Characterization of Magnetic Biochar Modified Using the One-Step and Electrochemical Methods and Its Impact on Phosphate Adsorption
Abstract
:1. Introduction
2. Materials and Methods
2.1. Material and Preparation of Raw Biochar
2.2. Preparation of Magnetic Biochar
2.3. Characterizations of Biochar
2.4. Phosphate Adsorption Studies
2.5. Data Analysis
3. Results and Discussion
3.1. Biochar Characterizations
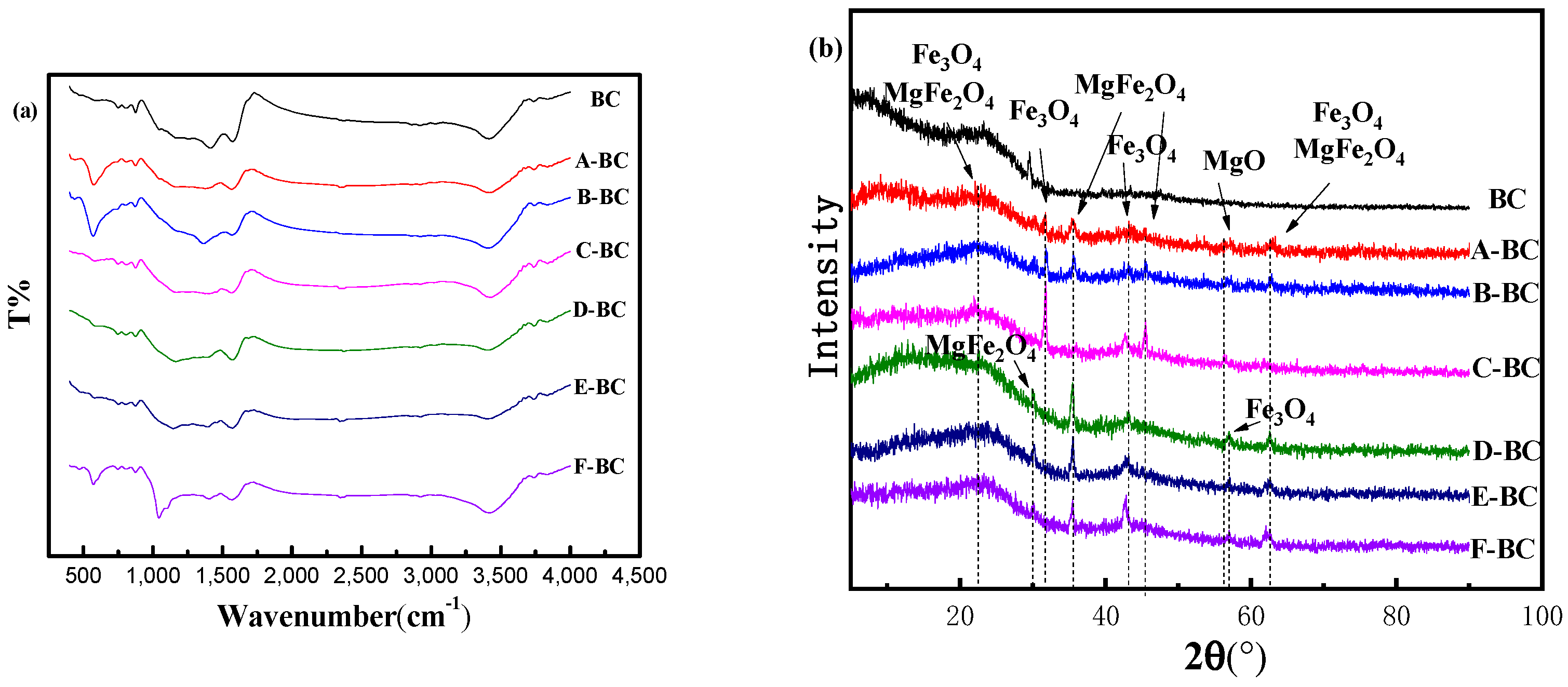
3.1.1. FTIR and XRD Analysis
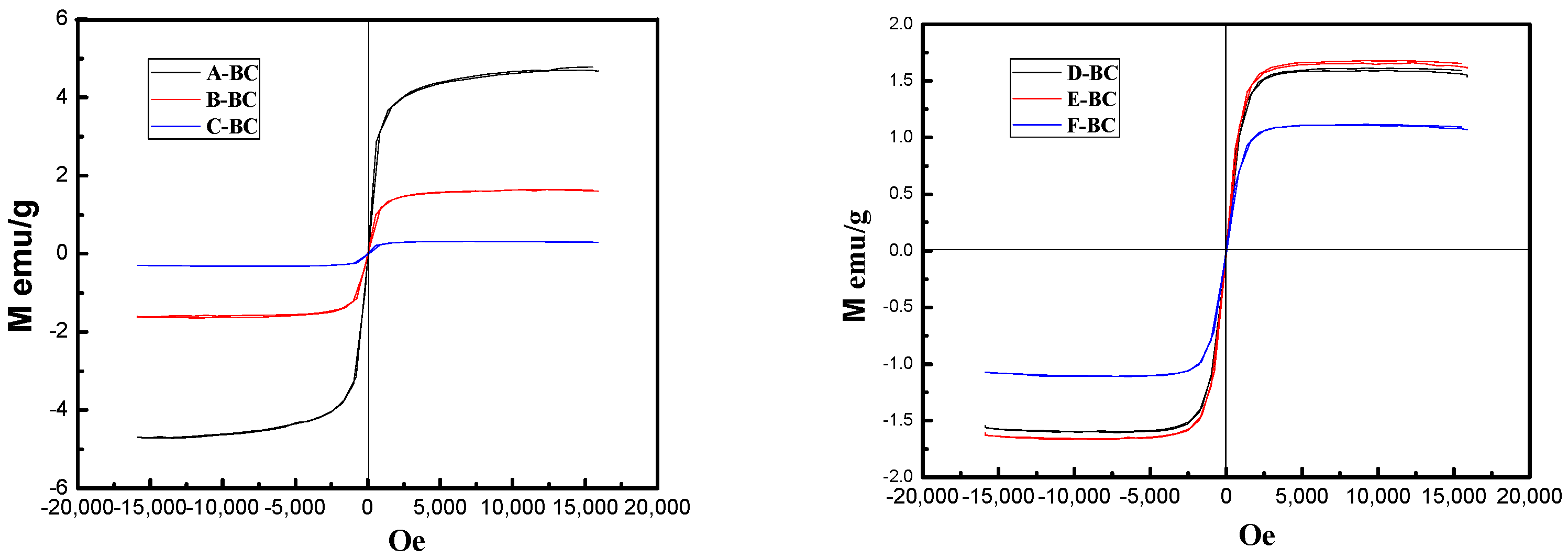
3.1.2. Hysteresis Loops
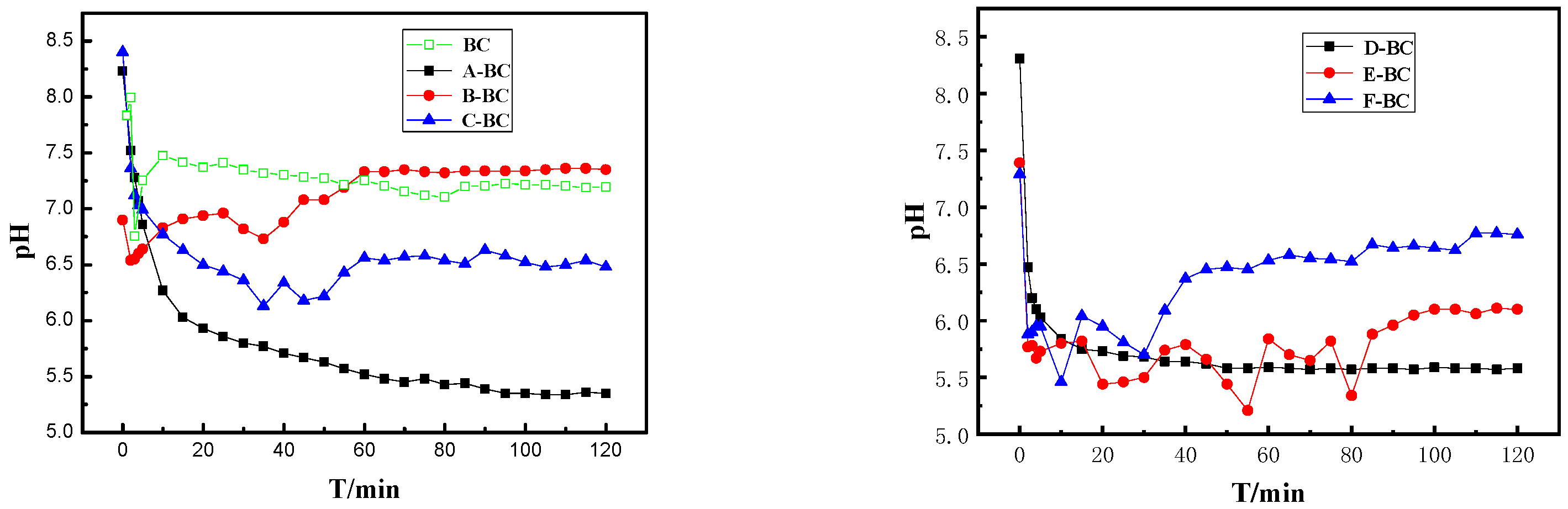
3.1.3. pHpzc and pH
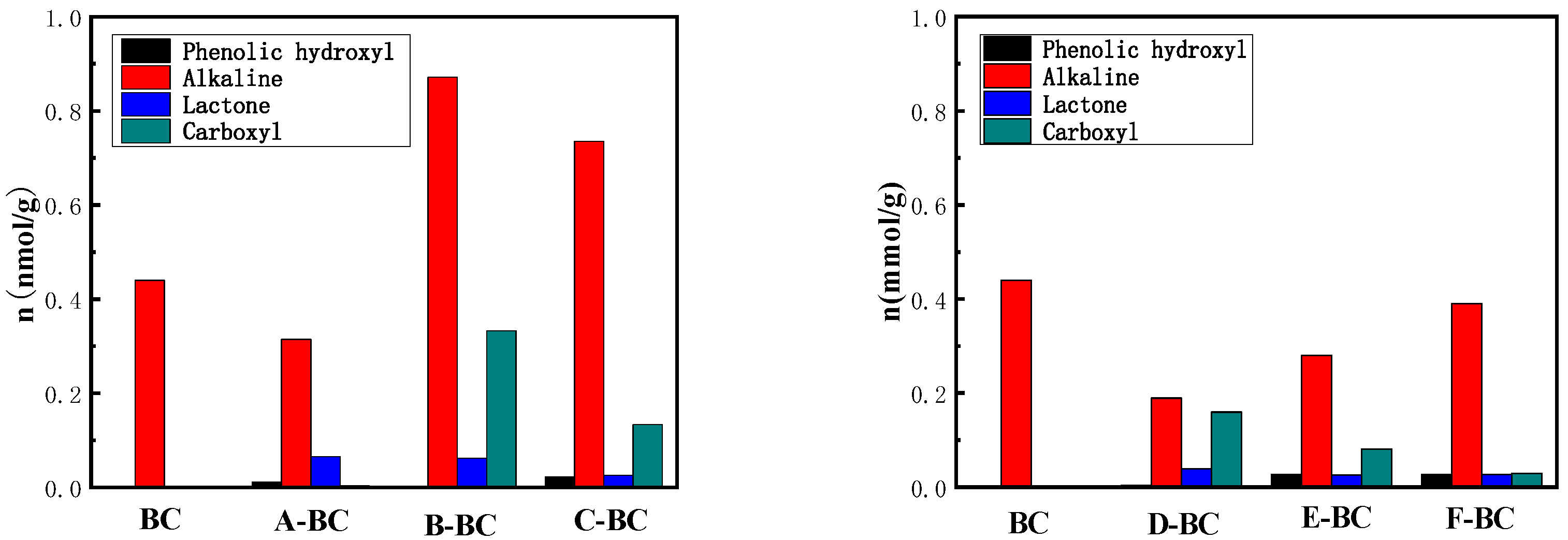
3.1.4. Boehm Titration
3.2. Adsorption of Phosphate
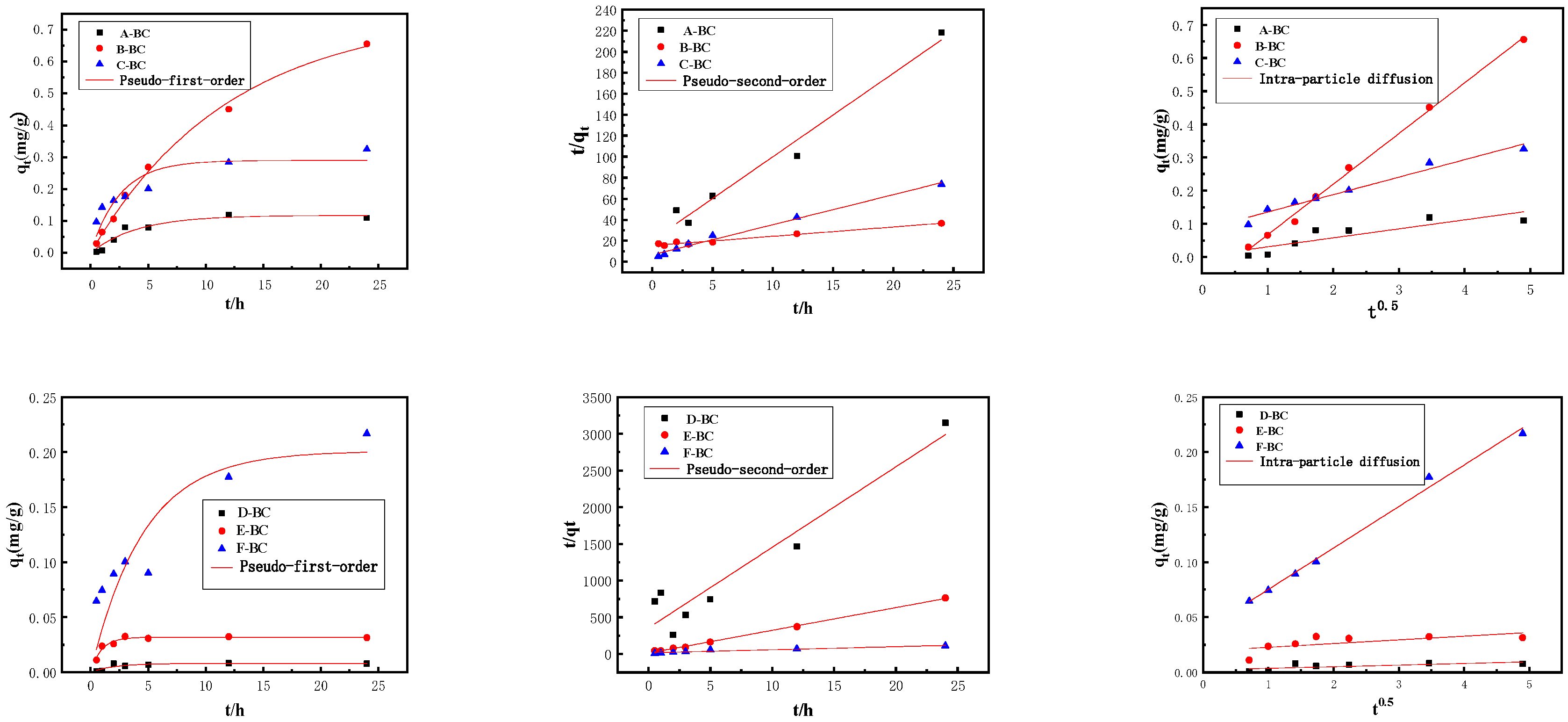
3.2.1. Adsorption Kinetics
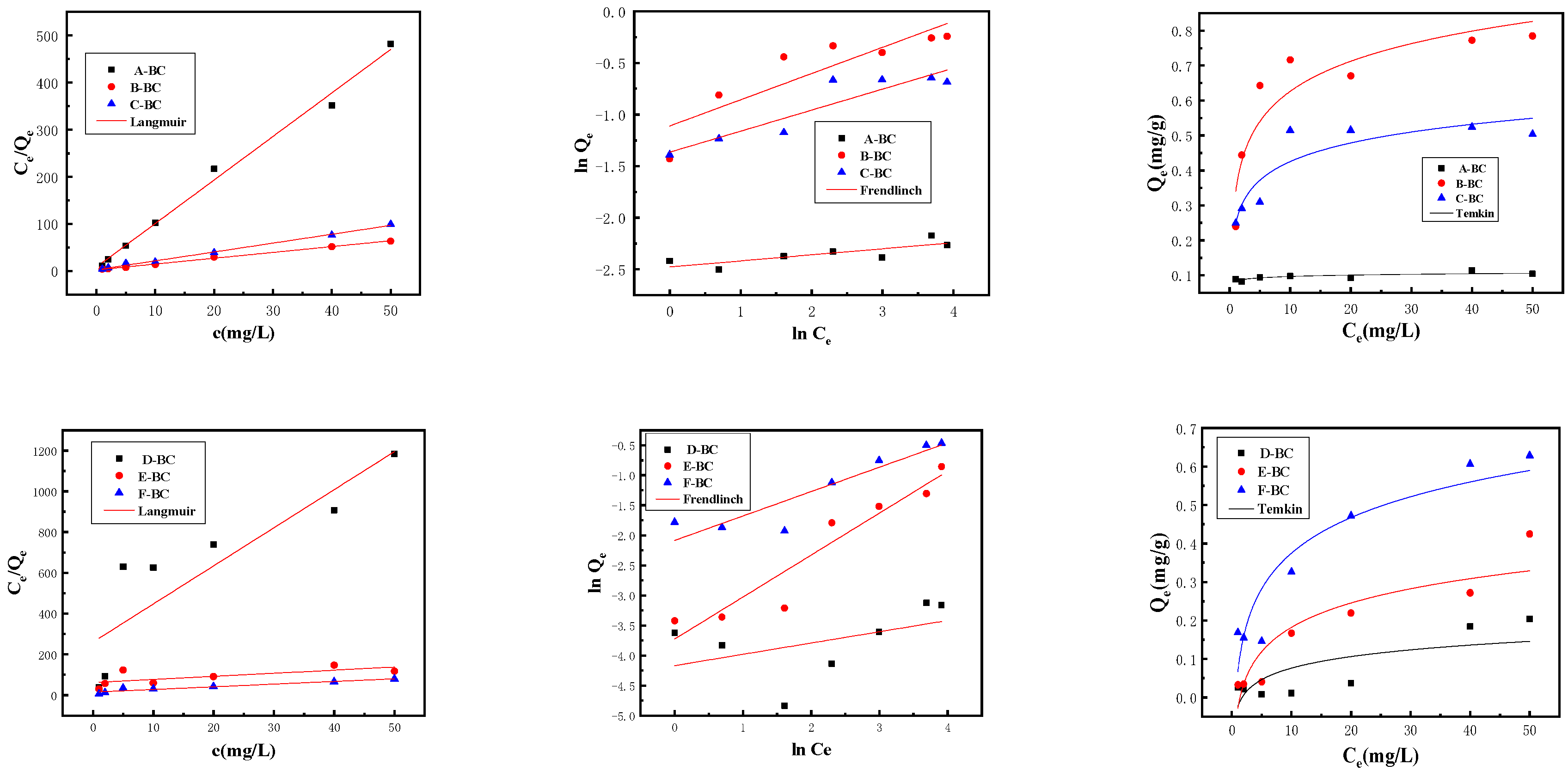
3.2.2. Adsorption Isotherm
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Lalley, J.; Han, C.; Li, X.; Dionysiou, D.D.; Nadagouda, M.N. Phosphate adsorption using modified iron oxide-based sorbents in lake water: Kinetics, equilibrium, and column tests. Chem. Eng. J. 2016, 284, 1386–1396. [Google Scholar] [CrossRef]
- Wu, J.; Wang, J.; Du, Y.; Li, H.; Jia, X. Adsorption mechanism and kinetics of azo dye chemicals on oxide nanotubes: A case study using porous CeO2 nanotubes. J. Nanopart. Res. 2016, 18, 191. [Google Scholar] [CrossRef]
- Ahmad, M.; Rajapaksha, A.U.; Lim, J.E.; Zhang, M.; Bolan, N.; Mohan, D.; Vithanage, M.; Lee, S.S.; Ok, Y.S. Biochar as a sorbent for contaminant management in soil and water: A review. Chemosphere 2014, 99, 19–33. [Google Scholar] [CrossRef]
