“Sweetwoods” Lignin as Promising Raw Material to Obtain Micro-Mesoporous Carbon Materials
Abstract
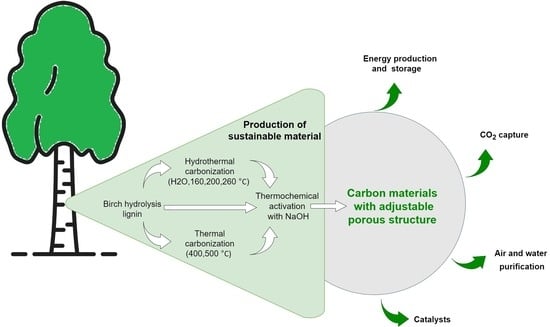
:1. Introduction
2. Materials and Methods
2.1. Materials and Their Carbonization
2.2. Analytical Methods Applied for the Materials Study
3. Results
3.1. Chemical Properties of SW Lignin before and after Hydrothermal Carbonization and Pyrolysis
3.2. The Porous Structure of Activated Carbons Depending on the Method of Carbonization
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Li, H.; Yuan, D.; Tang, C.; Wang, S.; Sun, J.; Li, Z.; Tang, T.; Wang, F.; Gong, H.; He, C. Lignin-Derived Interconnected Hierarchical Porous Carbon Monolith with Large Areal/Volumetric Capacitances for Supercapacitor. Carbon 2016, 100, 151–157. [Google Scholar] [CrossRef]
- Mohamad Nor, N.; Lau, L.C.; Lee, K.T.; Mohamed, A.R. Synthesis of Activated Carbon from Lignocellulosic Biomass and Its Applications in Air Pollution Control—A Review. J. Environ. Chem. Eng. 2013, 1, 658–666. [Google Scholar] [CrossRef]
- Khan, T.A.; Saud, A.S.; Jamari, S.S.; Rahim, M.H.A.; Park, J.W.; Kim, H.J. Hydrothermal Carbonization of Lignocellulosic Biomass for Carbon Rich Material Preparation: A Review. Biomass Bioenergy 2019, 130, 105384. [Google Scholar] [CrossRef]
- Pérez, E.; Tuck, C.O. Quantitative Analysis of Products from Lignin Depolymerisation in High-Temperature Water. Eur. Polym. J. 2018, 99, 38–48. [Google Scholar] [CrossRef]
- Dobele, G.; Dizhbite, T.; Gil, M.V.; Volperts, A.; Centeno, T.A. Production of Nanoporous Carbons from Wood Processing Wastes and Their Use in Supercapacitors and CO2 Capture. Biomass Bioenergy 2012, 46, 145–154. [Google Scholar] [CrossRef]
- Plavniece, A.; Volperts, A.; Dobele, G.; Zhurinsh, A.; Kaare, K.; Kruusenberg, I.; Kaprans, K.; Knoks, A.; Kleperis, J. Wood and Black Liquor-Based N-Doped Activated Carbon for Energy Application. Sustainability 2021, 13, 9237. [Google Scholar] [CrossRef]
- Schlee, P.; Hosseinaei, O.; Baker, D.; Landmér, A.; Tomani, P.; Mostazo-López, M.J.; Cazorla-Amorós, D.; Herou, S.; Titirici, M.M. From Waste to Wealth: From Kraft Lignin to Free-Standing Supercapacitors. Carbon 2019, 145, 470–480. [Google Scholar] [CrossRef]
- Antolini, E. Nitrogen-Doped Carbons by Sustainable N- and C-Containing Natural Resources as Nonprecious Catalysts and Catalyst Supports for Low Temperature Fuel Cells. Renew. Sustain. Energy Rev. 2016, 58, 34–51. [Google Scholar] [CrossRef]
- Espinoza-Acosta, J.L.; Torres-Chávez, P.I.; Olmedo-Martínez, J.L.; Vega-Rios, A.; Flores-Gallardo, S.; Zaragoza-Contreras, E.A. Lignin in Storage and Renewable Energy Applications: A Review. J. Energy Chem. 2018, 27, 1422–1438. [Google Scholar] [CrossRef]
- Xi, R.; Li, Y.; Zhang, Y.; Wang, P.; Hu, D. Effects of Activation Method on Biomass Carbon-Based Materials Used for Electrochemical Hydrogen Evolution Reaction Catalyst. Int. J. Hydrogen Energy 2023, in press. [Google Scholar] [CrossRef]
- Dessalle, A.; Quílez-Bermejo, J.; Fierro, V.; Xu, F.; Celzard, A. Recent Progress in the Development of Efficient Biomass-Based ORR Electrocatalysts. Carbon 2023, 203, 237–260. [Google Scholar] [CrossRef]
