New Water-Soluble (Iminomethyl)benzenesulfonates Derived from Biogenic Amines for Potential Biological Applications
Abstract
:1. Introduction
2. Experimental
2.1. Reagents and Apparatus
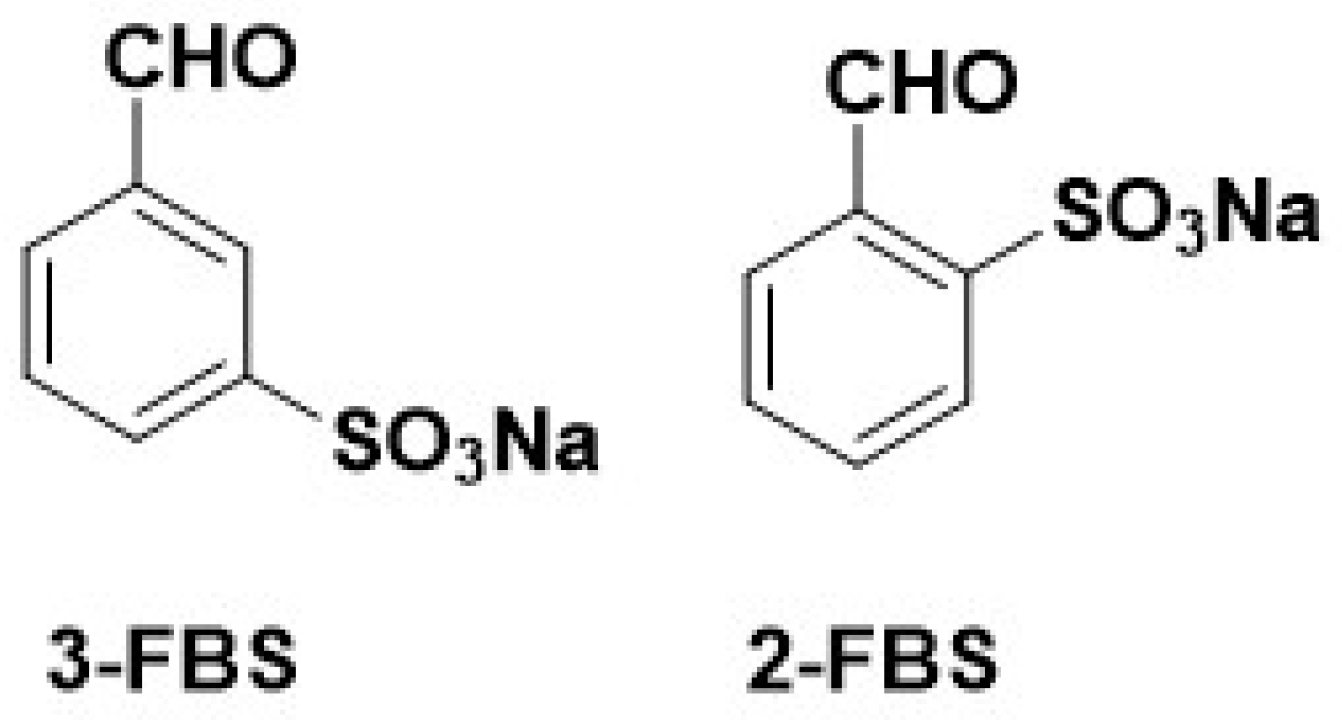
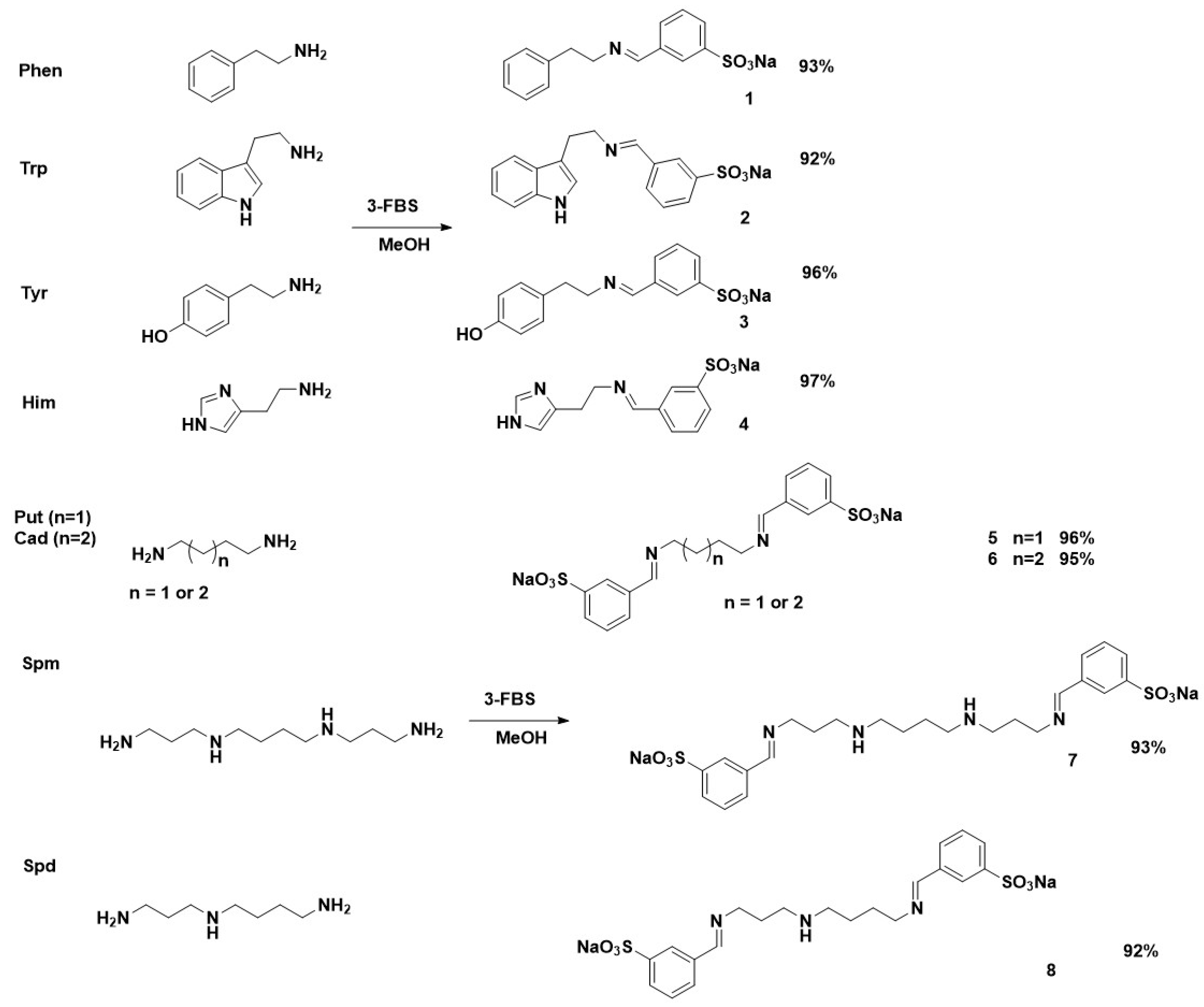
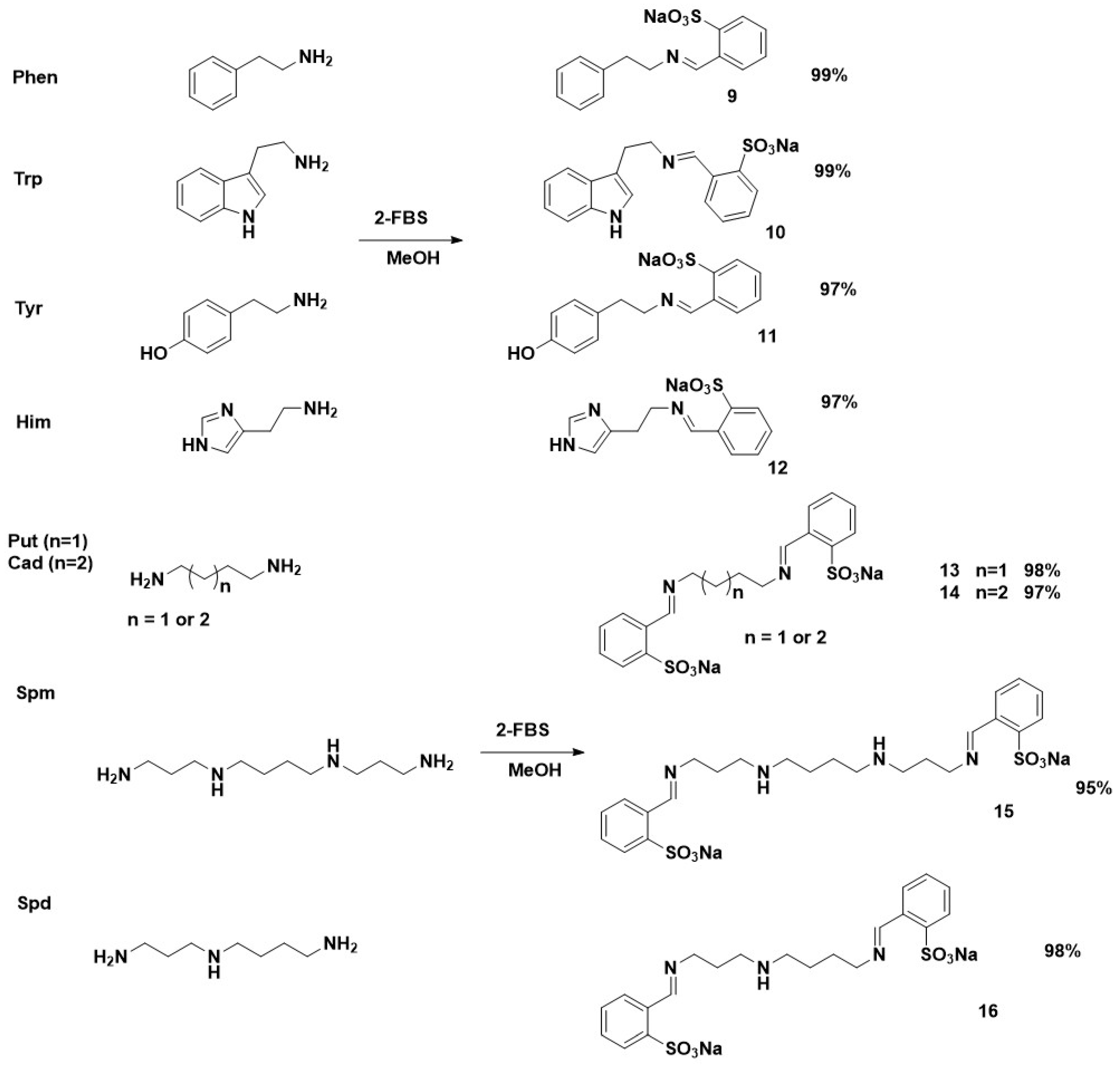
2.2. Synthesis of Imines Derived from Sodium 2- or 3-Formylbenzene Sulfonate and Biogenic Amine—General Procedure
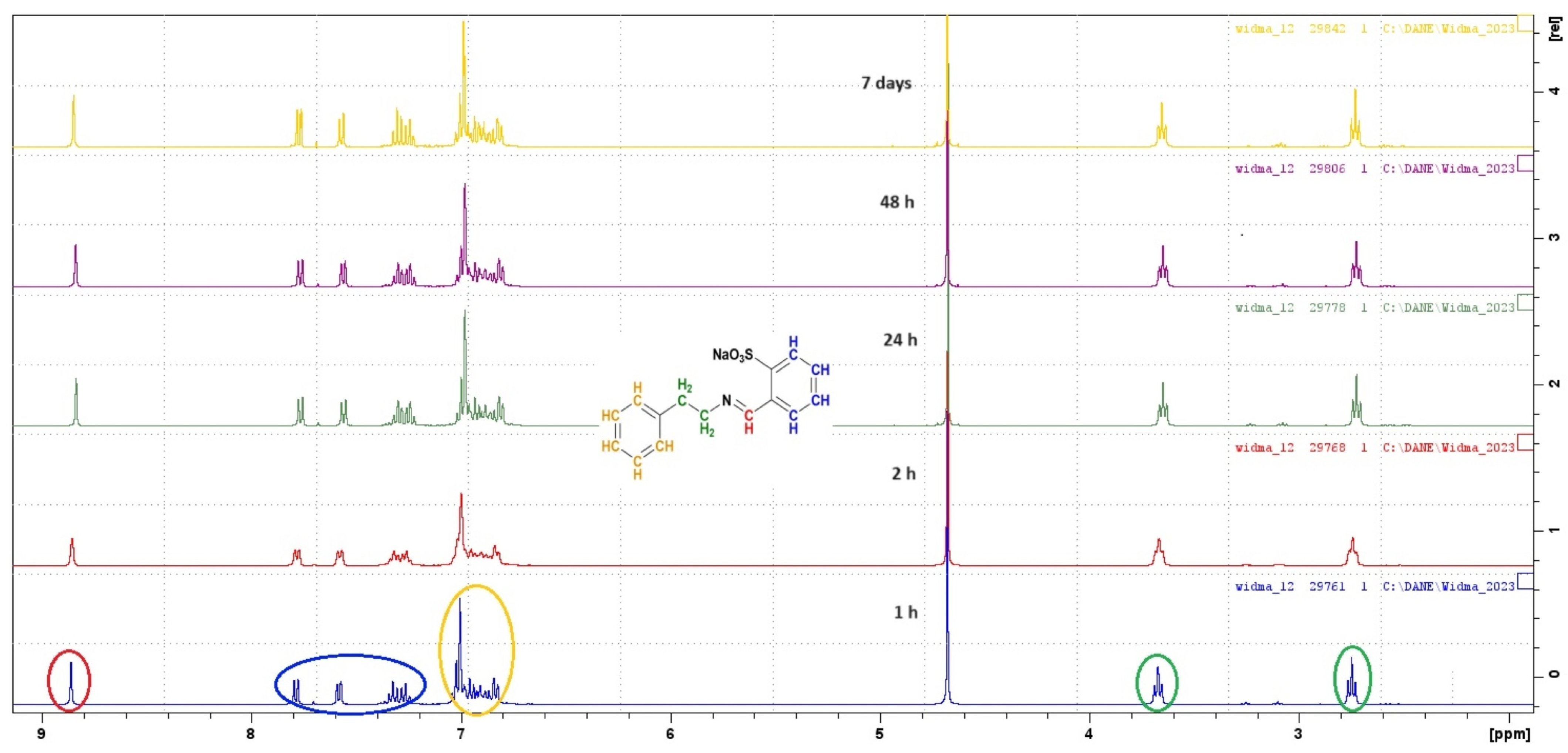
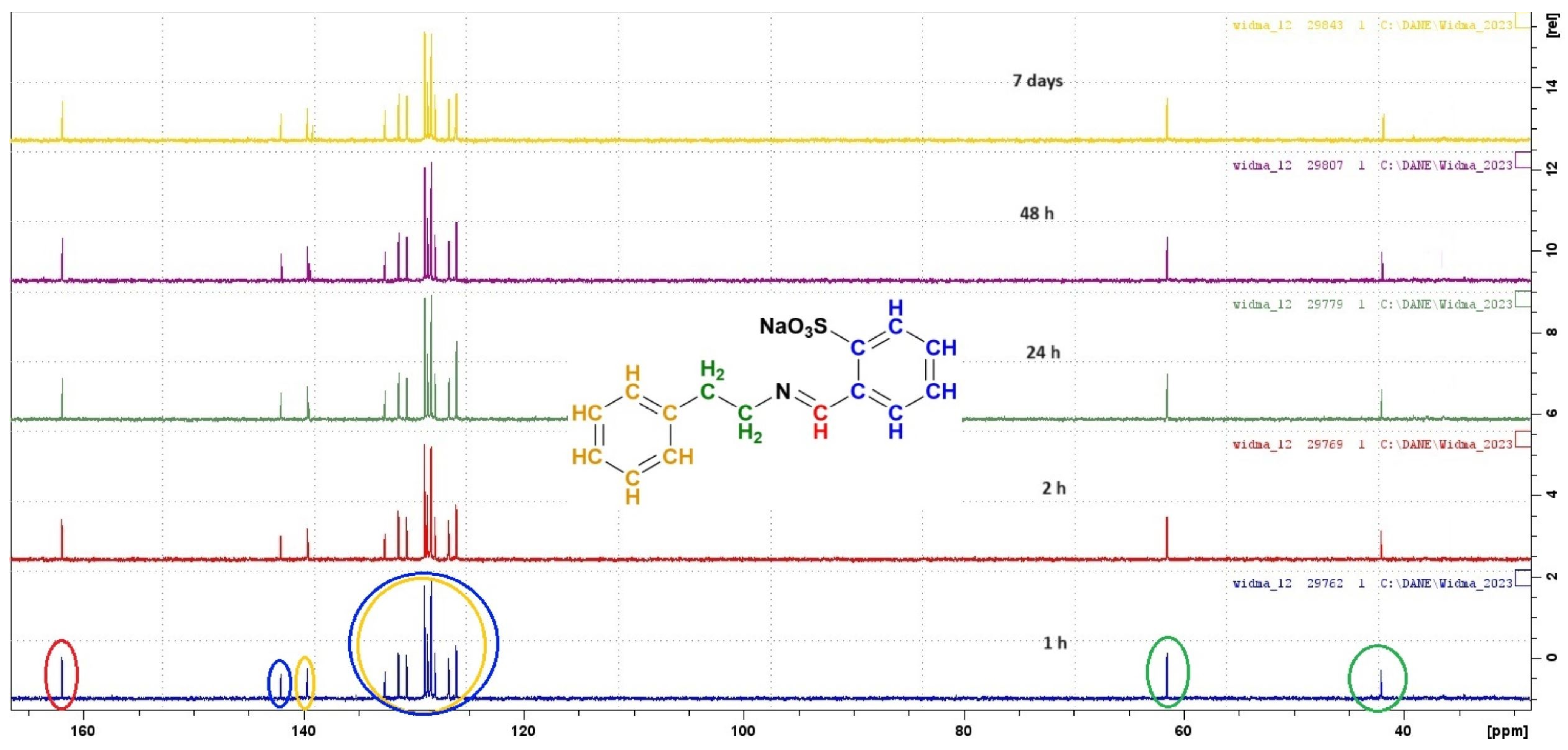
2.3. Stability of Imines in Aqueous Solution
2.4. Antimicrobial Activity of Tested Compounds
2.5. ABTS Assay of Tested Compounds
3. Results and Discussion
3.1. Structural Characteristics of the Imines Obtained
3.2. Stability in Water
3.3. Antimicrobial Activity
3.4. Antioxidant Activity (AA)
4. Future Prospects and Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Patil, R.D.; Adimurthy, S. Catalytic methods for imine synthesis. Asian J. Org. Chem. 2013, 2, 726–744. [Google Scholar] [CrossRef]
- Sun, D.; Ye, L.; Li, Z. Visible-light-assisted aerobic photocatalytic oxidation of amines to imines over NH2-MIL-125(Ti). Appl. Catal. B Environ. 2015, 64, 428–432. [Google Scholar] [CrossRef]