- Xiao, R.; Sun, X.; Wang, J.; Feng, J.; Li, R.; Zhang, Z.; Wang, J.J.; Amjad, A. Characteristics and phytotoxicity assay of biochars derived from a Zn-rich antibiotic residue. J. Anal. Appl. Pyrolysis 2015, 113, 575–583. [Google Scholar] [CrossRef]
- Li, R.; Wang, J.J.; Gaston, L.A.; Zhou, B.; Li, M.; Xiao, R.; Wang, Q.; Zhang, Z.; Huang, H.; Liang, W.; et al. An overview of carbothermal synthesis of metal–biochar composites for the removal of oxyanion contaminants from aqueous solution. Carbon 2018, 129, 674–687. [Google Scholar] [CrossRef]
- Chen, B.; Chen, Z.; Lv, S. A novel magnetic biochar efficiently sorbs organic pollutants and phosphate. Bioresour. Technol. 2011, 102, 716–723. [Google Scholar] [CrossRef]
- Zhang, M.; Gao, B.; Yao, Y.; Xue, Y.; Inyang, M. Synthesis of porous MgO-biochar nanocomposites for removal of phosphate and nitrate from aqueous solutions. Chem. Eng. J. 2012, 210, 26–32. [Google Scholar] [CrossRef]
- Yao, Y.; Gao, B.; Inyang, M.; Zimmerman, A.R.; Cao, X.; Pullammanappallil, P.; Yang, L. Removal of phosphate from aqueous solution by biochar derived from anaerobically digested sugar beet tailings. J. Hazard. Mater. 2011, 190, 501–507. [Google Scholar] [CrossRef]
- Fang, C.; Zhang, T.; Li, P.; Jiang, R.; Wu, S.; Nie, H.; Wang, Y. Phosphorus recovery from biogas fermentation liquid by Ca–Mg loaded biochar. J. Environ. Sci. 2015, 29, 106–114. [Google Scholar] [CrossRef]
