Resorcinol–Formaldehyde-Derived Carbon Xerogels: Preparation, Functionalization, and Application Aspects
Abstract
:1. Introduction
2. Sol–Gel Synthesis of Nanostructured Materials: From Oxides to Carbon Xerogels and Aerogels
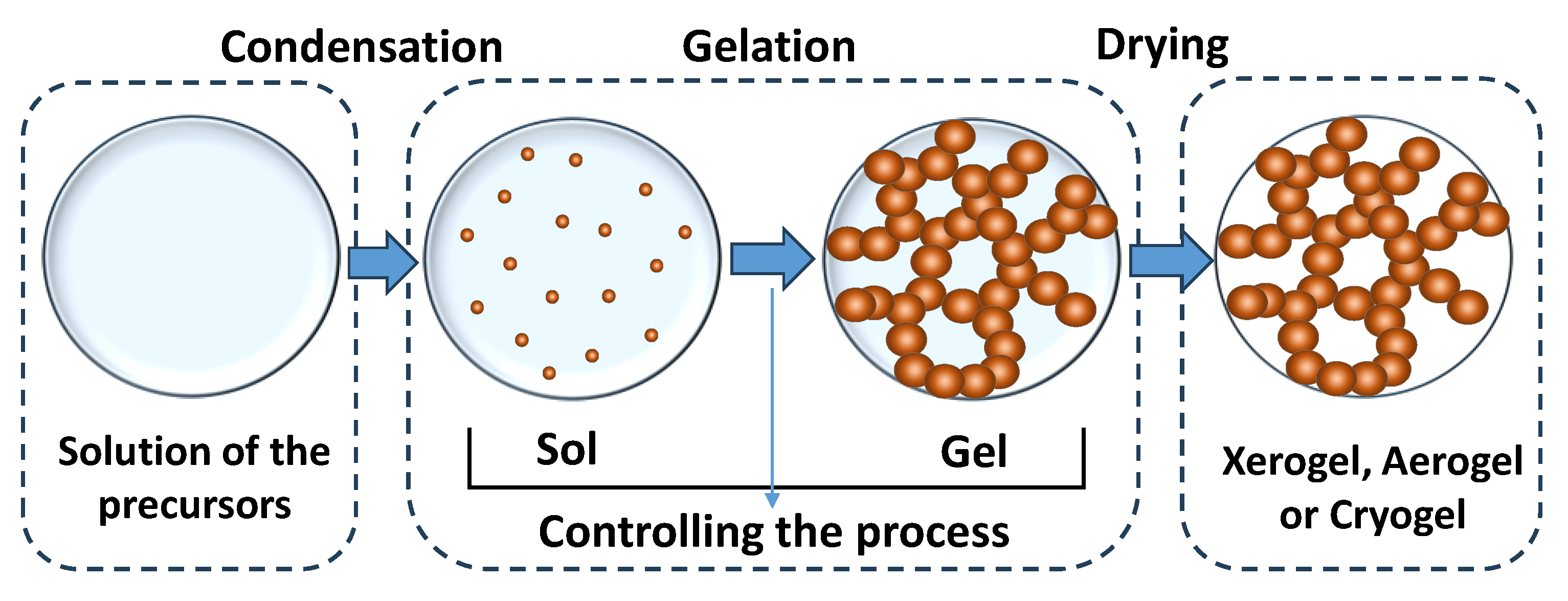
2.1. General Features of the Sol–Gel Process
- -
- Thermal drying under atmospheric pressure conditions to obtain xerogels. In this case, there is a significant loss of porosity during drying due to the influence of capillary forces.
- -
- Cyclic freezing/heating to produce materials called cryogels.
- -
- Drying under supercritical conditions, which are the gentlest for the porous structure. Supercritical fluids have zero surface tension, which contributes to the preservation of porosity. The aerogels formed in this way have the most developed surface and the largest pore volume. At the same time, the structure of aerogels may be more brittle and have less stability at high temperatures.
2.2. Sol–Gel Synthesis of Organic and Carbon Xerogels
3. Effect of the Synthesis Conditions on the Textural Properties of Carbon Xerogels and Aerogels
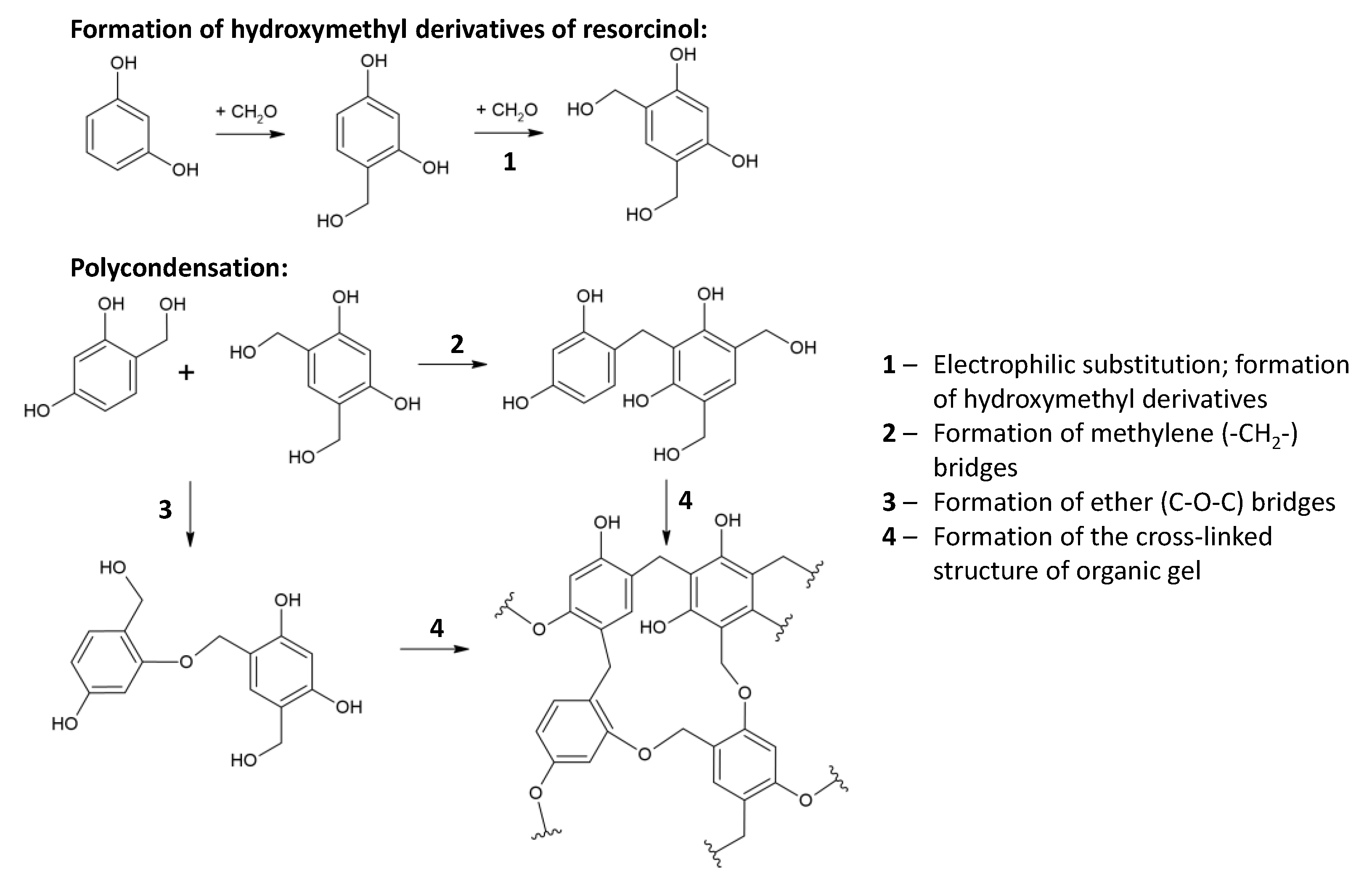
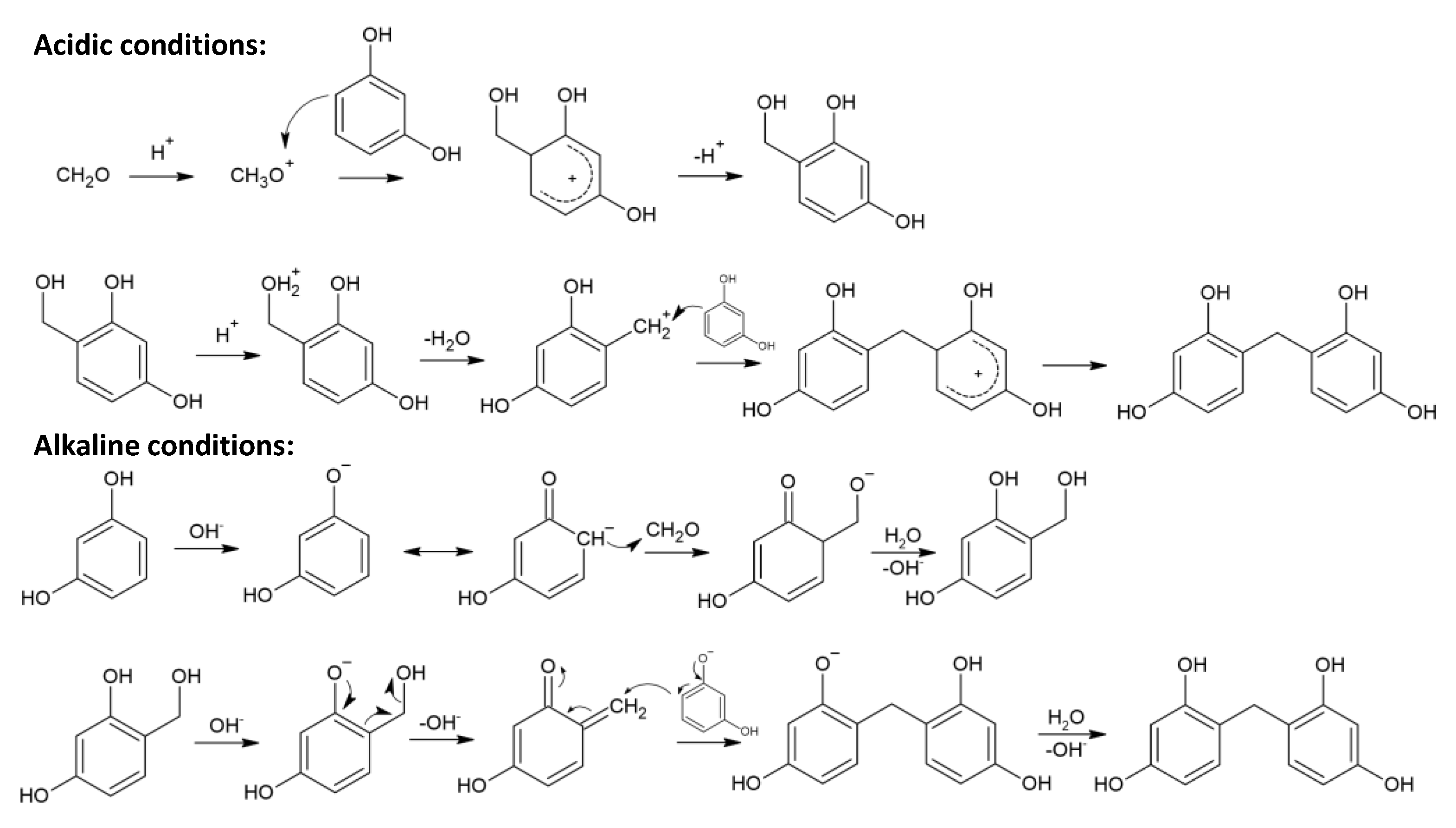
3.1. Polycondensation/Gelation Stage
3.2. Inverse Emulsification Process
3.3. Drying Stage
- -
- Step drying, e.g., 60 °C (24 h) → 80 °C (24 h) → 100 °C (24 h) → 120 °C (24 h) [54].
- -
- Drying under reduced pressure (~1000 Pa), which allows one to reduce the drying time to ~20 h [35].
- -
- Convection drying at a constant temperature of 70 °C. In this case, the removal of the solvent is accelerated due to the presence of airflow passing through the sample at a flow rate of 1–2 m/s [44].
- -
- -
- The exchange of the solvent for acetone before thermal drying. The lower surface tension of acetone reduces the loss in porosity [58].
- -
- The exchange of the solvent for acetone and then for carbon dioxide and the removal of carbon dioxide under supercritical conditions. This is the most time-consuming and expensive method, but it allows for obtaining aerogels with the largest pore volume and specific surface area [5].
3.4. Carbonization Stage
3.5. Activation of CXs
3.6. CXs Derived from Other Precursors
4. Methods for Doping CXs with Heteroatoms
4.1. Nitrogen-Containing Functional Groups
- (1)
- Methods based on the use of nitrogen-containing precursors in the polycondensation stage;
- (2)
- Methods based on the post-treatment of organic or carbon xerogels.