- Venu, B.; Shirisha, V.; Vishali, B.; Naresh, G.; Kishore, R.; Sreedhar, I.; Venugopal, A. A Cu-BTC metal-organic framework (MOF) as an efficient heterogeneous catalyst for the aerobic oxidative synthesis of imines from primary amines under solvent free conditions. New J. Chem. 2020, 44, 5972–5979. [Google Scholar] [CrossRef]
- Bermejo-López, A.; Carrasco, S.; Tortajada, P.J.; Kopf, K.P.M.; Sanz-Marco, A.; Hvid, M.S.; Lock, N.; Martín-Matute, B. Selective synthesis of imines by photo-oxidative amine cross-condensation catalyzed by PCN-222 (Pd). ACS Sustain. Chem. Eng. 2021, 9, 14405–14415. [Google Scholar] [CrossRef]
- Zhu, J.; Xiong, Y.; Mu, X.; Wan, J.; Li, T.; Jin, Y.; Li, R. Cobalt-Doped g-C3N4 Nanosheets for one-pot synthesis of imines under mild conditions. ACS Appl. Nano Mater. 2023, 6, 5127–5135. [Google Scholar] [CrossRef]
- Tran, C.C.; Kawaguchi, S.; Kobiki, Y.; Matsubara, H.; Tran, D.P.; Kodama, S.; Nomoto, A.; Ogawa, A. Palladium-catalyzed diarylation of isocyanides with tetraarylleads for the selective synthesis of imines and α-diimines. J. Org. Chem. 2019, 84, 11741–11751. [Google Scholar] [CrossRef]
- Iwanejko, J.; Wojaczyńska, E. Cyclic imines—Preparation and application in synthesis. Org. Biomol. Chem. 2018, 16, 7296–7314. [Google Scholar] [CrossRef]
- Pathan, I.R.; Patel, M.K. A comprehensive review on the synthesis and applications of Schiff base ligand and metal complexes: A comparative study of conventional heating, microwave heating, and sonochemical methods. Inorg. Chem. Commun. 2023, 158, 111464. [Google Scholar] [CrossRef]
