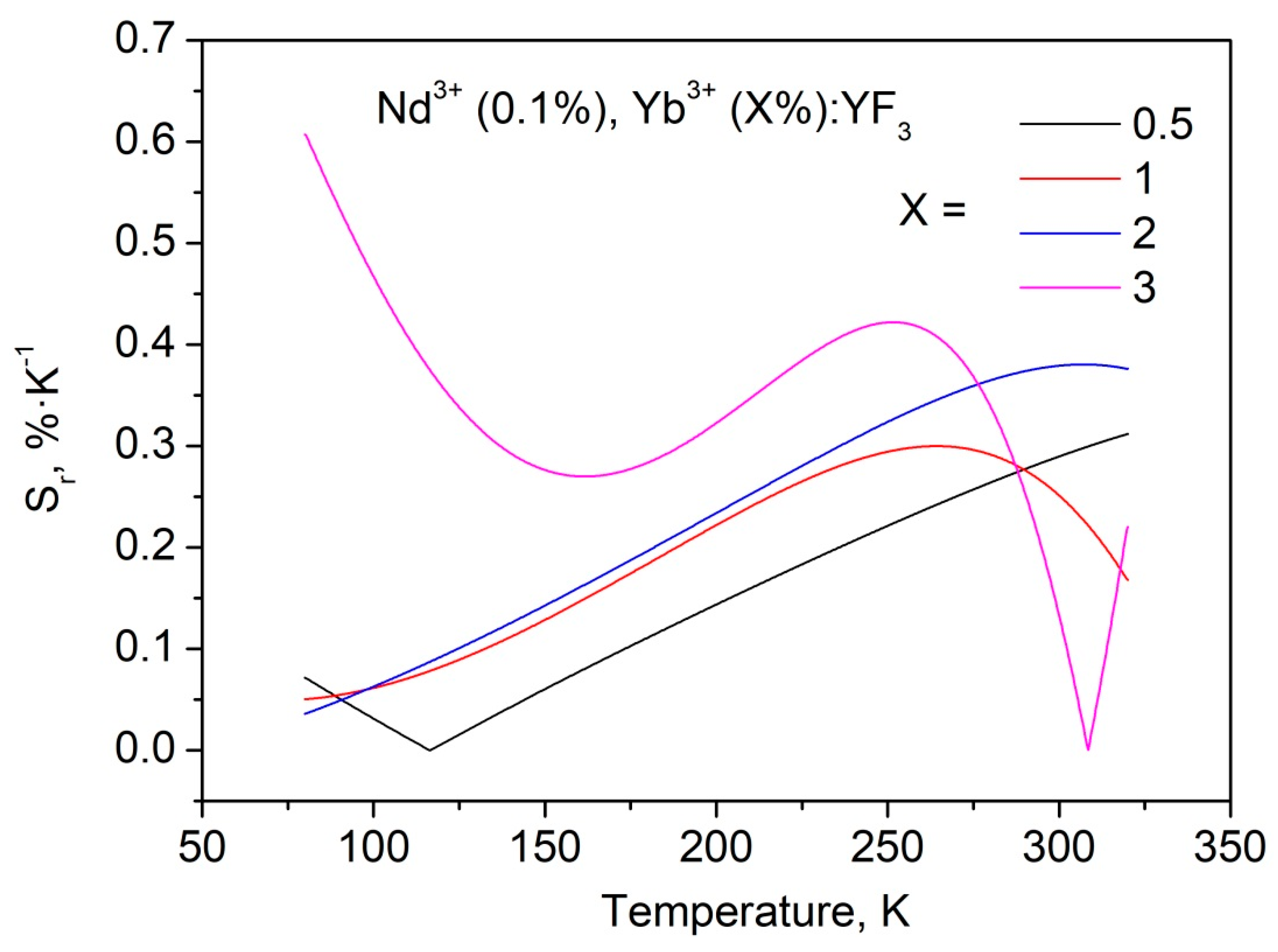
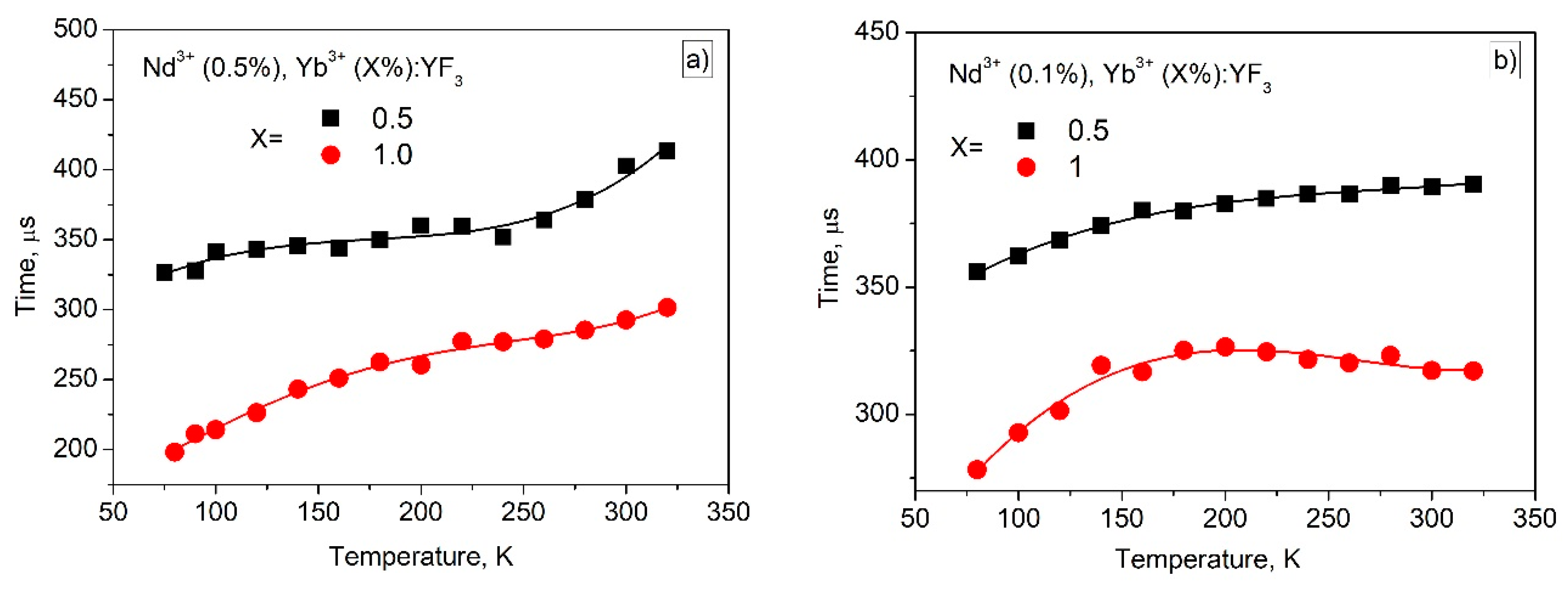
3.3. Temperature Dependent Spectral-Kinetic Characterization of Double-Doped Nd3+, Yb3+:YF3 Nanoparticles
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Jaque, D.; Vetrone, F. Luminescence nanothermometry. Nanoscale 2012, 4, 4301–4326. [Google Scholar] [CrossRef] [PubMed]
- Brites, C.D.; Lima, P.P.; Silva, N.J.; Millán, A.; Amaral, V.S.; Palacio, F.; Carlos, L.D. Thermometry at the nanoscale. Nanoscale 2012, 4, 4799–4829. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, X.D.; Wolfbeis, O.S.; Meier, R.J. Luminescent probes and sensors for temperature. Chem. Soc. Rev. 2013, 42, 7834–7869. [Google Scholar] [CrossRef] [PubMed]
- Hossain, M.K.; Ahmed, M.H.; Khan, M.I.; Miah, M.S.; Hossain, S. Recent Progress of Rare Earth Oxides for Sensor, Detector, and Electronic Device Applications: A Review. ACS Appl. Electron. Mater. 2021, 3, 4255–4283. [Google Scholar] [CrossRef]
- Hossain, M.K.; Hossain, S.; Ahmed, M.H.; Khan, M.I.; Haque, N.; Raihan, G.A. A Review on Optical Applications, Prospects, and Challenges of Rare-Earth Oxides. ACS Appl. Electron. Mater. 2021, 3, 3715–3746. [Google Scholar] [CrossRef]
- Piñol, R.; Brites, C.D.; Silva, N.J.; Carlos, L.D.; Millán, A. Nanoscale thermometry for hyperthermia applications. In Nanomaterials for Magnetic and Optical Hyperthermia Applications; Elsevier: Amsterdam, The Netherlands, 2019; pp. 139–172. [Google Scholar]
- Bazhukova, I.N.; Pustovarov, V.A.; Myshkina, A.V.; Ulitko, M.V. Luminescent Nanomaterials Doped with Rare Earth Ions and Prospects for Their Biomedical Applications (A Review). Opt. Spectrosc. 2020, 128, 2050–2068. [Google Scholar] [CrossRef]
- Fedorov, P.P.; Luginina, A.A.; Kuznetsov, S.V.; Osiko, V.V. Nanofluorides. J. Fluor. Chem. 2011, 132, 1012–1039. [Google Scholar] [CrossRef]