| No. | Sample | Functionalization Procedure | Heteroatom Content, % | Functional Groups by XPS, % | Ref. |
|---|---|---|---|---|---|
| 1. | N-CXG-1 | Addition of urea to precursor solution | EA—2.9 N, 7.7 O; XPS—2.0 N, 10.7 O | 39.1—N6, 4.6—N5, 52.8—NG, 3.6—N-O | [84] |
| 2. | Fe-N-CXG-1-TT2 | EA—0.9 N, 4 O; XPS—1.1 N, 6.1 O | 17—N6, 9.4—N5, 27.9—NG, 21.3—N-O, Me-N—24.5 | [84] | |
| 3. | NCX-150-2 | Addition of melamine to precursor solution | EA—2.97 N | N6, N5, NG—49.2 | [85] |
| 4. | Carbon xerogel | Addition of ammonia to the precursor solution | XPS—1.33 N, 14.44 O | 37.15—N6, 54.79 -N5+NG, 8.06—N-O | [86] |
| 5. | Fe-doped carbon xerogel | XPS—1.16 N, 15.19 O | 40.95—N6, 48.94—N5+NG, 10.11—N-O | [86] | |
| 6. | HAM-500 | Co-gelation of hydroxyaniline with melamine and formaldehyde | EA—13 N, 9 O; XPS—20 N, 3 O | 53—N6, 41—N5, 5—NG | [14] |
| 7. | HAM-700 | EA—9 N, 8 O; XPS—11 N, 2 O | 44—N6, 34—N5, 22—NG | ||
| 8. | HAM-900 | EA—6 N, 8 O; XPS—6 N, 1 O | 28—N6, 33—N5, 39—NG | ||
| 9. | CX_NH3_950 | Post-treatment with ammonia | EA—2.1 N, 4.9 O; XPS—1.7 N, 4.6 O | 46.7—N6, 33.8—N5, 19.5— NG | [77] |
| 10. | CX_NH3_650_BM | Post-treatment with ammonia and ball milling | EA—2.6 N, 6.0 O; XPS—1.8 N, 6.2 O | 26.4—N6, 62.2—N5, 11.3—NG | |
| 11. | CX_NH3_950_BM | EA—2.2 N, 6.3 O; XPS—1.4 N, 8.0 O | 48.9—N6, 32.5—N5, 18.6—NG | ||
| 12. | NH3 treated CXG | NH3 plasma treatment | XPS—8.5 N | N6, N5 and NG | [87] |
| 13. | CX-HMTA6 | Addition of hexamethylenetetramine to the precursor solution | XPS—1.26 N, 15.78 O | N6, N5 and NG | [88] |
| 14. | N-CX-8-1000 | Sol–gel polycondensation of resorcinol with pyrrole-2-carboxaldehyde | EA—3.8 N, 13.36 O | 31—N6, 12—N5, 48—NG, 9—N-O | [89] |
| 15. | CX-750 N | Post-treatment with melamine of the carbonized CX | XPS—2.4 N, 10.4 O | 62.2—N6, 37.8—N5 | [90] |
| 16. | AX-1000 N | Post-treatment with melamine of activated carbonized CX | XPS—1.8 N, 5.4 O | 49.2—N6, 36.8—N5, 14.0—NG |
4.2. Oxygen-Containing Functional Groups
- (1)
- The number of functional groups on the surface of the CXs depends on the treatment conditions, including the concentration of HNO3, temperature, and loading of carbon material in the hydrothermal treatment stage.
- (2)
- Such textural characteristics of the CXs as SSA and pore volume are not significantly affected when the treatment temperature is quite low (20 °C). At a temperature of 100 °C, the specific surface area grows with increasing HNO3 concentration.
