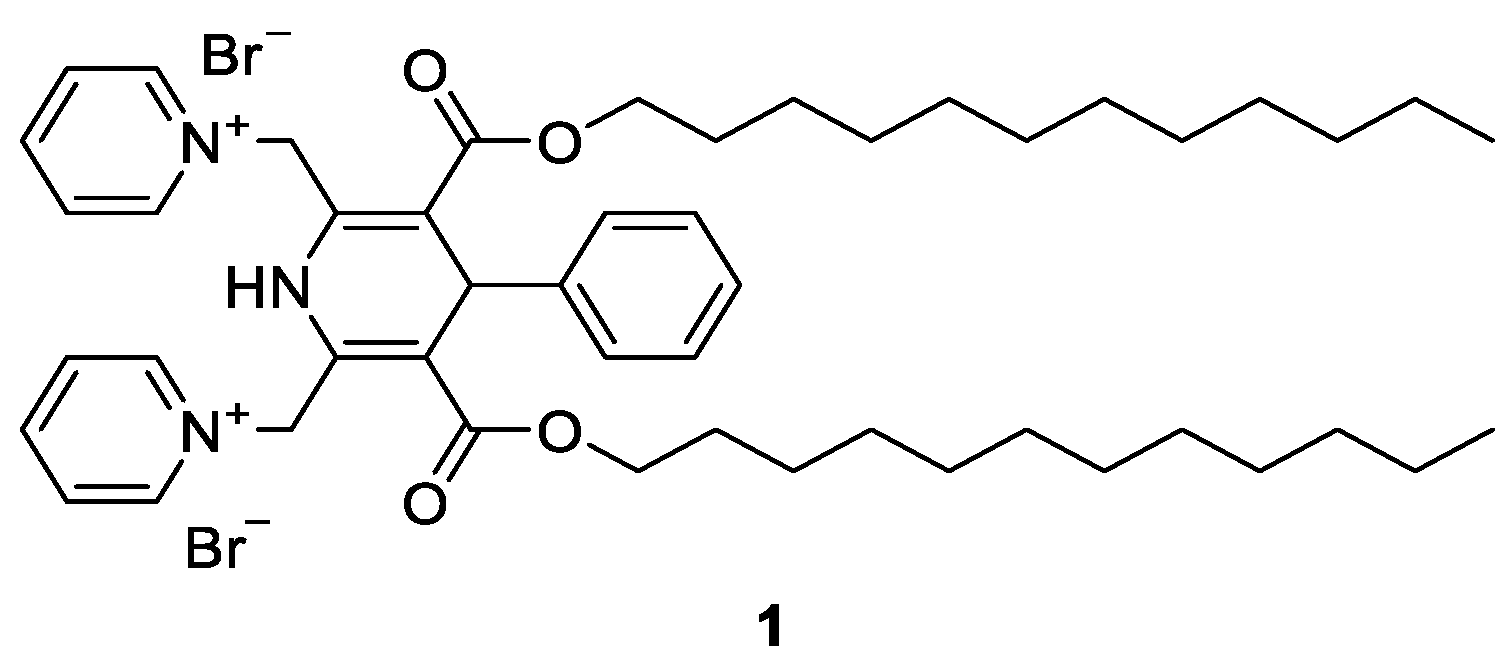
Synthesis and Characterization of Novel Amphiphilic N-Benzyl 1,4-Dihydropyridine Derivatives—Evaluation of Lipid Monolayer and Self-Assembling Properties
Abstract
:1. Introduction
2. Materials and Methods
2.1. General
2.2. Synthesis of Compounds
2.2.1. Didodecyl 1-Benzyl-2,6-dimethyl-4-phenyl-1,4-dihydropyridine-3,5-dicarboxylate (2)
2.2.2. Didodecyl 1-Benzyl-2,6-bis(bromomethyl)-4-phenyl-1,4-dihydropyridine-3,5-dicarboxylate (3)
2.2.3. General Procedure for Synthesis of 1,1′-((1-Benzyl-3,5-bis(dodecyloxycarbonyl)-4-phenyl-1,4-dihydropyridine-2,6-diyl)bis(methylene))bis(pyridin-1-ium) Dibromides 5
1,1′-((1-Benzyl-3,5-bis((dodecyloxy)carbonyl)-4-phenyl-1,4-dihydropyridine-2,6-diyl)bis(methylene))bis(pyridin-1-ium) Dibromide 5a
1,1′-((1-Benzyl-3,5-bis((dodecyloxy)carbonyl)-4-phenyl-1,4-dihydropyridine-2,6-diyl)bis(methylene))bis(4-methylpyridin-1-ium) 5b
1,1′-((1-Benzyl-3,5-bis((dodecyloxy)carbonyl)-4-phenyl-1,4-dihydropyridine-2,6-diyl)bis(methylene))bis(4-(dimethylamino)pyridin-1-ium) 5c
1,1′-((1-Benzyl-3,5-bis((dodecyloxy)carbonyl)-4-phenyl-1,4-dihydropyridine-2,6-diyl)bis(methylene))bis(4-phenylpyridin-1-ium) 5d
1,1′-((1-Benzyl-3,5-bis((dodecyloxy)carbonyl)-4-phenyl-1,4-dihydropyridine-2,6-diyl)bis(methylene))bis(4-propylpyridin-1-ium) 5e
2.3. Characterization of Monolayers Formed by 1,4-DHP Amphiphiles 5 or Surface Pressure–Area (π–A) Isotherms
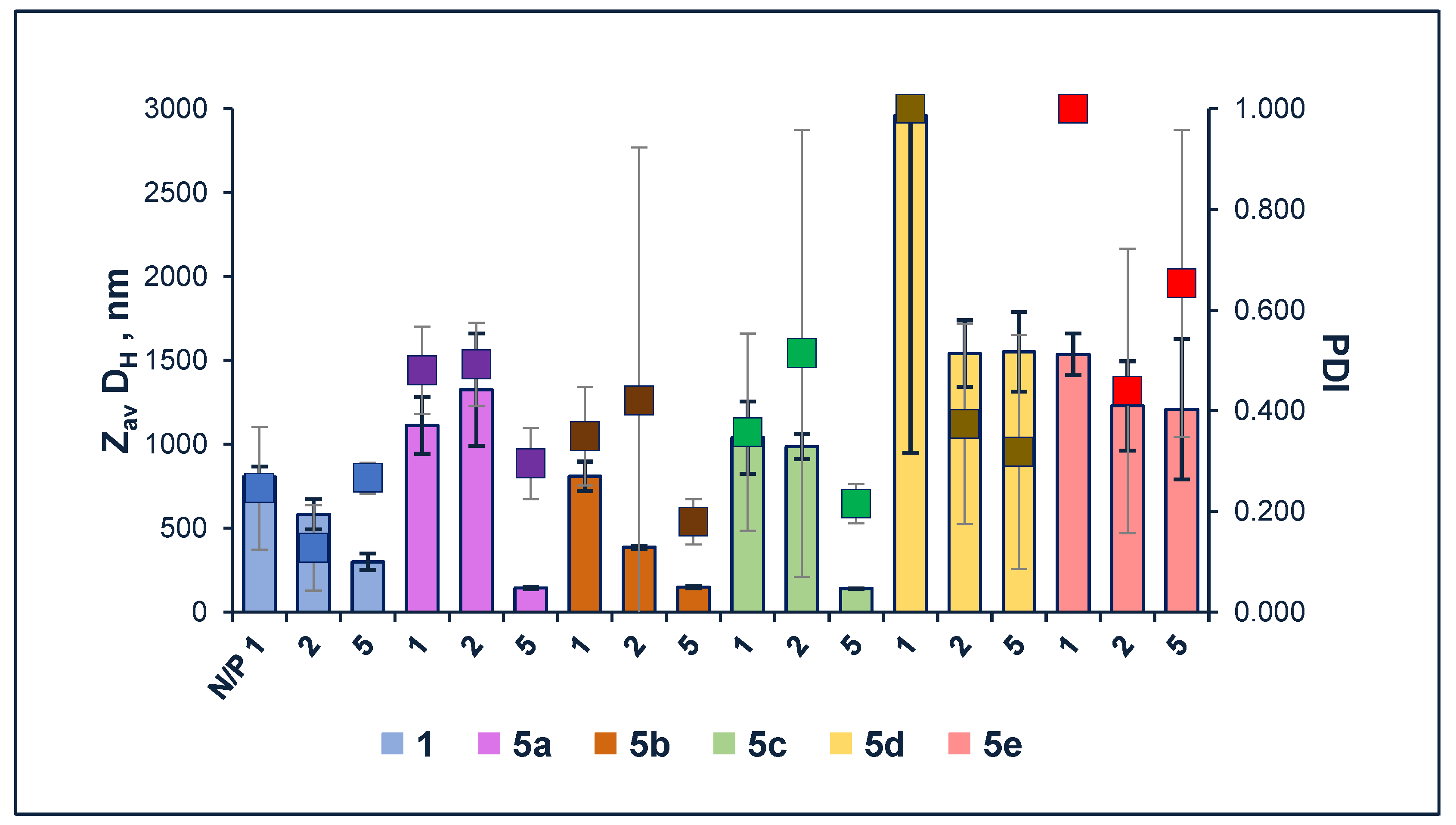
2.4. Self-Assembling Properties of Compounds 5 Using Dynamic Light Scattering Measurements
2.5. Determination of Critical Aggregation Concentrations
2.6. Formation of Lipoplexes
2.7. Statistical Analysis
3. Results and Discussion
3.1. Synthesis of N-Benzyl-1,4-dihydropyridines
3.2. Characterization of Monolayers
3.3. Self-Assembling Properties
3.3.1. Characterization of Size of Liposomes
3.3.2. Characterization of Lipoplexes
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Salamanca-Buentello, F.; Daar, A.S. Nanotechnology, equity and global health. Nat. Nanotechnol. 2021, 16, 358–361. [Google Scholar] [CrossRef] [PubMed]
- Soprano, E.; Polo, E.; Pelaz, B.; del Pino, P. Biomimetic cell-derived nanocarriers in cancer research. J. Nanobiotechnol 2022, 20, 538. [Google Scholar] [CrossRef] [PubMed]