- Pudovkin, M.S.; Zelenikhin, P.V.; Shtyreva, V.V.; Evtugyn, V.G.; Salnikov, V.V.; Nizamutdinov, A.S.; Semashko, V.V. Cellular uptake and cytotoxicity of unmodified Pr3+: LaF3 nanoparticles. J. Nanopart. Res. 2019, 21, 184. [Google Scholar] [CrossRef]
- Wang, X.; Li, Y. Rare-earth-compound nanowires, nanotubes, and fullerene-like nanoparticles: Synthesis, characterization, and properties. Chem.-A Eur. J. 2003, 9, 5627–5635. [Google Scholar] [CrossRef]
- Ximendes, E.C.; Rocha, U.; Kumar, K.U.; Jacinto, C.; Jaque, D. LaF3 core/shell nanoparticles for subcutaneous heating and thermal sensing in the second biological-window. Appl. Phys. Lett. 2016, 108, 253103. [Google Scholar] [CrossRef]
- Pudovkin, M.S.; Ginkel, A.K.; Lukinova, E.V. Temperature sensitivity of Nd3+, Yb3+: YF3 ratiometric luminescent thermometers at different Yb3+ concentration. Opt. Mater. 2021, 119, 111328. [Google Scholar] [CrossRef]
- Aarts, L.; Ende, B.V.D.; Reid, M.F.; Meijerink, A. Downconversion for solar cells in YF3: Pr3+, Yb3+. Spectrosc. Lett. 2010, 43, 373–381. [Google Scholar] [CrossRef]
- Marciniak, L.; Bednarkiewicz, A.; Trejgis, K.; Maciejewska, K.; Elzbieciak, K.; Ledwa, K. Enhancing the sensitivity of a Nd3+, Yb3+: YVO4 nanocrystalline luminescent thermometer by host sensitization. Phys. Chem. Chem. Phys. 2019, 21, 10532–10539. [Google Scholar] [CrossRef] [PubMed]
- Bednarkiewicz, A.; Stefanski, M.; Tomala, R.; Hreniak, D.; Strek, W. Near infrared absorbing near infrared emitting highly-sensitive luminescent nanothermometer based on Nd3+ to Yb3+ energy transfer. Phys. Chem. Chem. Phys. 2015, 17, 24315–24321. [Google Scholar]
- Pudovkin, M.S.; Ginkel, A.K.; Morozov, O.A.; Kiiamov, A.G.; Kuznetsov, M.D. Highly-sensitive lifetime optical thermometers based on Nd3+, Yb3+: YF3 phosphors. J. Lumin. 2022, 249, 119037. [Google Scholar] [CrossRef]
- Kaczmarek, A.M.; Kaczmarek, M.K.; Van Deun, R. Er3+-to-Yb3+ and Pr3+-to-Yb3+ energy transfer for highly efficient near-infrared cryogenic optical temperature sensing. Nanoscale 2019, 11, 833–837. [Google Scholar] [CrossRef]