4.3. Functional Groups Containing Sulfur, Boron, and Phosphorus
| Heteroatom | Functionalization Procedure | Functional Groups | Application | Ref. |
|---|---|---|---|---|
| Oxygen | O2 plasma treatment | Hydroxyl, carbonyl, and carboxyl groups | Photocatalysis (photodegradation of Rhodamine B) | [17,87] |
| Liquid-phase oxidation with HNO3 | Carboxyl, phenolic, lactone, and carbonyl groups; quinones; and anhydrides | Oxidation and hydrolysis processes, fuel cell catalysis | [93,94,95] | |
| Gas-phase oxidation with O2 | More thermally stable groups like phenolic hydroxyls | [94,95] | ||
| Boron | Addition of boric acid or phenyl boronic acid to the precursor solution | Boron oxides, oxycarbides, boron carbides, and B in the carbon lattice | - | [96] |
| Addition of boric acid to the precursor solution | B–C2O (60.4), B–N (36.5), B2O3 (3.1) | Electrochemical desalination of brackish water | [97] | |
| Phosphorus | Post-treatment with H3PO4 | C-P-O and C-O-P species | Dehydration of fructose to 5-hydroxymethylfurfural | [98] |
| Post-treatment with H3PO4 | P-O and P-C bonds | Oxygen reduction reaction | [102] | |
| Gas-phase condensation of red P | - | anode for sodium-ion batteries | [101] | |
| Post-treatment with H3PO4 | C–O–P-type groups | Capacitor material | [99] | |
| Polycondensation reaction of tyrosine and pyrrole-2-carbaldehyde using phosphoric acid as the catalyst | Pentavalent tetracoordinated phosphate (PO4) and pyrophosphate groups (PO3) | Electrode for supercapacitor | [100] | |
| Sulfur | H2SO4 treatment | -SO3H | Glycerol acetylation | [54] |
| 4-benzenediazonium sulfonate treatment | ||||
| Addition of sulfur-containing aldehydes like 2-thiophenecarboxaldehyde to the precursor solution | C-S-C, other forms of C-S, and some oxidized species like sulfates and sulfonates | Oxygen reduction reaction (Fe-N-S-CX) | [103,104] |
5. Doping of CXs with Metals
5.1. Conventional Impregnation-Based Approaches
5.2. Approaches Based on One-Step Sol–Gel Synthesis
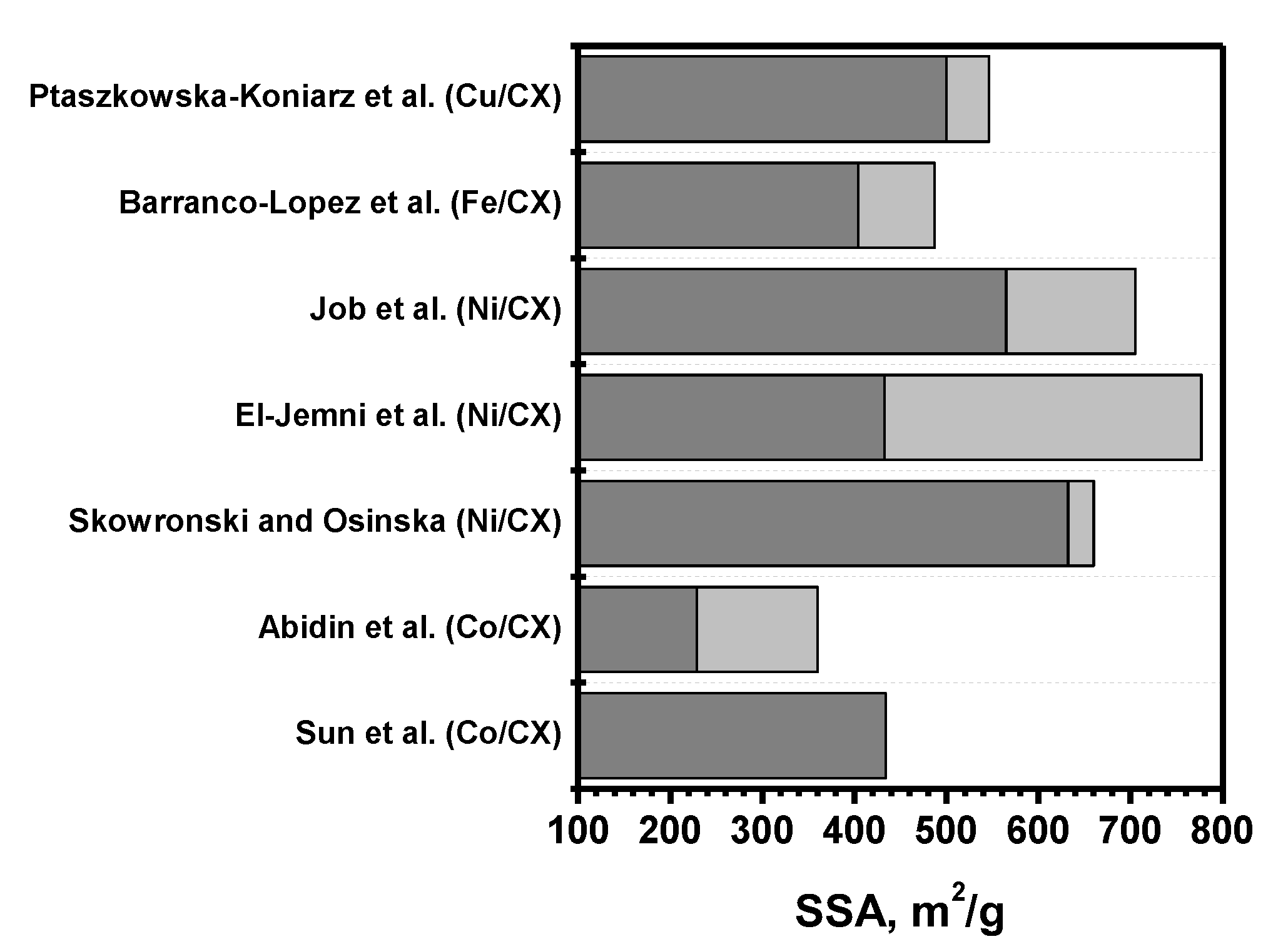
| No. | Material | Preparation Method | Application | Ref. |
|---|---|---|---|---|
| 1 | Co-CX | Doping Co2+ ions into wet organic gel | Heterogeneous oxidation of phenol | [110] |
| 2 | Co-CX | Solubilization of Co(NO3)2 in the precursor solution | Oxygen reduction reaction | [111] |
| 3 | Ni-CX | Solubilization of nickel acetate in the precursor solution | Electrode materials for supercapacitor | [112] |
| 4 | Ni-CX | Solubilization of nickel acetate in the precursor solution | Ethanol electro-oxidation | [113] |
| 5 | Fe-CX | Solubilization of iron acetate in the precursor solution | Electro-reduction of O2 to H2O2 combined with H2O2 catalytic decomposition | [115] |
| 6 | Cu-N-CX | Solubilization of copper acetate in the precursor solution | Adsorption of caffeine | [116] |
| 7 | Fe-N-CX and Fe-N-S-CX | Solubilization of iron chloride in the precursor solution | Oxidation and adsorption of chlorophenols | [121] |
| 8 | Co-N-CX | Solubilization of cobalt acetate in the precursor solution | Oxygen reduction reaction | [122] |
6. Application of Carbon Xerogels
6.1. Anodes for Li-Ion Batteries
6.2. Supercapacitor Materials
6.3. Fuel Cell Catalysts
6.4. Adsorbents
6.5. Photocatalytically Active Composite Materials
6.6. Other Catalytic Processes
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Lacarbonara, G.; Chini, S.; Ratso, S.; Kruusenberg, I.; Arbizzani, C. A MnOx–graphitic carbon composite from CO2 for sustainable Li-ion battery anodes. Mater. Adv. 2022, 3, 7087–7097. [Google Scholar] [CrossRef]
- Najafli, E.; Ratso, S.; Ivanov, Y.P.; Gatalo, M.; Pavko, L.; Yörük, C.R.; Walke, P.; Divitini, G.; Hodnik, N.; Kruusenberg, I. Sustainable CO2-Derived Nanoscale Carbon Support to a Platinum Catalyst for Oxygen Reduction Reaction. ACS Appl. Nano Mater. 2023, 6, 5772–5780. [Google Scholar] [CrossRef]
- Lee, J.; Kim, J.; Hyeon, T. Recent Progress in the Synthesis of Porous Carbon Materials. Adv. Mater. 2006, 18, 2073–2094. [Google Scholar] [CrossRef]
- Wang, C.; Kim, J.; Tang, J.; Kim, M.; Lim, H.; Malgras, V.; You, J.; Xu, Q.; Li, J.; Yamauchi, Y. New Strategies for Novel MOF-Derived Carbon Materials Based on Nanoarchitectures. Chem 2020, 6, 19–40. [Google Scholar] [CrossRef]
- Pekala, R. Organic aerogels from the polycondensation of resorcinol with formaldehyde. J. Mater. Sci. 1989, 24, 3221–3227. [Google Scholar] [CrossRef]
- Pillai, A.; Kandasubramanian, B. Carbon Xerogels for Effluent Treatment. J. Chem. Eng. Data 2020, 65, 2255–2270. [Google Scholar] [CrossRef]
- Job, N.; Pirard, R.; Marien, J.; Pirard, J.-P. Porous carbon xerogels with texture tailored by pH control during sol–gel process. Carbon 2004, 42, 619–628. [Google Scholar] [CrossRef]
- Al-Muhtaseb, S.A.; Ritter, J.A. Preparation and Properties of Resorcinol–Formaldehyde Organic and Carbon Gels. Adv. Mater. 2003, 15, 101–114. [Google Scholar] [CrossRef]
- Li, L.; Hu, T.; Li, A.; Zhang, J. Electrically Conductive Carbon Aerogels with High Salt-Resistance for Efficient Solar-Driven Interfacial Evaporation. ACS Appl. Mater. Interf. 2020, 12, 32143–32153. [Google Scholar] [CrossRef]
- María, C.-R.; Menéndez, J.A.; Ana, A. Carbon Xerogels: The Bespoke Nanoporous Carbons. In Porosity; Chapter 3; Taher Hcine, G., Ed.; IntechOpen: Rijeka, Croatia, 2017. [Google Scholar]
- Jiang, X.; Zhao, Z.; Zhou, S.; Zou, H.; Liu, P. Anisotropic and Lightweight Carbon/Graphene Composite Aerogels for Efficient Thermal Insulation and Electromagnetic Interference Shielding. ACS Appl. Mater. Interf. 2022, 14, 45844–45852. [Google Scholar] [CrossRef]
- Ibarra Torres, C.E.; Serrano Quezada, T.E.; Kharissova, O.V.; Kharisov, B.I.; Gómez de la Fuente, M.I. Carbon-based aerogels and xerogels: Synthesis, properties, oil sorption capacities, and DFT simulations. J. Environ. Chem. Eng. 2021, 9, 104886. [Google Scholar] [CrossRef]
- Job, N.; Berthon-Fabry, S.; Chatenet, M.; Marie, J.; Brigaudet, M.; Pirard, J.-P. Nanostructured carbons as platinum catalyst supports for proton exchange membrane fuel cell electrodes. Top. Catal. 2009, 52, 2117–2122. [Google Scholar] [CrossRef]
- Perez-Cadenas, M.; Moreno-Castilla, C.; Carrasco-Marin, F.; Perez-Cadenas, A.F. Surface chemistry, porous texture, and morphology of N-doped carbon xerogels. Langmuir 2009, 25, 466–470. [Google Scholar] [CrossRef]
- Gorgulho, H.F.; Gonçalves, F.; Pereira, M.F.R.; Figueiredo, J.L. Synthesis and characterization of nitrogen-doped carbon xerogels. Carbon 2009, 47, 2032–2039. [Google Scholar] [CrossRef]