- Harish, V.; Tewari, D.; Gaur, M.; Yadav, A.B.; Swaroop, S.; Bechelany, M.; Barhoum, A. Review on Nanoparticles and Nanostructured Materials: Bioimaging, Biosensing, Drug Delivery, Tissue Engineering, Antimicrobial, and Agro-Food Applications. Nanomaterials 2022, 12, 457. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Chen, Y.; Guo, J.; Huang, Q. Liposomes for tumor targeted therapy: A review. Int. J. Mol. Sci. 2023, 24, 2643. [Google Scholar] [CrossRef]
- Yusuf, A.; Almotairy, A.R.Z.; Henidi, H.; Alshehri, O.Y.; Aldughaim, M.S. Nanoparticles as Drug Delivery Systems: A Review of the implication of nanoparticles’ physicochemical properties on responses in biological systems. Polymers 2023, 15, 1596. [Google Scholar] [CrossRef]
- Nikolova, M.P.; Kumar, E.M.; Chavali, M.S. Updates on responsive drug delivery based on liposome vehicles for cancer treatment. Pharmaceutics 2022, 14, 2195. [Google Scholar] [CrossRef]
- Panahi, Y.; Farshbaf, M.; Mohammadhosseini, M.; Mirahadi, M.; Khalilov, R.; Saghfi, S.; Akbarzadeh, A. Recent advances on liposomal nanoparticles: Synthesis, characterization and biomedical applications. Artif. Cells Nanomed. Biotechnol. 2017, 45, 788–799. [Google Scholar] [CrossRef] [Green Version]
- Wang, N.; Wang, T.; Li, T.; Deng, Y. Modulation of the physicochemical state of interior agents to prepare controlled release liposomes. Colloids Surf. B. 2009, 69, 232–238. [Google Scholar] [CrossRef]
- Liu, P.; Chen, G.; Zhang, J. A Review of liposomes as a drug delivery system: Current status of approved products, regulatory environments, and future perspectives. Molecules 2022, 27, 1372. [Google Scholar] [CrossRef]
- Tenchov, R.; Bird, R.; Curtze, A.E.; Zhou, Q. Lipid nanoparticles from liposomes to mRNA vaccine delivery, a landscape of research diversity and advancement. ACS Nano 2021, 15, 16982–17015. [Google Scholar] [CrossRef]
- Gregoriadis, G. Liposomes and mRNA: Two technologies together create a COVID-19 vaccine. Med. Drug Discov. 2021, 12, 100104. [Google Scholar] [CrossRef]
- Mathaes, R.; Winter, G.; Besheer, A.; Engert, J. Non-spherical micro- and nanoparticles: Fabrication, characterization and drug delivery applications. Expert Opin. Drug Deliv. 2015, 12, 481–492. [Google Scholar] [CrossRef] [PubMed]