- Li, R.; Wang, J.J.; Zhou, B.; Awasthi, M.K.; Ali, A.; Zhang, Z.; Gaston, L.A.; Lahori, A.H.; Mahar, A. Enhancing phosphate adsorption by Mg/Al layered double hydroxide functionalized biochar with different Mg/Al ratios. Sci. Total Environ. 2016, 559, 121–129. [Google Scholar] [CrossRef]
- Zhang, C.; Dong, Y.; Yang, D.; Jin, Q.; Lin, H. Synthesis of co-pyrolyzed biochar using red mud and peanut shell for removing phosphate from pickling wastewater: Performance and mechanism. Chemosphere 2023, 331, 138841. [Google Scholar] [CrossRef] [PubMed]
- Tao, X.; Huang, T.; Lv, B. Synthesis of Fe/Mg-Biochar Nanocomposites for Phosphate Removal. Materials 2020, 13, 816. [Google Scholar] [CrossRef] [PubMed]
- Reguyal, F.; Sarmah, A.K.; Gao, W. Synthesis of magnetic biochar from pine sawdust via oxidative hydrolysis of FeCl2 for the removal sulfamethoxazole from aqueous solution. J. Hazard. Mater. 2017, 321, 868–878. [Google Scholar] [CrossRef] [PubMed]
- Jung, K.-W.; Choi, B.H.; Song, K.G.; Choi, J.-W. Statistical optimization of preparing marine macroalgae derived activated carbon/iron oxide magnetic composites for sequestering acetylsalicylic acid from aqueous media using response surface methodologys. Chemosphere 2019, 215, 432–443. [Google Scholar] [CrossRef]
- Zoroufchi Benis, K.; Sokhansanj, A.; Norberto, J.; McPhedran, K.N.; Soltan, J. A binary oxide-biochar composite for adsorption of arsenic from aqueous solutions: Combined microwave pyrolysis and electrochemical modification. Chem. Eng. J. 2022, 446, 137024. [Google Scholar] [CrossRef]