- Bajema, E.; Roberts, K.; Meade, T. Cobalt-Schiff Base Complexes: Preclinical Research and Potential Therapeutic Uses. In Metal Ions in Life Sciences; De Gruyter GmbH: Berlin, Germany, 2019; pp. 267–301. [Google Scholar] [CrossRef]
- Nagar, S.; Raizada, S.; Tripathee, N. A review on various green methods for synthesis of Schiff base ligands and their metal complexes. Results Chem. 2023, 6, 101153. [Google Scholar] [CrossRef]
- de Marco, B.A.; Rechelo, B.S.; Tótoli, E.G.; Kogawa, A.C.; Salgado, H.R.N. Evolution of green chemistry and its multidimensional impacts: A review. Saudi Pharm. J. 2019, 27, 1–8. [Google Scholar] [CrossRef]
- Rubab, L.; Anum, A.; Al-Hussain, S.A.; Irfan, A.; Ahmad, S.; Ullah, S.; Al-Mutairi, A.A.; Zaki, M.E.A. Green chemistry in organic synthesis: Recent update on green catalytic approaches in synthesis of 1,2,4-thiadiazoles. Catalysts 2022, 12, 1329. [Google Scholar] [CrossRef]
- Prat, D.; Hayler, J.; Wells, A. A survey of solvent selection guides. Green Chem. 2014, 16, 4546–4551. [Google Scholar] [CrossRef]