- Dhiman, N.; Awasthi, R.; Sharma, B.; Kharkwal, H.; Kulkarni, G.T. Lipid nanoparticles as carriers for bioactive delivery. Front. Chem. 2021, 9, 268. [Google Scholar] [CrossRef]
- Nguyen, V.H.; Thuy, V.N.; Van, T.V.; Dao, A.H.; Lee, B.J. Nanostructured lipid carriers and their potential applications for versatile drug delivery via oral administration. OpenNano 2022, 8, 100064. [Google Scholar] [CrossRef]
- van der Koog, L.; Gandek, T.B.; Nagelkerke, A. Liposomes and extracellular vesicles as drug delivery systems: A comparison of composition, pharmacokinetics, and functionalization. Adv. Healthc. Mater. 2022, 11, 2100639. [Google Scholar] [CrossRef]
- Hyvönen, Z.; Plotniece, A.; Reine, I.; Chekavichus, B.; Duburs, G.; Urtti, A. Novel cationic amphiphilic 1,4-dihydropyridine derivatives for DNA delivery. Biochim. Biophys. Acta Biomembr. 2000, 1509, 451–466. [Google Scholar] [CrossRef] [Green Version]
- Pajuste, K.; Hyvonen, Z.; Petrichenko, O.; Kaldre, D.; Rucins, M.; Cekavicus, B.; Ose, V.; Skrivele, B.; Gosteva, M.; Morin-Picardat, E.; et al. Gene delivery agents possessing antiradical activity: Self-assembling cationic amphiphilic 1,4-dihydropyridine derivatives. New J. Chem. 2013, 37, 3062–3075. [Google Scholar] [CrossRef]
- Triggle, D.J. The 1,4-dihydropyridine nucleus: A pharmacophoric template part 1. Actions at ion channels. Mini Rev. Med. Chem. 2003, 3, 215–223. [Google Scholar] [CrossRef]
- Petrichenko, O.; Rucins, M.; Vezane, A.; Timofejeva, I.; Sobolev, A.; Cekavicus, B.; Pajuste, K.; Plotniece, M.; Gosteva, M.; Kozlovska, T.; et al. Studies of the physicochemical and structural properties of self-assembling cationic pyridine derivatives as gene delivery agents. Chem. Phys. Lipids 2015, 191, 25–37. [Google Scholar] [CrossRef]
- Apsite, G.; Timofejeva, I.; Vezane, A.; Vigante, B.; Rucins, M.; Sobolev, A.; Plotniece, M.; Pajuste, K.; Kozlovska, T.; Plotniece, A. Synthesis and comparative evaluation of novel cationic amphiphile C12-Man-Q as an efficient DNA delivery agent in vitro. Molecules 2018, 23, 1540. [Google Scholar] [CrossRef] [Green Version]
- Petrichenko, O.; Plotniece, A.; Pajuste, K.; Rucins, M.; Dimitrijevs, P.; Sobolev, A.; Sprugis, E.; Cēbers, A. Evaluation of physicochemical properties of amphiphilic 1,4-dihydropyridines and preparation of magnetoliposomes. Nanomaterials 2021, 11, 593. [Google Scholar] [CrossRef] [PubMed]