- Liu, Y.; Zhao, X.; Li, J.; Ma, D.; Han, R. Characterization of bio-char from pyrolysis of wheat straw and its evaluation on methylene blue adsorption. Desalination Water Treat. 2012, 46, 115–123. [Google Scholar] [CrossRef]
- Choudhary, M.; Kumar, R.; Neogi, S. Activated biochar derived from Opuntia ficus-indica for the efficient adsorption of malachite green dye, Cu+2 and Ni+2 from water. J. Hazard. Mater. 2020, 392, 122441. [Google Scholar] [CrossRef]
- Boehm, H.P. Chemical Identification of Surface Groups. In Advances in Catalysis; Eley, D.D., Pines, H., Weisz, P.B., Eds.; Academic Press: Cambridge, MA, USA, 1966; Volume 16, pp. 179–274. [Google Scholar]
- Schönherr, J.; Buchheim, J.R.; Scholz, P.; Adelhelm, P. Boehm Titration Revisited (Part I): Practical Aspects for Achieving a High Precision in Quantifying Oxygen-Containing Surface Groups on Carbon Materials. C 2018, 4, 21. [Google Scholar] [CrossRef]
- Fang, C.; Zhang, T.; Li, P.; Jiang, R.-f.; Wang, Y. Application of Magnesium Modified Corn Biochar for Phosphorus Removal and Recovery from Swine Wastewater. Int. J. Environ. Res. Public Health 2014, 11, 9217–9237. [Google Scholar] [CrossRef]
- Chen, B.; Zhou, D.; Zhu, L. Transitional Adsorption and Partition of Non-Polar and Polar Aromatic Contaminants by Biochars of Pine Needles with Different Pyrolytic Temperatures. Environ. Sci. Technol. 2008, 42, 5137–5143. [Google Scholar] [CrossRef]
- Gong, C.; Chen, D.; Jiao, X.; Wang, Q. Continuous hollow alpha-Fe2O3 and alpha-Fe fibers prepared by the sol-gel method. J. Mater. Chem. 2002, 112, 1844–1847. [Google Scholar] [CrossRef]