- Ganesh, K.N.; Zhang, D.; Miller, S.J.; Rossen, K.; Chirik, P.J.; Kozlowski, M.C.; Zimmerman, J.B.; Brooks, B.W.; Savage, P.E.; Allen, D.T.; et al. Green chemistry: A framework for a sustainable future. Org. Process Res. Dev. 2021, 25, 1455–1459. [Google Scholar] [CrossRef]
- Fang, J.; Zhai, F.; Guo, X.; Xu, H.; Okamoto, K. A facile approach for the preparation of cross-linked sulfonated polyimide membranes for fuel cell application. J. Mater. Chem. 2007, 17, 1102–1108. [Google Scholar] [CrossRef]
- Rabiej-Kozioł, D.; Krzemiński, M.P.; Szydłowska-Czerniak, A. Synthesis of steryl hydroxycinnamates to enhance antioxidant activity of rapeseed oil and emulsions. Materials 2020, 13, 4536. [Google Scholar] [CrossRef] [PubMed]
- Dash, A.C.; Dash, B.; Praharaj, S. Hydrolysis of imines: Kinetics and mechanism of spontaneous acid-, base-, and metal ion-induced hydrolysis of N-salicylidene-2-aminothiazole. J. Chem. Soc. Dalton Trans. 1981, 2063–2069. [Google Scholar] [CrossRef]
- Saggiomo, V.; Lüning, U. Remarkable Stability of Imino Macrocycles in Water. Eur. J. Org. Chem. 2008, 2008, 4329–4333. [Google Scholar] [CrossRef]
- Ceramella, J.; Iacopetta, D.; Catalano, A.; Cirillo, F.; Lappano, R.; Sinicropi, M.S. A Review on the antimicrobial activity of Schiff bases: Data collection and recent studies. Antibiotics 2022, 11, 191. [Google Scholar] [CrossRef]