- Samant, P.V.; Gonçalves, F.; Freitas, M.M.A.; Pereira, M.F.R.; Figueiredo, J.L. Surface activation of a polymer based carbon. Carbon 2004, 42, 1321–1325. [Google Scholar] [CrossRef]
- Mahata, N.; Pereira, M.; Suárez-García, F.; Martínez-Alonso, A.; Tascón, J.; Figueiredo, J.L. Tuning of texture and surface chemistry of carbon xerogels. J. Colloid Interf. Sci. 2008, 324, 150–155. [Google Scholar] [CrossRef] [PubMed]
- Bedilo, A.; Klabunde, K. Synthesis of high surface area zirconia aerogels using high temperature supercritical drying. Nanostruct. Mater. 1997, 8, 119–135. [Google Scholar] [CrossRef]
- Pant, B.; Park, M.; Park, S.-J. Recent advances in TiO2 films prepared by sol-gel methods for photocatalytic degradation of organic pollutants and antibacterial activities. Coatings 2019, 9, 613. [Google Scholar] [CrossRef]
- Ilyina, E.V.; Mishakov, I.V.; Vedyagin, A.A.; Bedilo, A.F.; Klabunde, K.J. Promoting effect of vanadium on CF2Cl2 destructive sorption over nanocrystalline mesoporous MgO. Microporous Mesoporous Mater. 2013, 175, 76–84. [Google Scholar] [CrossRef]
- Hu, B.; Yao, M.; Xiao, R.; Chen, J.; Yao, X. Optical properties of amorphous Al2O3 thin films prepared by a sol–gel process. Ceram. Int. 2014, 40, 14133–14139. [Google Scholar] [CrossRef]
- Dubey, R.; Rajesh, Y.; More, M. Synthesis and characterization of SiO2 nanoparticles via sol-gel method for industrial applications. Mater. Today Proc. 2015, 2, 3575–3579. [Google Scholar] [CrossRef]
- Veselov, G.B.; Karnaukhov, T.M.; Stoyanovskii, V.O.; Vedyagin, A.A. Preparation of the nanostructured Ni-Mg-O oxide system by a sol–gel technique at varied pH. Nanomaterials 2022, 12, 952. [Google Scholar] [CrossRef]
- Milea, C.; Bogatu, C.; Duta, A. The influence of parameters in silica sol-gel process. Bull. Transilvania Univ. Brasov 2011, 4, 53. [Google Scholar]
- Wu, S.-H.; Mou, C.-Y.; Lin, H.-P. Synthesis of mesoporous silica nanoparticles. Chem. Soc. Rev. 2013, 42, 3862–3875. [Google Scholar] [CrossRef]
- Hench, L.L.; West, J.K. The sol-gel process. Chem. Rev. 1990, 90, 33–72. [Google Scholar] [CrossRef]
- ElKhatat, A.M.; Al-Muhtaseb, S.A. Advances in Tailoring Resorcinol-Formaldehyde Organic and Carbon Gels. Adv. Mater. 2011, 23, 2887–2903. [Google Scholar] [CrossRef]
- Mulik, S.; Sotiriou-Leventis, C. Resorcinol–Formaldehyde Aerogels. In Aerogels Handbook; Aegerter, M.A., Leventis, N., Koebel, M.M., Eds.; Springer: New York, NY, USA, 2011; pp. 215–234. [Google Scholar]
- Mulik, S.; Sotiriou-Leventis, C.; Leventis, N. Time-Efficient Acid-Catalyzed Synthesis of Resorcinol–Formaldehyde Aerogels. Chem. Mater. 2007, 19, 6138–6144. [Google Scholar] [CrossRef]
- Li, T.; Cao, M.; Liang, J.; Xie, X.; Du, G. Theoretical Confirmation of the Quinone Methide Hypothesis for the Condensation Reactions in Phenol-Formaldehyde Resin Synthesis. Polymers 2017, 9, 45. [Google Scholar] [CrossRef] [PubMed]
- Li, T.; Cao, M.; Liang, J.; Xie, X.; Du, G. Mechanism of Base-Catalyzed Resorcinol-Formaldehyde and Phenol-Resorcinol-Formaldehyde Condensation Reactions: A Theoretical Study. Polymers 2017, 9, 426. [Google Scholar] [CrossRef] [PubMed]
- Lin, C.; Ritter, J.A. Effect of synthesis pH on the structure of carbon xerogels. Carbon 1997, 35, 1271–1278. [Google Scholar] [CrossRef]
- Horikawa, T.; Hayashi, J.i.; Muroyama, K. Controllability of pore characteristics of resorcinol–formaldehyde carbon aerogel. Carbon 2004, 42, 1625–1633. [Google Scholar] [CrossRef]
- Rey-Raap, N.; Arenillas, A.; Menéndez, J.A. A visual validation of the combined effect of pH and dilution on the porosity of carbon xerogels. Microporous Mesoporous Mater. 2016, 223, 89–93. [Google Scholar] [CrossRef]
- Zubizarreta, L.; Arenillas, A.; Pirard, J.-P.; Pis, J.J.; Job, N. Tailoring the textural properties of activated carbon xerogels by chemical activation with KOH. Microporous Mesoporous Mater. 2008, 115, 480–490. [Google Scholar] [CrossRef]
- Rey-Raap, N.; Menéndez, J.A.; Arenillas, A. RF xerogels with tailored porosity over the entire nanoscale. Microporous Mesoporous Mater. 2014, 195, 266–275. [Google Scholar] [CrossRef]
- Rey-Raap, N.; Menéndez, J.A.; Arenillas, A. Simultaneous adjustment of the main chemical variables to fine-tune the porosity of carbon xerogels. Carbon 2014, 78, 490–499. [Google Scholar] [CrossRef]
- Gaca, K.Z.; Sefcik, J. Mechanism and kinetics of nanostructure evolution during early stages of resorcinol–formaldehyde polymerisation. J. Colloid Interf. Sci. 2013, 406, 51–59. [Google Scholar] [CrossRef]
- Alonso-Buenaposada, I.D.; Rey-Raap, N.; Calvo, E.G.; Angel Menéndez, J.; Arenillas, A. Effect of methanol content in commercial formaldehyde solutions on the porosity of RF carbon xerogels. J. Non-Cryst. Solids 2015, 426, 13–18. [Google Scholar] [CrossRef]
- Chavhan, M.P.; Slovak, V.; Zelenkova, G.; Dominko, D. Revisiting the Effect of Pyrolysis Temperature and Type of Activation on the Performance of Carbon Electrodes in an Electrochemical Capacitor. Materials 2022, 15, 2431. [Google Scholar] [CrossRef] [PubMed]
- Job, N.; Gommes, C.J.; Pirard, R.; Pirard, J.-P. Effect of the counter-ion of the basification agent on the pore texture of organic and carbon xerogels. J. Non-Crystal. Solids 2008, 354, 4698–4701. [Google Scholar] [CrossRef]
- Calvo, E.G.; Menéndez, J.A.; Arenillas, A. Influence of alkaline compounds on the porosity of resorcinol-formaldehyde xerogels. J. Non-Crystal. Solids 2016, 452, 286–290. [Google Scholar] [CrossRef]
- Pandey, A.P.; Bhatnagar, A.; Shukla, V.; Soni, P.K.; Singh, S.; Verma, S.K.; Shaneeth, M.; Sekkar, V.; Srivastava, O.N. Hydrogen storage properties of carbon aerogel synthesized by ambient pressure drying using new catalyst triethylamine. Int. J. Hydrogen Energy 2020, 45, 30818–30827. [Google Scholar] [CrossRef]
- Job, N.; Panariello, F.; Marien, J.; Crine, M.; Pirard, J.-P.; Léonard, A. Synthesis optimization of organic xerogels produced from convective air-drying of resorcinol–formaldehyde gels. J. Non-Crystal. Solids 2006, 352, 24–34. [Google Scholar] [CrossRef]
- Kakunuri, M.; Sharma, C.S. Resorcinol-formaldehyde derived carbon xerogels: A promising anode material for lithium-ion battery. J. Mater. Res. 2018, 33, 1074–1087. [Google Scholar] [CrossRef]
- Sharma, C.S.; Kulkarni, M.M.; Sharma, A.; Madou, M. Synthesis of carbon xerogel particles and fractal-like structures. Chem. Eng. Sci. 2009, 64, 1536–1543. [Google Scholar] [CrossRef]
- Yamamoto, T.; Endo, A.; Ohmori, T.; Nakaiwa, M. The effects of different synthetic conditions on the porous properties of carbon cryogel microspheres. Carbon 2005, 43, 1231–1238. [Google Scholar] [CrossRef]