- Aryee, A.A.; Dovi, E.; Han, R.; Li, Z.; Qu, L. One novel composite based on functionalized magnetic peanut husk as adsorbent for efficient sequestration of phosphate and Congo red from solution: Characterization, equilibrium, kinetic and mechanism studies. J. Colloid Interface Sci. 2021, 598, 69–82. [Google Scholar] [CrossRef] [PubMed]
- Ghasemzadeh, M.A.; Mirhosseini-Eshkevari, B.; Sanaei-Rad, S. ZIF-8-incorporated nanoparticles of MgFe2O4 supported on graphene oxide: A ternary hybrid catalyst for the efficient synthesis of pyrazole-based pyrido[2,3-d]pyrimidine-diones. Polyhedron 2022, 212, 115588. [Google Scholar] [CrossRef]
- Huynh, N.C.; Nguyen, T.T.T.; Nguyen, D.T.C.; Tran, T.V. Production of MgFe2O4/activated carbons derived from a harmful grass Cynodon dactylon and their utilization for ciprofloxacin removal. Chemosphere 2023, 343, 139891. [Google Scholar] [CrossRef]
- Wu, L.; Wei, C.; Zhang, S.; Wang, Y.; Kuzyakov, Y.; Ding, X. MgO-modified biochar increases phosphate retention and rice yields in saline-alkaline soil. J. Clean. Prod. 2019, 235, 901–909. [Google Scholar] [CrossRef]
- Jung, K.-W.; Lee, S.; Lee, Y.J. Synthesis of novel magnesium ferrite (MgFe2O4)/biochar magnetic composites and its adsorption behavior for phosphate in aqueous solutions. Bioresour. Technol. 2017, 245, 751–759. [Google Scholar] [CrossRef]
- Sewu, D.D.; Tran, H.N.; Ohemeng-Boahen, G.; Woo, S.H. Facile magnetic biochar production route with new goethite nanoparticle precursor. Sci. Total Environ. 2020, 717, 137091. [Google Scholar] [CrossRef]