- Inclán, M.; Torres Hernández, N.; Martínez Serra, A.; Torrijos Jabón, G.; Blasco, S.; Andreu, C.; del Olmo, M.l.; Jávega, B.; O’Connor, J.-E.; García-España, E. Antimicrobial properties of new polyamines conjugated with oxygen-containing aromatic functional groups. Molecules 2023, 28, 7678. [Google Scholar] [CrossRef]
- Douglas, E.J.A.; Alkhzem, A.H.; Wonfor, T.; Li, S.; Woodman, T.J.; Blagbrough, I.S.; Laabei, M. Antibacterial activity of novel linear polyamines against Staphylococcus aureus. Front. Microbiol. 2022, 13, 948343. [Google Scholar] [CrossRef]
- Muchaamba, F.; Stephan, R.; Tasara, T. β-phenylethylamine as a natural food additive shows antimicrobial activity against listeria monocytogenes on ready-to-eat foods. Foods 2020, 9, 1363. [Google Scholar] [CrossRef] [PubMed]
- Hettiarachchi, S.S.; Perera, Y.; Dunuweera, S.P.; Dunuweera, A.N.; Rajapakse, S.; Rajapakse, R.M.G. Comparison of antibacterial activity of nanocurcumin with bulk curcumin. ACS Omega 2022, 7, 46494–46500. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Tang, J.; Tang, X.; Wang, C.; Lian, Y.; Shao, Z.; Yao, X.; Gao, H. New phenethylamine derivatives from Arenibacter nanhaiticus sp. nov. NH36AT and their antimicrobial activity. J. Antibiot. 2013, 66, 655–661. [Google Scholar] [CrossRef] [PubMed]
- Kwon, D.H.; Lu, C.D. Polyamine effects on antibiotic susceptibility in bacteria. Antimicrob. Agents Chemother. 2007, 51, 2070–2077. [Google Scholar] [CrossRef] [PubMed]