- Kim, S.-I.; Yamamoto, T.; Endo, A.; Ohmori, T.; Nakaiwa, M. Influence of nonionic surfactant concentration on physical characteristics of resorcinol-formaldehyde carbon cryogel microspheres. J. Ind. Eng. Chem. 2006, 12, 484–488. [Google Scholar]
- Wang, X.; Wang, X.; Liu, L.; Bai, L.; An, H.; Zheng, L.; Yi, L. Preparation and characterization of carbon aerogel microspheres by an inverse emulsion polymerization. J. Non-Crystal. Solids 2011, 357, 793–797. [Google Scholar] [CrossRef]
- Bailón-García, E.; Elmouwahidi, A.; Ribeiro, F.; Henriques, C.; Pérez-Cadenas, A.F.; Carrasco-Marín, F.; Maldonado-Hódar, F.J. Reduction of NO with new vanadium-carbon xerogel composites. Effect of the oxidation state of vanadium species. Carbon 2020, 156, 194–204. [Google Scholar] [CrossRef]
- Kim, H.; Fortunato, M.E.; Xu, H.; Bang, J.H.; Suslick, K.S. Carbon microspheres as supercapacitors. J. Phys. Chem. C 2011, 115, 20481–20486. [Google Scholar] [CrossRef]
- Xu, C.; Cheng, L.; Shen, P.; Liu, Y. Methanol and ethanol electrooxidation on Pt and Pd supported on carbon microspheres in alkaline media. Electrochem. Commun. 2007, 9, 997–1001. [Google Scholar] [CrossRef]
- Zhou, J.; Lian, J.; Hou, L.; Zhang, J.; Gou, H.; Xia, M.; Zhao, Y.; Strobel, T.A.; Tao, L.; Gao, F. Ultrahigh volumetric capacitance and cyclic stability of fluorine and nitrogen co-doped carbon microspheres. Nature Commun. 2015, 6, 8503. [Google Scholar] [CrossRef] [PubMed]
- Malaika, A.; Kozłowski, M. Glycerol conversion towards valuable fuel blending compounds with the assistance of SO3H-functionalized carbon xerogels and spheres. Fuel Proc. Technol. 2019, 184, 19–26. [Google Scholar] [CrossRef]
- Rey-Raap, N.; Menéndez, J.A.; Arenillas, A. Optimization of the process variables in the microwave-induced synthesis of carbon xerogels. J. Sol-Gel Sci. Technol. 2014, 69, 488–497. [Google Scholar] [CrossRef]
- Zubizarreta, L.; Arenillas, A.; Menéndez, J.A.; Pis, J.J.; Pirard, J.P.; Job, N. Microwave drying as an effective method to obtain porous carbon xerogels. J. Non-Crystal. Solids 2008, 354, 4024–4026. [Google Scholar] [CrossRef]
- Calvo, E.G.; Juárez-Pérez, E.J.; Menéndez, J.A.; Arenillas, A. Fast microwave-assisted synthesis of tailored mesoporous carbon xerogels. J. Colloid Interf. Sci. 2011, 357, 541–547. [Google Scholar] [CrossRef]
- Lin, C.; Ritter, J.A. Carbonization and activation of sol–gel derived carbon xerogels. Carbon 2000, 38, 849–861. [Google Scholar] [CrossRef]
- Eskenazi, D.; Kreit, P.; Pirard, J.-P.; Job, N.; Compère, P. Toward a continuous synthesis of porous carbon xerogel beads. AIChE J. 2018, 64, 1049–1058. [Google Scholar] [CrossRef]
- Jiang, H.; Wang, J.; Wu, S.; Yuan, Z.; Hu, Z.; Wu, R.; Liu, Q. The pyrolysis mechanism of phenol formaldehyde resin. Polym. Degrad. Stabil. 2012, 97, 1527–1533. [Google Scholar] [CrossRef]
- Moreno, A.H.; Arenillas, A.; Calvo, E.G.; Bermúdez, J.M.; Menéndez, J.A. Carbonisation of resorcinol–formaldehyde organic xerogels: Effect of temperature, particle size and heating rate on the porosity of carbon xerogels. J. Analyt. Appl. Pyrol. 2013, 100, 111–116. [Google Scholar] [CrossRef]
- Lin, C.; Ritter, J.A.; Popov, B.N. Correlation of Double-Layer Capacitance with the Pore Structure of Sol-Gel Derived Carbon Xerogels. J. Electrochem. Soc. 1999, 146, 3639. [Google Scholar] [CrossRef]
- Wang, J.; Kaskel, S. KOH activation of carbon-based materials for energy storage. J. Mater. Chem. 2012, 22, 23710–23725. [Google Scholar] [CrossRef]
- Scherdel, C.; Reichenauer, G. Carbon xerogels synthesized via phenol–formaldehyde gels. Micropor. Mesopor. Mater. 2009, 126, 133–142. [Google Scholar] [CrossRef]
- Jirglová, H.; Pérez-Cadenas, A.F.; Maldonado-Hódar, F.J. Synthesis and Properties of Phloroglucinol−Phenol−Formaldehyde Carbon Aerogels and Xerogels. Langmuir 2009, 25, 2461–2466. [Google Scholar] [CrossRef] [PubMed]
- Molchanov, V.V.; Shchuchkin, M.N.; Zaikovskii, V.I.; Bogdanov, S.V.; Zaitseva, N.A. Sorbents and supports based on nanoporous carbon xerogels. Kinet. Catal. 2008, 49, 702–707. [Google Scholar] [CrossRef]
- Teng, H.; Wang, S.-C. Preparation of porous carbons from phenol–formaldehyde resins with chemical and physical activation. Carbon 2000, 38, 817–824. [Google Scholar] [CrossRef]
- Peikolainen, A.-L.; Uibu, M.; Kozlova, J.; Mändar, H.; Tamm, A.; Aabloo, A. Carbon xerogel from 5-methylresorcinol-formaldehyde gel: The controllability of structural properties. Carbon Trends 2021, 3, 100037. [Google Scholar] [CrossRef]
- Mikova, N.M.; Ivanov, I.P.; Zhizhaev, A.M.; Tsyganova, S.I.; Kuznetsov, B.N. Synthesis and Properties of Carbon Gels Based on Larch Bark Tannins and Hydrolysis Lignin. Russ. J. Appl. Chem. 2022, 95, 393–400. [Google Scholar] [CrossRef]
- Mikova, N.M.; Ivanov, I.P.; Fetisova, O.Y.; Kazachenko, A.S.; Kuznetsov, B.N. Characterization of the pine biomass derived tannin–furfuryl carbon xerogels. Biores. Technol. Rep. 2023, 22, 101454. [Google Scholar] [CrossRef]
- Wu, D.; Fu, R.; Yu, Z. Organic and carbon aerogels from the NaOH-catalyzed polycondensation of resorcinol–furfural and supercritical drying in ethanol. J. Appl. Polymer Sci. 2005, 96, 1429–1435. [Google Scholar] [CrossRef]
- Kiciński, W.; Szala, M.; Nita, M. Structurally tailored carbon xerogels produced through a sol–gel process in a water–methanol–inorganic salt solution. J. Sol-Gel Sci. Technol. 2011, 58, 102–113. [Google Scholar] [CrossRef]
- Tian, H.Y.; Buckley, C.E.; Paskevicius, M.; Sheppard, D.A. Acetic acid catalysed carbon xerogels derived from resorcinol-furfural for hydrogen storage. Int. J. Hydrogen Energy 2011, 36, 671–679. [Google Scholar] [CrossRef]
- Figueiredo, J.L. Functionalization of porous carbons for catalytic applications. J. Mater. Chem. A 2013, 1, 9351–9364. [Google Scholar] [CrossRef]
- Jin, H.; Zhang, H.; Zhong, H.; Zhang, J. Nitrogen-doped carbon xerogel: A novel carbon-based electrocatalyst for oxygen reduction reaction in proton exchange membrane (PEM) fuel cells. Energy Environ. Sci. 2011, 4, 3389–3394. [Google Scholar] [CrossRef]
- Shen, W.; Fan, W. Nitrogen-containing porous carbons: Synthesis and application. J. Mater. Chem. A 2013, 1, 999–1013. [Google Scholar] [CrossRef]
- Morawa Eblagon, K.; Rey-Raap, N.; Figueiredo, J.L.; Pereira, R.M.F. Relationships between texture, surface chemistry and performance of N-doped carbon xerogels in the oxygen reduction reaction. Appl. Surf. Sci. 2021, 548, 149242. [Google Scholar] [CrossRef]
- Hulicova-Jurcakova, D.; Seredych, M.; Lu, G.Q.; Bandosz, T.J. Combined Effect of Nitrogen- and Oxygen-Containing Functional Groups of Microporous Activated Carbon on its Electrochemical Performance in Supercapacitors. Adv. Func. Mater. 2009, 19, 438–447. [Google Scholar] [CrossRef]