- Bourikas, K.; Kordulis, C.; Lycourghiotis, A. Differential potentiometric titration: Development of a methodology for determining the point of zero charge of metal (hydr)oxides by one titration curve. Environ. Sci. Technol. 2005, 39, 4100–4108. [Google Scholar] [CrossRef]
- Álvarez-Merino, M.A.; Fontecha-Cámara, M.A.; López-Ramón, M.V.; Moreno-Castilla, C. Temperature dependence of the point of zero charge of oxidized and non-oxidized activated carbons. Carbon 2008, 46, 778–787. [Google Scholar] [CrossRef]
- Kosmulski, M. The pH dependent surface charging and points of zero charge. VIII. Update. Adv. Colloid Interface Sci. 2019, 275, 102064. [Google Scholar] [CrossRef]
- Li, X.; Shen, Q.; Zhang, D.; Mei, X.; Ran, W.; Xu, Y.; Yu, G. Functional Groups Determine Biochar Properties (pH and EC) as Studied by Two-Dimensional (13)C NMR Correlation Spectroscopy. PLoS ONE 2013, 8, e65949. [Google Scholar] [CrossRef]
- Mohan, D.; Pittman, C.U.; Bricka, M.; Smith, F.; Yancey, B.; Mohammad, J.; Steele, P.H.; Alexandre-Franco, M.F.; Gómez-Serrano, V.; Gong, H. Sorption of arsenic, cadmium, and lead by chars produced from fast pyrolysis of wood and bark during bio-oil production. J. Colloid Interface Sci. 2007, 310, 57–73. [Google Scholar] [CrossRef] [PubMed]
- Tseng, R.-L.; Wu, F.-C. Inferring the favorable adsorption level and the concurrent multi-stage process with the Freundlich constant. J. Hazard. Mater. 2008, 155, 277–287. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Yan, L.; Yu, H.; Yan, T.; Li, X. Adsorption of phosphate from aqueous solution by vegetable biochar/layered double oxides: Fast removal and mechanistic studies. Bioresour. Technol. 2019, 284, 65–71. [Google Scholar] [CrossRef]