- Bacci, A.; Runfola, M.; Sestito, S.; Rapposelli, S. Beyond antioxidant effects: Nature-based templates unveil new strategies for neurodegenerative diseases. Antioxidants 2021, 10, 367. [Google Scholar] [CrossRef] [PubMed]
- Hirano, R.; Shirasawa, H.; Kurihara, S. Health-promoting effects of dietary polyamines. Med. Sci. 2021, 9, 8. [Google Scholar] [CrossRef] [PubMed]
- Melchiorre, C.; Bolognesi, M.L.; Minarini, A.; Rosini, M.; Tumiatti, V. Polyamines in drug discovery: From the universal template approach to the multitarget-directed ligand design strategy. J. Med. Chem. 2010, 53, 5906–5914. [Google Scholar] [CrossRef] [PubMed]
- Vrijsen, S.; Houdou, M.; Cascalho, A.; Eggermont, J.; Vangheluwe, P. Polyamines in Parkinson’s disease: Balancing between neurotoxicity and neuroprotection. Annu. Rev. Biochem. 2023, 92, 435–464. [Google Scholar] [CrossRef]
- Lenis, Y.; Elmetwally, M.; Maldonado-Estrada, J.; Bazer, F. Physiological importance of polyamines. Zygote 2017, 25, 244–255. [Google Scholar] [CrossRef]
- Jastrząb, R.; Kaczmarek, M.T.; Nowak, M.; Trojanowska, A.; Zabiszak, M. Complexes of polyamines and their derivatives as living system active compounds. Coord. Chem. Rev. 2017, 351, 32–44. [Google Scholar] [CrossRef]