- Niemirowicz-Laskowska, K.; Głuszek, K.; Piktel, E.; Pajuste, K.; Durnaś, B.; Król, G.; Wilczewska, A.Z.; Janmey, P.A.; Plotniece, A.; Bucki, R. Bactericidal and immunomodulatory properties of magnetic nanoparticles functionalized by 1,4-dihydropyridines. Int. J. Nanomed. 2018, 13, 3411–3424. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rucins, M.; Smits, R.; Sipola, A.; Vigante, B.; Domracheva, I.; Turovska, B.; Muhamadejev, R.; Pajuste, K.; Plotniece, M.; Sobolev, A.; et al. Pleiotropic properties of amphiphilic dihydropyridines, dihydropyridones, and aminovinylcarbonyl compounds. Oxid. Med. Cell. Longev. 2020, 2020, 8413713. [Google Scholar] [CrossRef]
- Rucins, M.; Kaukulis, M.; Plotniece, A.; Pajuste, K.; Pikun, N.; Sobolev, A. 1,1′-{[3,5-Bis(dodecyloxycarbonyl)-4-(naphthalen-2-yl)-1,4-dihydropyridine-2,6-diyl]bis(methylene)}bis{4-[(E)-2-(naphthalen-2-yl)vinyl]pyridin-1-ium}dibromide. Molbank 2022, 2022, M1396. [Google Scholar] [CrossRef]
- Ozolins, R.; Plotniece, M.; Pajuste, K.; Putralis, R.; Pikun, N.; Sobolev, A.; Plotniece, A.; Rucins, M. 1,1′-{[3,5-Bis((dodecyloxycarbonyl)-4-phenyl-1,4-dihydropyridine-2,6-diyl]bis(methylene)}bis [4-(anthracen-9-yl)pyridin-1-ium] Dibromide. Molbank 2022, 2022, M1438. [Google Scholar] [CrossRef]
- Topel, Ö.; Çakir, B.A.; Budama, L.; Hoda, N. Determination of critical micelle concentration of polybutadiene-block- poly(ethyleneoxide) diblock copolymer by fluorescence spectroscopy and dynamic light scattering. J. Mol. Liq. 2013, 177, 40–43. [Google Scholar] [CrossRef]
- Rucins, M.; Pajuste, K.; Sobolev, A.; Plotniece, M.; Pikun, N.; Pajuste, K.; Plotniece, A. Data for the synthesis and characterisation of 2,6-di(bromomethyl)-3,5-bis(alkoxycarbonyl)-4-aryl-1,4-dihydropyridines as important intermediates for synthesis of amphiphilic 1,4-dihydropyridines. Data Br. 2020, 30, 105532. [Google Scholar] [CrossRef]
- Leshchiner, I.; Agina, E.; Boiko, N.; Richardson, R.M.; Edler, K.J.; Shibaev, V.P. Liquid crystal codendrimers with a statistical distribution of phenolic and mesogenic groups: Behavior as langmuir and langmuir-Blodgett films. Langmuir 2008, 24, 11082–11088. [Google Scholar] [CrossRef] [PubMed]