- Zhang, M.; Zhang, J.; Ran, S.; Sun, W.; Zhu, Z. Biomass-Derived Sustainable Carbon Materials in Energy Conversion and Storage Applications: Status and Opportunities. A Mini Review. Electrochem. Commun. 2022, 138, 107283. [Google Scholar] [CrossRef]
- Feng, Y.; Jiang, J.; Xu, Y.; Wang, S.; An, W.; Chai, Q.; Prova, U.H.; Wang, C.; Huang, G. Biomass Derived Diverse Carbon Nanostructure for Electrocatalysis, Energy Conversion and Storage. Carbon 2023, 211, 118105. [Google Scholar] [CrossRef]
- Fierro, V.; Torné-Fernández, V.; Celzard, A. Methodical Study of the Chemical Activation of Kraft Lignin with KOH and NaOH. Microporous Mesoporous Mater. 2007, 101, 419–431. [Google Scholar] [CrossRef]
- Hayashi, J.; Kazehaya, A.; Muroyama, K.; Watkinson, A.P. Preparation of Activated Carbon from Lignin by Chemical Activation. Carbon 2000, 38, 1873–1878. [Google Scholar] [CrossRef]
- García-Mateos, F.J.; Ruiz-Rosas, R.; Rosas, J.M.; Rodríguez-Mirasol, J.; Cordero, T. Controlling the Composition, Morphology, Porosity, and Surface Chemistry of Lignin-Based Electrospun Carbon Materials. Front. Mater. 2019, 6, 114. [Google Scholar] [CrossRef]
- Salinas-Torres, D.; Ruiz-Rosas, R.; Morallón, E.; Cazorla-Amorós, D. Strategies to Enhance the Performance of Electrochemical Capacitors Based on Carbon Materials. Front. Mater. 2019, 6, 115. [Google Scholar] [CrossRef]
- Sudakova, I.G.; Levdansky, A.V.; Kuznetsov, B.N. Methods of Chemical and Thermochemical Processing of Hydrolytic Lignin. J. Sib. Fed. Univ. Chem. 2021, 14, 263–275. [Google Scholar] [CrossRef]
- Dharmaraja, J.; Shobana, S.; Arvindnarayan, S.; Francis, R.R.; Jeyakumar, R.B.; Saratale, R.G.; Ashokkumar, V.; Bhatia, S.K.; Kumar, V.; Kumar, G. Lignocellulosic Biomass Conversion via Greener Pretreatment Methods towards Biorefinery Applications. Bioresour. Technol. 2023, 369, 128328. [Google Scholar] [CrossRef]
- Gao, N.; Li, Z.; Quan, C.; Miskolczi, N.; Egedy, A. A New Method Combining Hydrothermal Carbonization and Mechanical Compression In-Situ for Sewage Sludge Dewatering: Bench-Scale Verification. J. Anal. Appl. Pyrolysis 2019, 139, 187–195. [Google Scholar] [CrossRef]
- Kawamoto, H. Lignin Pyrolysis Reactions. J. Wood Sci. 2017, 63, 117–132. [Google Scholar] [CrossRef]
- Mankar, A.R.; Pandey, A.; Modak, A.; Pant, K.K. Pretreatment of Lignocellulosic Biomass: A Review on Recent Advances. Bioresour. Technol. 2021, 334, 125235. [Google Scholar] [CrossRef]
- Tofani, G.; Cornet, I.; Tavernier, S. Separation and Recovery of Lignin and Hydrocarbon Derivatives from Cardboard. Biomass Convers. Biorefinery 2020, 12, 3409–3424. [Google Scholar] [CrossRef]
- Project—Sweetwoods. Available online: https://sweetwoods.eu/ (accessed on 31 August 2023).
- Azadi, P.; Inderwildi, O.R.; Farnood, R.; King, D.A. Liquid Fuels, Hydrogen and Chemicals from Lignin: A Critical Review. Renew. Sustain. Energy Rev. 2013, 21, 506–523. [Google Scholar] [CrossRef]
- Sweetwater Energy Enabling the Sustainable Carbon Economy. Available online: https://fas-europe.org/wp-content/uploads/2018/07/1-Sweetwater-Energy-Business-Case.pdf (accessed on 22 August 2023).