- Maciejewska, K.; Bednarkiewicz, A.; Marciniak, L. NIR luminescence lifetime nanothermometry based on phonon assisted Yb 3+–Nd 3+ energy transfer. Nanoscale Adv. 2021, 3, 4918–4925. [Google Scholar] [CrossRef]
- Rocha, U.; Upendra Kumar, K.; Jacinto, C.; Ramiro, J.; Caamano, A.J.; Garcia Sole, J.; Jaque, D. Nd3+ doped LaF3 nanoparticles as self-monitored photo-thermal agents. Appl. Phys. Lett. 2014, 104, 053703. [Google Scholar] [CrossRef] [Green Version]
- Wang, X.; Xu, T.; Cai, P.; Vu, T.; Seo, H.J. Controlled synthesis, multicolor luminescence, and optical thermometer of bifunctional NaYbF4: Nd3+@ NaYF4: Yb3+ active-core/active-shell colloidal nanoparticles. J. Alloys Compound. 2017, 691, 530–536. [Google Scholar] [CrossRef]
- Vanetsev, A.; Kaldvee, K.; Puust, L.; Keevend, K.; Nefedova, A.; Fedorenko, S.; Orlovskii, Y. Relation of Crystallinity and Fluorescent Properties of LaF3: Nd3+ Nanoparticles Synthesized with Different Water-Based Techniques. Chem. Select 2017, 2, 4874–4881. [Google Scholar] [CrossRef]
- Khadiev, A.R.; Korableva, S.L.; Ginkel, A.K.; Morozov, O.A.; Nizamutdinov, A.S.; Semashko, V.V.; Pudovkin, M.S. Down-conversion based Tm3+: LiY1-XYbXF4 temperature sensors. Opt. Mater. 2022, 134, 113118. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pudovkin, M.; Oleynikova, E.; Kiiamov, A.; Cherosov, M.; Gafurov, M. Nd3+, Yb3+:YF3 Optical Temperature Nanosensors Operating in the Biological Windows. Materials 2023, 16, 39. https://doi.org/10.3390/ma16010039
Pudovkin M, Oleynikova E, Kiiamov A, Cherosov M, Gafurov M. Nd3+, Yb3+:YF3 Optical Temperature Nanosensors Operating in the Biological Windows. Materials. 2023; 16(1):39. https://doi.org/10.3390/ma16010039
Chicago/Turabian StylePudovkin, Maksim, Ekaterina Oleynikova, Airat Kiiamov, Mikhail Cherosov, and Marat Gafurov. 2023. "Nd3+, Yb3+:YF3 Optical Temperature Nanosensors Operating in the Biological Windows" Materials 16, no. 1: 39. https://doi.org/10.3390/ma16010039