- Jurak, M.; Szafran, K.; Cea, P.; Martín, S. Analysis of molecular interactions between components in phospholipid-immunosuppressant-antioxidant mixed Langmuir films. Langmuir 2021, 37, 5601–5616. [Google Scholar] [CrossRef]
- Charcosset, C.; Juban, A.; Valour, J.P.; Urbaniak, S.; Fessi, H. Preparation of liposomes at large scale using the ethanol injection method: Effect of scale-up and injection devices. Chem. Eng. Res. Des. 2015, 94, 508–515. [Google Scholar] [CrossRef]
- Fan, Y.; Marioli, M.; Zhang, K. Analytical characterization of liposomes and other lipid nanoparticles for drug delivery. J. Pharm. Biomed. Anal. 2021, 192, 113642. [Google Scholar] [CrossRef] [PubMed]
- Freitas, C.; Müller, R.H. Effect of light and temperature on zeta potential and physical stability in solid lipid nanoparticle (SLN®) dispersions. Int. J. Pharm. 1998, 168, 221–229. [Google Scholar] [CrossRef]
- Perinelli, D.R.; Cespi, M.; Lorusso, N.; Palmieri, G.F.; Bonacucina, G.; Blasi, P. Surfactant self-assembling and critical micelle concentration: One approach fits All? Langmuir 2020, 36, 5745–5753. [Google Scholar] [CrossRef] [PubMed]
- Szutkowski, K.; Kołodziejska, Z.; Pietralik, Z.; Zhukov, I.; Skrzypczak, A.; Materna, K.; Kozak, M. Clear distinction between CAC and CMC revealed by high-resolution NMR diffusometry for a series of bis-imidazolium gemini surfactants in aqueous solutions. RSC Adv. 2018, 8, 38470–38482. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Felice, B.; Prabhakaran, M.P.; Rodríguez, A.P.; Ramakrishna, S. Drug delivery vehicles on a nano-engineering perspective. Mater. Sci. Eng. C 2014, 41, 178–195. [Google Scholar] [CrossRef]
- Gindy, M.E.; Feuston, B.; Glass, A.; Arrington, L.; Haas, R.M.; Schariter, J.; Stirdivant, S.M. Stabilization of ostwald ripening in low molecular weight amino lipid nanoparticles for systemic delivery of siRNA Therapeutics. Mol. Pharm. 2014, 11, 4143–4153. [Google Scholar] [CrossRef]
- Jakubek, Z.J.; Chen, S.; Zaifman, J.; Tam, Y.Y.C.; Zou, S. Lipid nanoparticle and liposome reference materials: Assessment of size homogeneity and long-term −70 °C and 4 °C Storage Stability. Langmuir 2023, 39, 2509–2519. [Google Scholar] [CrossRef]
- Jaafar-Maalej, C.; Diab, R.; Andrieu, V.; Elaissari, A.; Fessi, H. Ethanol injection method for hydrophilic and lipophilic drug-loaded liposome preparation. J. Liposome Res. 2010, 20, 228–243. [Google Scholar] [CrossRef]