- Khan, M.A.R.; Habib, M.A.; Naime, J.; Rumon, M.M.H.; Mamun, M.S.A.; Islam, A.B.M.N.; Mahiuddin, M.; Karim, K.M.R.; Ara, M.H. A review on synthesis, characterizations, and applications of Schiff base functionalized nanoparticles. Results Chem. 2023, 6, 101160. [Google Scholar] [CrossRef]
- Burmistrov, V.; Batrakova, A.; Aleksandriiskii, V.; Novikov, I.; Belov, K.; Khodov, I.; Koifman, O. Conformational and Supramolecular Aspects in Chirality of Flexible Camphor-Containing Schiff Base as an Inducer of Helical Liquid Crystals. Molecules 2023, 28, 2388. [Google Scholar] [CrossRef]
- Romanowski, G.; Budka, J.; Inkielewicz-Stepniak, I. Synthesis, Spectroscopic Characterization, Catalytic and Biological Activity of Oxidovanadium(V) Complexes with Chiral Tetradentate Schiff Bases. Molecules 2023, 28, 7408. [Google Scholar] [CrossRef]
- Singh, P.; Sodhi, K.K.; Tomer, A.; Mehta, S.B. Advancement in the synthesis of metal complexes with special emphasis on Schiff base ligands and their important biological aspects. Results Chem. 2024, 7, 101222. [Google Scholar] [CrossRef]
- Khalaf, M.M.; Abd El-Lateef, H.M.; Gouda, M.; Sayed, F.N.; Mohamed, G.G.; Abu-Dief, A.M. Design, Structural Inspection and Bio-Medicinal Applications of Some Novel Imine Metal Complexes Based on Acetylferrocene. Materials 2022, 15, 4842. [Google Scholar] [CrossRef]