- Plavniece, A.; Dobele, G.; Volperts, A.; Zhurinsh, A. Hydrothermal Carbonization vs. Pyrolysis: Effect on the Porosity of the Activated Carbon Materials. Sustainbility 2022, 14, 15982. [Google Scholar] [CrossRef]
- Plavniece, A.; Dobele, G.; Volperts, A.; Djachkovs, D.; Jashina, L.; Bikovens, O.; Zhurinsh, A. Effect of the Pretreatment on the Porosity of the Hybrid Activated Carbons Prepared from Wood-Based Solid and Liquid Precursors. Wood Sci. Technol. 2022, 56, 1743–1759. [Google Scholar] [CrossRef]
- Volperts, A.; Plavniece, A.; Dobele, G.; Zhurinsh, A.; Kruusenberg, I.; Kaare, K.; Locs, J.; Tamasauskaite-Tamasiunaite, L.; Norkus, E. Biomass Based Activated Carbons for Fuel Cells. Renew. Energy 2019, 141, 40–45. [Google Scholar] [CrossRef]
- Du, J.; Zhang, Y.; Wu, H.; Hou, S.; Chen, A. N-Doped Hollow Mesoporous Carbon Spheres by Improved Dissolution-Capture for Supercapacitors. Carbon 2020, 156, 523–528. [Google Scholar] [CrossRef]
- Saverettiar, G.; Li, D.; Gross, A.; Ho, G. Hydrothermal Carbonization of Cattle Paunch Waste: Process Conditions and Product Characteristics. J. Environ. Chem. Eng. 2020, 8, 104487. [Google Scholar] [CrossRef]
- Faix, O. Condensation Indices of Lignins Determined by FTIR-Spectroscopy. Holz als Roh-Werkst. 1991, 49, 356. [Google Scholar] [CrossRef]
- Tolvaj, L. Traditions, Anomalies, Mistakes and Recommendations in Infrared Spectrum Measurement for Wood. Wood Sci. Technol. 2022, 56, 1819–1834. [Google Scholar] [CrossRef]
- Latham, K.G.; Matsakas, L.; Figueira, J.; Rova, U.; Christakopoulos, P.; Jansson, S. Examination of How Variations in Lignin Properties from Kraft and Organosolv Extraction Influence the Physicochemical Characteristics of Hydrothermal Carbon. J. Anal. Appl. Pyrolysis 2021, 155, 105095. [Google Scholar] [CrossRef]
- Kim, D.Y.; Ma, C.H.; Jang, Y.; Radhakrishnan, S.; Ko, T.H.; Kim, B.S. A Simple and Green Approach to Develop Porous Carbons from Biomass Lignin for Advanced Asymmetric Supercapacitors. Colloids Surf. A Physicochem. Eng. Asp. 2022, 652, 129785. [Google Scholar] [CrossRef]
- Wang, L.; Feng, X.; Li, X.; Wang, H.; Wu, J.; Ma, H.; Zhou, J. Hydrothermal, KOH-Assisted Synthesis of Lignin-Derived Porous Carbon for Supercapacitors: Value-Added of Lignin and Constructing Texture Properties/Specific Capacitance Relationships. J. Mater. Res. Technol. 2022, 16, 570–580. [Google Scholar] [CrossRef]
- Tian, Y.; Zhou, H. A Novel Nitrogen-Doped Porous Carbon Derived from Black Liquor for Efficient Removal of Cr(VI) and Tetracycline: Comparison with Lignin Porous Carbon. J. Clean. Prod. 2022, 333, 130106. [Google Scholar] [CrossRef]
- Lim, G.H.; Lee, J.W.; Choi, J.H.; Kang, Y.C.; Roh, K.C. Efficient Utilization of Lignin Residue for Activated Carbon in Supercapacitor Applications. Mater. Chem. Phys. 2022, 284, 126073. [Google Scholar] [CrossRef]
- Gong, L.; Bao, A. High-Value Utilization of Lignin to Prepare N,O-Codoped Porous Carbon as a High-Performance Adsorbent for Carbon Dioxide Capture. J. CO2 Util. 2023, 68, 102374. [Google Scholar] [CrossRef]