- Cheng, X.; Huang, X.; Wang, X.; Sun, D. Influence of calcination on the adsorptive removal of phosphate by Zn–Al layered double hydroxides from excess sludge liquor. J. Hazard. Mater. 2010, 177, 516–523. [Google Scholar] [CrossRef]
- Jung, K.-W.; Ahn, K.-H. Fabrication of porosity-enhanced MgO/biochar for removal of phosphate from aqueous solution: Application of a novel combined electrochemical modification method. Bioresour. Technol. 2016, 200, 1029–1032. [Google Scholar] [CrossRef]

| Materials | BC | A-BC | B-BC | C-BC | D-BC | E-BC | F-BC |
|---|---|---|---|---|---|---|---|
| pHpzc | 5.45 | 6.49 | 9.08 | 8.81 | 4.52 | 8.71 | 9.90 |
| Kinetic Model | Parameters | A-BC | B-BC | C-BC | D-BC | E-BC | F-BC |
|---|---|---|---|---|---|---|---|
| Pseudo-First-Order | qe (mg/g) | 0.1174 | 0.7502 | 0.2901 | 0.0079 | 0.0318 | 0.2009 |
| K1 (1/h) | 0.2487 | 0.0831 | 0.3946 | 0.4933 | 1.0729 | 0.0218 | |
| R2 | 0.9125 | 0.9958 | 0.7295 | 0.7419 | 0.9258 | 0.6528 | |
| Pseudo-Second-Order | qe (mg/g) | 0.1257 | 1.1305 | 0.3495 | 0.0091 | 0.0323 | 0.2411 |
| K2 [g/(mg·h)] | 3.1028 | 0.0509 | 1.2387 | 33.9153 | 81.0668 | 1.0928 | |
| R2 | 0.9695 | 0.9688 | 0.9576 | 0.9060 | 0.9980 | 0.9202 | |
| Intra-Particle Diffusion | K [g/(mg·h1/2)] | 0.0271 | 0.1529 | 0.0527 | 0.0018 | 0.0036 | 0.0376 |
| C | 0.0031 | 0.0869 | 0.0825 | 0.0215 | 0.0194 | 0.0332 | |