- Leng, L.; Xu, S.; Liu, R.; Yu, T.; Zhuo, X.; Leng, S.; Xiong, Q.; Huang, H. Nitrogen containing functional groups of biochar: An overview. Biores. Technol. 2020, 298, 122286. [Google Scholar] [CrossRef]
- Lim, G.; Lee, K.B.; Ham, H.C. Effect of N-Containing Functional Groups on CO2 Adsorption of Carbonaceous Materials: A Density Functional Theory Approach. J. Phys. Chem. C 2016, 120, 8087–8095. [Google Scholar] [CrossRef]
- Yang, X.; Wan, Y.; Zheng, Y.; He, F.; Yu, Z.; Huang, J.; Wang, H.; Ok, Y.S.; Jiang, Y.; Gao, B. Surface functional groups of carbon-based adsorbents and their roles in the removal of heavy metals from aqueous solutions: A critical review. Chem. Eng. J. 2019, 366, 608–621. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Xu, Y.; Zhang, G.; Wu, C.; Liu, J.; Lv, Y. Nitrogen-doped porous carbons derived from sustainable biomass via a facile post-treatment nitrogen doping strategy: Efficient CO2 capture and DRM. Int. J. Hydrogen Energy 2022, 47, 24388–24397. [Google Scholar] [CrossRef]
- Rocha, R.P.; Restivo, J.; Sousa, J.P.S.; Órfão, J.J.M.; Pereira, M.F.R.; Figueiredo, J.L. Nitrogen-doped carbon xerogels as catalysts for advanced oxidation processes. Catal. Today 2015, 241, 73–79. [Google Scholar] [CrossRef]
- Álvarez-Manuel, L.; Alegre, C.; Sebastián, D.; Eizaguerri, A.; Napal, P.F.; Lázaro, M.J. N-doped carbon xerogels from urea-resorcinol-formaldehyde as carbon matrix for Fe-N-C catalysts for oxygen reduction in fuel cells. Catal. Today 2023, 418, 114067. [Google Scholar] [CrossRef]
- Yang, B.; Yu, C.; Yu, Q.; Zhang, X.; Li, Z.; Lei, L. N-doped carbon xerogels as adsorbents for the removal of heavy metal ions from aqueous solution. RSC Adv. 2015, 5, 7182–7191. [Google Scholar] [CrossRef]
- Liu, S.; Deng, C.; Yao, L.; Zhong, H.; Zhang, H. The key role of metal dopants in nitrogen-doped carbon xerogel for oxygen reduction reaction. J. Power Sources 2014, 269, 225–235. [Google Scholar] [CrossRef]
- Haye, E.; Job, N.; Wang, Y.; Penninckx, S.; Stergiopoulos, V.; Tumanov, N.; Cardinal, M.; Busby, Y.; Colomer, J.-F.; Su, B.-L.; et al. ZnO/Carbon xerogel photocatalysts by low-pressure plasma treatment, the role of the carbon substrate and its plasma functionalization. J. Colloid Interf. Sci. 2020, 570, 312–321. [Google Scholar] [CrossRef]
- Wang, S.; Miao, J.; Liu, M.; Zhang, L.; Liu, Z. Hierarchical porous N-doped carbon xerogels for high performance CO2 capture and supercapacitor. Colloids Surf. A Physicochem. Eng. Asp. 2021, 616, 126285. [Google Scholar] [CrossRef]
- Kiciński, W.; Norek, M.; Jankiewicz, B.J. Heterogeneous Carbon Gels: N-Doped Carbon Xerogels from Resorcinol and N-Containing Heterocyclic Aldehydes. Langmuir 2014, 30, 14276–14285. [Google Scholar] [CrossRef]
- Canal-Rodríguez, M.; Rey-Raap, N.; Menéndez, J.Á.; Montes-Morán, M.A.; Figueiredo, J.L.; Pereira, M.F.R.; Arenillas, A. Effect of porous structure on doping and the catalytic performance of carbon xerogels towards the oxygen reduction reaction. Microporous Mesoporous Mater. 2020, 293, 109811. [Google Scholar] [CrossRef]
- Lázaro, M.J.; Alegre, C.; Nieto-Monge, M.J.; Sebastián, D.; Gálvez, M.E.; Pastor, E.; Moliner, R. Nitrogen Doped and Functionalized Carbon Materials as Supports for Catalysts in Electro-Oxidation of Methanol. Adv. Sci. Technol. 2014, 93, 41–49. [Google Scholar] [CrossRef]
- Rocha, R.P.; Pereira, M.F.R.; Figueiredo, J.L. Characterisation of the surface chemistry of carbon materials by temperature-programmed desorption: An assessment. Catal. Today 2023, 418, 114136. [Google Scholar] [CrossRef]
- Silva, A.M.T.; Machado, B.F.; Figueiredo, J.L.; Faria, J.L. Controlling the surface chemistry of carbon xerogels using HNO3-hydrothermal oxidation. Carbon 2009, 47, 1670–1679. [Google Scholar] [CrossRef]
- Mager, N.; Meyer, N.; Léonard, A.F.; Job, N.; Devillers, M.; Hermans, S. Functionalization of carbon xerogels for the preparation of palladium supported catalysts applied in sugar transformations. Appl. Catal. B Environ. 2014, 148–149, 424–435. [Google Scholar] [CrossRef]
- Alegre, C.; Gálvez, M.E.; Baquedano, E.; Moliner, R.; Pastor, E.; Lázaro, M.J. Oxygen-Functionalized Highly Mesoporous Carbon Xerogel Based Catalysts for Direct Methanol Fuel Cell Anodes. J. Phys. Chem. C 2013, 117, 13045–13058. [Google Scholar] [CrossRef]
- Zapata-Benabihe, Z.; Moreno-Castilla, C.; Carrasco-Marín, F. Influence of the Boron Precursor and Drying Method on Surface Properties and Electrochemical Behavior of Boron-Doped Carbon Gels. Langmuir 2014, 30, 1716–1722. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Feng, J.; Meng, Q.; Cao, B.; Tian, G. Study on boron and nitrogen co-doped graphene xerogel for high-performance electrosorption application. J. Solid State Electrochem. 2019, 23, 2377–2390. [Google Scholar] [CrossRef]
- Eblagon, K.M.; Arenillas, A.; Malaika, A.; Fernando, R.; Pereira, M.; Figueiredo, J.L. The influence of the surface chemistry of phosphorylated carbon xerogel catalysts on the production of HMF from fructose in water. Fuel 2023, 334, 126610. [Google Scholar] [CrossRef]
- Annamalai, K.P.; Fathy, N.A.; Tao, Y. Synthesis and capacitance performance of phosphorous-enriched carbon xerogel. J. Sol-Gel Sci. Technol. 2017, 84, 515–521. [Google Scholar] [CrossRef]
- Ma, Y.; Wu, D.; Wang, T.; Jia, D. Nitrogen, Phosphorus Co-doped Carbon Obtained from Amino Acid Based Resin Xerogel as Efficient Electrode for Supercapacitor. ACS Appl. Energy Mater. 2020, 3, 957–969. [Google Scholar] [CrossRef]
- Deng, C.; Lu, W. A facile process to fabricate phosphorus/carbon xerogel composite as anode for sodium ion batteries. J. Electrochem. Soc. 2021, 168, 080529. [Google Scholar] [CrossRef]
- Wu, J.; Yang, Z.; Sun, Q.; Li, X.; Strasser, P.; Yang, R. Synthesis and electrocatalytic activity of phosphorus-doped carbon xerogel for oxygen reduction. Electrochim. Acta 2014, 127, 53–60. [Google Scholar] [CrossRef]
- Kiciński, W.; Norek, M.; Dziura, A.; Polański, M. Copolycondensation of heterocyclic aldehydes: A general approach to sulfur and nitrogen dually-doped carbon gels. Microporous Mesoporous Mater. 2016, 225, 198–209. [Google Scholar] [CrossRef]
- Kiciński, W.; Dembinska, B.; Norek, M.; Budner, B.; Polański, M.; Kulesza, P.J.; Dyjak, S. Heterogeneous iron-containing carbon gels as catalysts for oxygen electroreduction: Multifunctional role of sulfur in the formation of efficient systems. Carbon 2017, 116, 655–669. [Google Scholar] [CrossRef]