- Šturm, L.; Ulrih, N.P. Basic methods for preparation of liposomes and studying their interactions with different compounds, with the emphasis on polyphenols. Int. J. Mol. Sci. 2021, 22, 6547. [Google Scholar] [CrossRef]
- Lombardo, D.; Kiselev, M.A. Methods of liposomes preparation: Formation and control factors of versatile nanocarriers for biomedical and nanomedicine application. Pharmaceutics 2022, 14, 543. [Google Scholar] [CrossRef]


| Entry | Comp. | Cs−1, mN/m | P, mN/m | MMA, Å2 |
|---|---|---|---|---|
| 1 | 1 | 169.64 ± 2.05 | 24.61 ± 0.71 | 96.05 ± 4.69 |
| 2 | 5a * | 211.94 ± 20.35 | 37.93 ± 1.44 | 138.11 ± 3.57 |
| 3 | 5b | 154.67 ± 1.88 | 27.74 ± 0.63 | 132.58 ± 4.90 |
| 4 | 5c | 201.03 ± 33.56 | 35.61 ± 1.22 | 131.15 ± 7.15 |
| 5 | 5d * | 167.73 ± 0.42 | 36.05 ± 1.19 | 139.06 ± 1.94 |
| 6 | 5e # | 146.16 ± 20.59 | 36.02 ± 0.54 | 191.26 ± 8.21 |
| 102.16 ± 25.46 | 44.05 ± 1.36 | |||
| 142.22 ± 7.45 | 54.94 ± 1.33 |
| Entry | Value | Comp. 1 | Comp. 5a | Comp. 5b | Comp. 5c | Comp. 5d | Comp. 5e |
|---|---|---|---|---|---|---|---|
| 1 | Zpot, mV | 75.4 ± 7.5 | 40.6 ± 0.9 | 89.0 ± 1.1 | 56.8 ± 0.4 | 35.7 ± 2.5 | 90.9 ± 9.1 |
| 2 | CAC, µM | 1.06 | 0.46 | 1.16 | 0.01 | 0.95 | 2.65 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Krapivina, A.; Lacis, D.; Rucins, M.; Plotniece, M.; Pajuste, K.; Sobolev, A.; Plotniece, A. Synthesis and Characterization of Novel Amphiphilic N-Benzyl 1,4-Dihydropyridine Derivatives—Evaluation of Lipid Monolayer and Self-Assembling Properties. Materials 2023, 16, 4206. https://doi.org/10.3390/ma16124206
Krapivina A, Lacis D, Rucins M, Plotniece M, Pajuste K, Sobolev A, Plotniece A. Synthesis and Characterization of Novel Amphiphilic N-Benzyl 1,4-Dihydropyridine Derivatives—Evaluation of Lipid Monolayer and Self-Assembling Properties. Materials. 2023; 16(12):4206. https://doi.org/10.3390/ma16124206
Chicago/Turabian StyleKrapivina, Anna, Davis Lacis, Martins Rucins, Mara Plotniece, Karlis Pajuste, Arkadij Sobolev, and Aiva Plotniece. 2023. "Synthesis and Characterization of Novel Amphiphilic N-Benzyl 1,4-Dihydropyridine Derivatives—Evaluation of Lipid Monolayer and Self-Assembling Properties" Materials 16, no. 12: 4206. https://doi.org/10.3390/ma16124206