| Phen | 1 | 9 | Spd | 8 | 16 | Spm | 7 | 15 | CFT | SPT | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| S. aureus | 11.47 A ±0.37 | 11.42 A ±0.58 | 11.29 A ±0.58 | 20.79 B ±0.58 | 16.51 A ±0.92 | 16.05 A ±0.65 | 23.62 B ±1.63 | 17.56 A ±1.81 | 16.91 A ±1.48 | 22.96 ±0.63 | 22.27 ±0.32 |
| E. coli | 12.32 A ±0.42 | 12.25 A ±0.36 | 12.08 A ±0.65 | - | - | - | 22.10 B ±2.18 | 9.22 A ±0.64 | 8.76 A ±0.52 | 21.86 ±0.40 | 22.15 ±1.10 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kmieciak, A.; Krzemiński, M.P.; Hodii, A.; Gorczyca, D.; Jastrzębska, A. New Water-Soluble (Iminomethyl)benzenesulfonates Derived from Biogenic Amines for Potential Biological Applications. Materials 2024, 17, 520. https://doi.org/10.3390/ma17020520
Kmieciak A, Krzemiński MP, Hodii A, Gorczyca D, Jastrzębska A. New Water-Soluble (Iminomethyl)benzenesulfonates Derived from Biogenic Amines for Potential Biological Applications. Materials. 2024; 17(2):520. https://doi.org/10.3390/ma17020520
Chicago/Turabian StyleKmieciak, Anna, Marek P. Krzemiński, Anastasiia Hodii, Damian Gorczyca, and Aneta Jastrzębska. 2024. "New Water-Soluble (Iminomethyl)benzenesulfonates Derived from Biogenic Amines for Potential Biological Applications" Materials 17, no. 2: 520. https://doi.org/10.3390/ma17020520