- Thakur, A.K.; Kurtyka, K.; Majumder, M.; Yang, X.; Ta, H.Q.; Bachmatiuk, A.; Liu, L.; Trzebicka, B.; Rummeli, M.H. Recent Advances in Boron- and Nitrogen-Doped Carbon-Based Materials and Their Various Applications. Adv. Mater. Interf. 2022, 9, 2101964. [Google Scholar] [CrossRef]
- Radkevich, V.Z.; Senko, T.L.; Wilson, K.; Grishenko, L.M.; Zaderko, A.N.; Diyuk, V.Y. The influence of surface functionalization of activated carbon on palladium dispersion and catalytic activity in hydrogen oxidation. Appl. Catal. A Gen. 2008, 335, 241–251. [Google Scholar] [CrossRef]
- Zhu, E.; Wu, M.; Xu, H.; Peng, B.; Liu, Z.; Huang, Y.; Li, Y. Stability of Platinum-Group-Metal-Based Electrocatalysts in Proton Exchange Membrane Fuel Cells. Adv. Func. Mater. 2022, 32, 2203883. [Google Scholar] [CrossRef]
- Lambert, S.; Job, N.; D’Souza, L.; Pereira, M.F.R.; Pirard, R.; Heinrichs, B.; Figueiredo, J.L.; Pirard, J.-P.; Regalbuto, J.R. Synthesis of very highly dispersed platinum catalysts supported on carbon xerogels by the strong electrostatic adsorption method. J. Catal. 2009, 261, 23–33. [Google Scholar] [CrossRef]
- Zubizarreta, L.; Menéndez, J.A.; Job, N.; Marco-Lozar, J.P.; Pirard, J.P.; Pis, J.J.; Linares-Solano, A.; Cazorla-Amorós, D.; Arenillas, A. Ni-doped carbon xerogels for H2 storage. Carbon 2010, 48, 2722–2733. [Google Scholar] [CrossRef]
- Sun, H.; Tian, H.; Hardjono, Y.; Buckley, C.E.; Wang, S. Preparation of cobalt/carbon-xerogel for heterogeneous oxidation of phenol. Catal. Today 2012, 186, 63–68. [Google Scholar] [CrossRef]
- Zainul Abidin, A.F.; Loh, K.S.; Wong, W.Y.; Mohamad, A.B.; Puspasari, I. Effect of carbon precursor and initial pH on cobalt-doped carbon xerogel for oxygen reduction. Int. J. Hydrogen Energy 2018, 43, 11047–11055. [Google Scholar] [CrossRef]
- Skowroński, J.M.; Osińska, M. Effect of nickel catalyst on physicochemical properties of carbon xerogels as electrode materials for supercapacitor. Current Appl. Phys. 2012, 12, 911–918. [Google Scholar] [CrossRef]
- El-Jemni, M.A.; Hassan, H.H.; Abd El Rehim, S.S.; Abdel-Samad, H.S. Nano-Nickel Doped Carbon Xerogel: Synthesis, Characterization and Catalytic Performance towards Ethanol Fuel Electro-oxidation. ChemistrySelect 2023, 8, e202300809. [Google Scholar] [CrossRef]
- Job, N.; Pirard, R.; Marien, J.; Pirard, J.-P. Synthesis of transition metal-doped carbon xerogels by solubilization of metal salts in resorcinol–formaldehyde aqueous solution. Carbon 2004, 42, 3217–3227. [Google Scholar] [CrossRef]
- Barranco-López, A.; Moral-Rodríguez, A.I.; Fajardo-Puerto, E.; Elmouwahidi, A.; Bailón-García, E. Highly graphitic Fe-doped carbon xerogels as dual-functional electro-Fenton catalysts for the degradation of tetracycline in wastewater. Environ. Res. 2023, 228, 115757. [Google Scholar] [CrossRef] [PubMed]
- Ptaszkowska-Koniarz, M.; Goscianska, J.; Pietrzak, R. Synthesis of carbon xerogels modified with amine groups and copper for efficient adsorption of caffeine. Chem. Eng. J. 2018, 345, 13–21. [Google Scholar] [CrossRef]
- Job, N.; Pirard, R.; Vertruyen, B.; Colomer, J.-F.; Marien, J.; Pirard, J.-P. Synthesis of transition metal-doped carbon xerogels by cogelation. J. Non-Crystal. Solids 2007, 353, 2333–2345. [Google Scholar] [CrossRef]
- Chen, L.; Deng, J.; Hong, S.; Lian, H. Rapid, tunable synthesis of porous carbon xerogels with expanded graphite and their application as anodes for Li-ion batteries. J. Colloid Interf. Sci. 2020, 565, 368–377. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Deng, J.; Yuan, Y.; Hong, S.; Yan, B.; He, S.; Lian, H. Hierarchical porous graphitized carbon xerogel for high performance supercapacitor. Diam. Relat. Mater. 2022, 121, 108781. [Google Scholar] [CrossRef]
- Skowroński, J.M.; Osińska, M. The influence of thermal treatment on the electrochemical properties of carbon–Ni–Pd composites. J. Sol-Gel Sci. Technol. 2014, 71, 109–117. [Google Scholar] [CrossRef]
- Kuśmierek, K.; Kiciński, W.; Norek, M.; Polański, M.; Budner, B. Oxidative and adsorptive removal of chlorophenols over Fe-, N- and S-multi-doped carbon xerogels. J. Environ. Chem. Eng. 2021, 9, 105568. [Google Scholar] [CrossRef]
- Zainul Abidin, A.F.; Loh, K.S.; Wong, W.Y.; Mohamad, A.B. Nitrogen-doped carbon xerogels catalyst for oxygen reduction reaction: Improved structural and catalytic activity by enhancing nitrogen species and cobalt insertion. Int. J. Hydrogen Energy 2019, 44, 28789–28802. [Google Scholar] [CrossRef]
- Piedboeuf, M.-L.C.; Léonard, A.F.; Reichenauer, G.; Balzer, C.; Job, N. How do the micropores of carbon xerogels influence their electrochemical behavior as anodes for lithium-ion batteries? Microporous Mesoporous Mater. 2019, 275, 278–287. [Google Scholar] [CrossRef]
- Gaikwad, M.M.; Kakunuri, M.; Sharma, C.S. Enhanced catalytic graphitization of resorcinol formaldehyde derived carbon xerogel to improve its anodic performance for lithium ion battery. Mater. Today Commun. 2019, 20, 100569. [Google Scholar] [CrossRef]
- Gaikwad, M.M.; Sharma, C.S. In situ graphitized hard carbon xerogel: A promising high-performance anode material for Li-ion batteries. J. Mater. Res. 2020, 35, 2989–3003. [Google Scholar] [CrossRef]
- Kakunuri, M.; Kali, S.; Sharma, C.S. Catalytic graphitization of resorcinol-formaldehyde xerogel and its effect on lithium ion intercalation. J. Analyt. Appl. Pyrol. 2016, 117, 317–324. [Google Scholar] [CrossRef]
- Shi, Q.; Zhou, J.; Ullah, S.; Yang, X.; Tokarska, K.; Trzebicka, B.; Ta, H.Q.; Rümmeli, M.H. A review of recent developments in Si/C composite materials for Li-ion batteries. Energy Stor. Mater. 2021, 34, 735–754. [Google Scholar] [CrossRef]
- Santos-Gómez, L.d.; Cuesta, N.; Cameán, I.; García-Granda, S.; García, A.B.; Arenillas, A. A promising silicon/carbon xerogel composite for high-rate and high-capacity lithium-ion batteries. Electrochim. Acta 2022, 426, 140790. [Google Scholar] [CrossRef]
- Huang, M.; Yoo, S.J.; Lee, J.-S.; Yoon, T.-H. Electrochemical properties of an activated carbon xerogel monolith from resorcinol–formaldehyde for supercapacitor electrode applications. RSC Adv. 2021, 11, 33192–33201. [Google Scholar] [CrossRef]
- Osińska, M.; Krawczyk, P.; Łuczak, T.; Rozmanowski, T. Nitrogen, nickel and graphene oxide doped carbon xerogel as an active electrode of an electrochemical capacitor. J. Sol-Gel Sci. Technol. 2023, 106, 827–836. [Google Scholar] [CrossRef]
- Chen, L.; Deng, J.; Song, Y.; Hong, S.; Lian, H. Highly Stable Dispersion of Carbon Nanotubes in Deep Eutectic Solvent for the Preparation of CNT-Embedded Carbon Xerogels for Supercapacitors. ChemElectroChem 2019, 6, 5750–5758. [Google Scholar] [CrossRef]