- Yu, B.; Chang, Z.; Wang, C. The Key Pre-Pyrolysis in Lignin-Based Activated Carbon Preparation for High Performance Supercapacitors. Mater. Chem. Phys. 2016, 181, 187–193. [Google Scholar] [CrossRef]
- Said, B.; Bacha, O.; Rahmani, Y.; Harfouche, N.; Kheniche, H.; Zerrouki, D.; Belkhalfa, H.; Henni, A. Activated Carbon Prepared by Hydrothermal Pretreatment-Assisted Chemical Activation of Date Seeds for Supercapacitor Application. Inorg. Chem. Commun. 2023, 155, 111012. [Google Scholar] [CrossRef]
- Zhang, B.; Jiang, Y.; Balasubramanian, R. Synthesis of Biowaste-Derived Carbon Foam for CO2 Capture. Resour. Conserv. Recycl. 2022, 185, 106453. [Google Scholar] [CrossRef]
- Hu, J.; Hong, C.; Zhao, C.; Si, Y.; Xing, Y.; Ling, W.; Zhang, B.; Li, Z.; Wang, Y.; Feng, L.; et al. Nitrogen Self-Doped Hierarchical Porous Carbon via Penicillin Fermentation Residue (PR) Hydrothermal Carbonization (HTC) and Activation for Supercapacitance. J. Alloys Compd. 2022, 918, 165452. [Google Scholar] [CrossRef]





| Sample | Synthesis Conditions: Temperature and Time | Yield, % | C, % | H, % | O, % * | O/C | H/C |
|---|---|---|---|---|---|---|---|
| SW | - | - | 63.83 | 6.01 | 29.54 | 0.31 | 1.13 |
| HTC (160, 4 h) | 160, 4 h (H2O) | 88.7 | 65.68 | 5.88 | 27.87 | 0.30 | 1.07 |
| HTC (200, 4 h) | 200, 4 h (H2O) | 64.3 | 67.81 | 5.43 | 26.35 | 0.30 | 0.96 |
| HTC (260, 2 h) | 260, 2 h (H2O) | 62.0 | 68.35 | 5.29 | 25.94 | 0.31 | 0.93 |
| HTC (260, 4 h) | 260, 4 h (H2O) | 59.9 | 69.55 | 5.30 | 24.78 | 0.29 | 0.91 |
| HTC (260, 6 h) | 260, 6 h (H2O) | 59.4 | 70.12 | 5.30 | 24.09 | 0.28 | 0.91 |
| HTC (260, 8 h) | 260, 8 h (H2O) | 58.8 | 73.99 | 5.35 | 20.07 | 0.23 | 0.87 |
| PYR (400) | 400, 1 h (pyrolysis) | 51.8 | 80.65 | 3.64 | 14.72 | 0.25 | 0.54 |
| PYR (500) | 500, 1 h (pyrolysis | 45.9 | 87.10 | 3.55 | 8.36 | 0.15 | 0.49 |
| SW | HTC 160 | HTC 200 | HTC 260 | ||||
|---|---|---|---|---|---|---|---|
| 2h | 4h | 6h | 8h | ||||
| Carbohydrates, % | 7.26 | 4.23 | 3.38 | 2.06 | 2.49 | 2.50 | 2.36 |
| Acid, Ester | 2.76 | 1.78 | 1.45 | 1.45 | 1.74 | 1.75 | 1.61 |
| Aldehyde, Ketone | 2.59 | 1.21 | 0.92 | - | - | - | - |
| Cyclopentane derivates | 0.29 | 0.23 | 0.43 | 0.39 | 0.50 | 0.45 | 0.52 |
| Furan | 1.62 | 0.94 | 0.58 | 0.22 | 0.25 | 0.30 | 0.23 |
| Pyran | trace | 0.07 | - | - | - | - | - |
| Lignin derivates, % | 61.21 | 61.49 | 59.32 | 59.28 | 56.34 | 56.46 | 56.83 |
| Phenyl and benzyl derivates | 4.82 | 4.92 | 6.07 | 6.96 | 8.98 | 8.99 | 8.05 |
| Guaiacyl derivates | 17.69 | 17.93 | 16.97 | 16.01 | 15.78 | 16.22 | 16.09 |
| Syringyl derivates | 38.70 | 38.64 | 36.28 | 36.31 | 31.58 | 31.25 | 32.69 |
| Summary: Carbon dioxide, Water, Methanol, % | 28.21 | 31.06 | 32.53 | 33.97 | 35.72 | 35.38 | 35.80 |
| N-containing, % | 0.68 | 0.47 | 0.40 | 0.02 | 0.03 | 0.03 | 0.02 |
| Other compounds, % | 1.61 | 1.60 | 3.08 | 3.40 | 4.35 | 4.84 | 4.09 |
| Identified, % | 98.97 | 98.85 | 98.71 | 98.73 | 98.93 | 99.21 | 99.10 |