| R2 | 0.7045 | 0.9966 | 0.9616 | 0.6970 | 0.6498 | 0.9751 |
| Isotherm Model | Parameters | A-BC | B-BC | C-BC | D-BC | E-BC | F-BC |
|---|---|---|---|---|---|---|---|
| Langmuir | qmax (mg/g) | 0.3093 | 0.8083 | 0.5307 | 0.0535 | 0.6586 | 0.7620 |
| KL (L/mg) | 0.3729 | 0.4975 | 0.6683 | 0.0718 | 0.0245 | 0.0885 | |
| R2 | 0.9912 | 0.9968 | 0.9953 | 0.7318 | 0.3947 | 0.9143 | |
| Freundlich | KF (L/mg) | 0.0834 | 0.3292 | 0.2554 | 0.0155 | 0.0241 | 0.1246 |
| 1/n | 0.5883 | 0.2536 | 0.2037 | 0.1880 | 0.6986 | 0.4060 | |
| R2 | 0.6023 | 0.7316 | 0.8217 | 0.0663 | 0.8922 | 0.8284 | |
| Temkin | βT (J/mol) | 2.4179 | 15.4922 | 25.1650 | 0.5818 | 0.7342 | 1.6556 |
| AT (L/mg) | 0.0056 | 0.1241 | 0.0769 | 0.0432 | 0.0913 | 0.1336 | |
| R2 | 0.5901 | 0.8321 | 0.8030 | 0.4794 | 0.8060 | 0.8452 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mei, C.; Wang, L.; Tao, W. Characterization of Magnetic Biochar Modified Using the One-Step and Electrochemical Methods and Its Impact on Phosphate Adsorption. Materials 2023, 16, 7092. https://doi.org/10.3390/ma16227092
Mei C, Wang L, Tao W. Characterization of Magnetic Biochar Modified Using the One-Step and Electrochemical Methods and Its Impact on Phosphate Adsorption. Materials. 2023; 16(22):7092. https://doi.org/10.3390/ma16227092
Chicago/Turabian StyleMei, Changgen, Lulu Wang, and Wei Tao. 2023. "Characterization of Magnetic Biochar Modified Using the One-Step and Electrochemical Methods and Its Impact on Phosphate Adsorption" Materials 16, no. 22: 7092. https://doi.org/10.3390/ma16227092