| Lignin | Carbonization Conditions: Temperature, Environment | Activation Conditions: Temperature, Activator (Activator to Carbon Material) | Porous Structure | Ref. | ||
|---|---|---|---|---|---|---|
| SBET, m2g−1 | Vt, cm3 g−1 | Vmeso from Vt, % | ||||
| Black liquor | 800 °C NaOH (2:1) | * 2104 | * 2.35 | * 71 | [6] | |
| Lignin (Kraft lignin, USA) | 180 °C HTC (acidic) | 800 °C KOH (1:1) | 1374 | 0.75 | 72 | [35] |
|
Lignin (Tokyo Chemical Industry Co., Ltd., Tokyo, Japan) | 100 °C 150 °C 200 °C 250 °C HTC (H2O) | 900 °C KOH (2:1) | 2448 3033 3178 2694 | 1.32 1.93 1.94 1.55 | 17.42 34.72 29.90 24.52 | [36] |
| Precipitated lignin | 800 °C KOH | * 1043 | * 0.58 | 20.69 | [37] | |
| Dealkalized lignin | 350 °C | 800 °C 600 °C KOH (2:1) | 2050 | 0.84 | 9.52 | [39] |
| 1659 | 0.71 | 11.27 | ||||
| 450 °C | ||||||
| 1493 | 0.60 | 10 | ||||
| 550 °C | ||||||
| 1407 | 0.57 | 8.77 | ||||
| Lignin (chemical plant in Shandong province, China) | - 600°C | 800 °C KOH (3:1) | * 2889 2233 | * 1.44 1.06 | 78.57 28.30 | [40] |
| Larch Klason lignin Organosolv lignin | 600°C | 900 °C | 2451 2304 2388 | 1.01 0.94 0.97 | 5.9 5.3 6.2 | [38] |
| Sample | Yield (After Carbonization and Activation), % | Specific Surface Area (BET), m2 g−1 | Pore Volume, cm3 g−1 | Average Pore Width, nm | Mesopores from Vt, % | N, % | C, % | H, % | O, % | ||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Total | Micro | Meso | |||||||||
| A-SW | 5 | 2655 | 1.7 | 0.8 | 0.9 | 2.6 | 52.8 | 0.72 | 93.98 | 1.61 | 3.69 |
| A-HTC (160, 4 h) | 5.3 | 2000 | 1.5 | 0.6 | 0.9 | 3.2 | 60.0 | 0.89 | 85.49 | 0.70 | 12.92 |
| A-HTC (200, 4 h) | 6.9 | 2485 | 1.8 | 0.8 | 1.0 | 2.9 | 56.6 | 0.66 | 90.16 | 1.31 | 7.87 |
| A-HTC (260, 2 h) | 9.1 | 2778 | 1.9 | 0.9 | 1.0 | 2.7 | 53.8 | 0.47 | 92.85 | 0.30 | 6.38 |
| A-HTC (260, 4 h) | 10.5 | 2555 | 2.1 | 0.8 | 1.3 | 3.3 | 61.5 | 1.29 | 92.23 | 0.38 | 6.11 |
| A-HTC (260, 6 h) | 10.8 | 2727 | 1.8 | 0.8 | 1.0 | 2.7 | 54.3 | 1.30 | 92.20 | 0.34 | 6.16 |
| A-HTC (260, 8 h) | 11.5 | 2286 | 1.8 | 0.7 | 1.1 | 3.1 | 60.4 | 1.19 | 89.48 | 0.82 | 8.52 |
| A-PYR (400) | 14.8 | 2729 | 1.4 | 0.9 | 0.5 | 2.1 | 38.0 | 0.58 | 91.82 | 0.26 | 7.34 |
| A-PYR (500) | 15.5 | 2674 | 1.4 | 0.9 | 0.6 | 2.2 | 39.5 | 0.73 | 94.53 | 0.28 | 4.46 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Plavniece, A.; Dobele, G.; Djachkovs, D.; Jashina, L.; Bikovens, O.; Volperts, A.; Zhurinsh, A. “Sweetwoods” Lignin as Promising Raw Material to Obtain Micro-Mesoporous Carbon Materials. Materials 2023, 16, 6024. https://doi.org/10.3390/ma16176024
Plavniece A, Dobele G, Djachkovs D, Jashina L, Bikovens O, Volperts A, Zhurinsh A. “Sweetwoods” Lignin as Promising Raw Material to Obtain Micro-Mesoporous Carbon Materials. Materials. 2023; 16(17):6024. https://doi.org/10.3390/ma16176024
Chicago/Turabian StylePlavniece, Ance, Galina Dobele, Dmitrijs Djachkovs, Lilija Jashina, Oskars Bikovens, Aleksandrs Volperts, and Aivars Zhurinsh. 2023. "“Sweetwoods” Lignin as Promising Raw Material to Obtain Micro-Mesoporous Carbon Materials" Materials 16, no. 17: 6024. https://doi.org/10.3390/ma16176024