- Khammar, H.; Abdelwahab, A.; Abdel-Samad, H.S.; Hassan, H.H. Synergistic performance of simply fabricated polyaniline/carbon xerogel composite as supercapacitor electrode. J. Electroanalyt. Chem. 2021, 880, 114848. [Google Scholar] [CrossRef]
- Wasfey, M.A.; Abdelwahab, A.; Carrasco-Marín, F.; Pérez-Cadenas, A.F.; Abdullah, H.H.; Yahia, S.I.; Farghali, A.A. Nickel Cobaltite Functionalized Silver Doped Carbon Xerogels as Efficient Electrode Materials for High Performance Symmetric Supercapacitor. Materials 2020, 13, 4906. [Google Scholar] [CrossRef] [PubMed]
- Abdelwahab, A.; Farghali, A.A.; Enaiet Allah, A. Synergy between iron oxide sites and nitrogen-doped carbon xerogel/diamond matrix for boosting the oxygen reduction reaction. Nanoscale Adv. 2022, 4, 837–848. [Google Scholar] [CrossRef]
- Abdelrazek, G.M.; El-Deeb, M.M.; Farghali, A.A.; Pérez-Cadenas, A.F.; Abdelwahab, A. Design of Self-Supported Flexible Nanostars MFe-LDH@ Carbon Xerogel-Modified Electrode for Methanol Oxidation. Materials 2021, 14, 5271. [Google Scholar] [CrossRef] [PubMed]
- Abdelwahab, A.; Carrasco-Marín, F.; Pérez-Cadenas, A.F. Carbon Xerogels Hydrothermally Doped with Bimetal Oxides for Oxygen Reduction Reaction. Materials 2019, 12, 2446. [Google Scholar] [CrossRef] [PubMed]
- Abdelrazek, G.M.; Sayed, M.A.; El-Deeb, M.M.; Farghali, A.A.; Abdelwahab, A. 3D Nanohybrid of MoS2 nanocoils functionalized carbon xerogels for methanol electro-oxidation. J. Alloys Compnd. 2022, 921, 166077. [Google Scholar] [CrossRef]
- Medina, O.E.; Galeano-Caro, D.; Castelo-Quibén, J.; Ocampo-Pérez, R.; Perez-Cadenas, A.F.; Carrasco-Marín, F.; Franco, C.A.; Corteś, F.B. Monolithic carbon xerogels-metal composites for crude oil removal from oil in-saltwater emulsions and subsequent regeneration through oxidation process: Composites synthesis, adsorption studies, and oil decomposition experiments. Microporous Mesoporous Mater 2021, 319, 111039. [Google Scholar] [CrossRef]
- Rashed, Y.; Messele, S.A.; Zeng, H.; Gamal El-Din, M. Mesoporous carbon xerogel material for the adsorption of model naphthenic acids: Structure effect and kinetics modelling. Environ. Technol. 2020, 41, 3534–3543. [Google Scholar] [CrossRef]
- Benally, C.; Messele, S.A.; Gamal El-Din, M. Adsorption of organic matter in oil sands process water (OSPW) by carbon xerogel. Water Res. 2019, 154, 402–411. [Google Scholar] [CrossRef] [PubMed]
- Girgis, B.S.; Attia, A.A.; Fathy, N.A. Potential of nano-carbon xerogels in the remediation of dye-contaminated water discharges. Desalination 2011, 265, 169–176. [Google Scholar] [CrossRef]
- Carrales-Alvarado, D.H.; Leyva-Ramos, R.; Bailón-García, E.; Carrasco-Marín, F.; Villela-Martinez, D.E. Synthesis, characterization, and application of pristine and clay-templated carbon xerogel microspheres for removing diclofenac and heavy metals from water solution. Environ. Sci. Pollut. Res. 2023, 30, 34684–34697. [Google Scholar] [CrossRef] [PubMed]
- Segovia-Sandoval, S.J.; Pastrana-Martínez, L.M.; Ocampo-Pérez, R.; Morales-Torres, S.; Berber-Mendoza, M.S.; Carrasco-Marín, F. Synthesis and characterization of carbon xerogel/graphene hybrids as adsorbents for metronidazole pharmaceutical removal: Effect of operating parameters. Separ. Purif. Technol. 2020, 237, 116341. [Google Scholar] [CrossRef]
- Ptaszkowska-Koniarz, M.; Goscianska, J.; Bazan-Wozniak, A.; Pietrzak, R. Amine-Modified Carbon Xerogels as Effective Carbon-Based Adsorbents of Anionic Dye from Aqueous Solutions. Materials 2022, 15, 5736. [Google Scholar] [CrossRef]
- Ptaszkowska-Koniarz, M.; Goscianska, J.; Pietrzak, R. Removal of rhodamine B from water by modified carbon xerogels. Colloids Surf. A Physicochem. Eng. Asp. 2018, 543, 109–117. [Google Scholar] [CrossRef]
- Rastegar, A.; Gholami, M.; Jafari, A.J.; Hosseini-Bandegharaei, A.; Kermani, M.; Hashemi, Y.K. Use of NH4Cl for activation of carbon xerogel to prepare a novel efficacious adsorbent for benzene removal from contaminated air streams in a fixed-bed column. J. Environ. Health Sci. Eng. 2020, 18, 1141–1149. [Google Scholar] [CrossRef]
- Vivo-Vilches, J.F.; Pérez-Cadenas, A.F.; Maldonado-Hódar, F.J.; Carrasco-Marín, F.; Regufe, M.J.; Ribeiro, A.M.; Ferreira, A.F.P.; Rodrigues, A.E. Resorcinol–formaldehyde carbon xerogel as selective adsorbent of carbon dioxide present on biogas. Adsorption 2018, 24, 169–177. [Google Scholar] [CrossRef]
- Zheivot, V.I.; Molchanov, V.V.; Zaikovskii, V.I.; Krivoruchko, V.N.; Zaitseva, N.A.; Shchuchkin, M.N. Carbon xerogels: Nano- and adsorption textures, chemical nature of the surface and gas chromatography properties. Microporous Mesoporous Mater. 2010, 130, 7–13. [Google Scholar] [CrossRef]
- De Sousa, J.G.M.; da Silva, T.V.C.; de Moraes, N.P.; Caetano Pinto da Silva, M.L.; da Silva Rocha, R.; Landers, R.; Rodrigues, L.A. Visible light-driven ZnO/g-C3N4/carbon xerogel ternary photocatalyst with enhanced activity for 4-chlorophenol degradation. Mater. Chem. Phys. 2020, 256, 123651. [Google Scholar] [CrossRef]
- de Moraes, N.P.; Valim, R.B.; da Silva Rocha, R.; da Silva, M.L.C.P.; Campos, T.M.B.; Thim, G.P.; Rodrigues, L.A. Effect of synthesis medium on structural and photocatalytic properties of ZnO/carbon xerogel composites for solar and visible light degradation of 4-chlorophenol and bisphenol A. Colloids Surf. A Physicochem. Eng. Asp. 2020, 584, 124034. [Google Scholar] [CrossRef]
- De Moraes, N.P.; Goes, C.M.; Sperandio, D.C.; Rocha, R.d.S.; Landers, R.; Paramasivam, T.; Rodrigues, L.A. Development of a new zinc oxide/tin oxide/carbon xerogel photocatalyst for visible light photodegradation of 4-chlorophenol. Mater. Sci. Eng. B 2021, 269, 115183. [Google Scholar] [CrossRef]
- Safri, A.; Fletcher, A.J.; Safri, R.; Rasheed, H. Integrated Adsorption–Photodegradation of Organic Pollutants by Carbon Xerogel/Titania Composites. Molecules 2022, 27, 8483. [Google Scholar] [CrossRef]
- Do Carmo Batista, W.V.F.; da Cunha, R.; dos Santos, A.C.; dos Reis, P.M.; Furtado, C.A.; Silva, M.C.; de Fátima Gorgulho, H. Synthesis of a reusable magnetic photocatalyst based on carbon xerogel/TiO2 composites and its application on acetaminophen degradation. Ceram. Int. 2022, 48, 34395–34404. [Google Scholar] [CrossRef]
- De Moraes, N.P.; Torezin, F.A.; Jucá Dantas, G.V.; de Sousa, J.G.M.; Valim, R.B.; da Silva Rocha, R.; Landers, R.; da Silva, M.L.C.P.; Rodrigues, L.A. TiO2/Nb2O5/carbon xerogel ternary photocatalyst for efficient degradation of 4-chlorophenol under solar light irradiation. Ceram. Int. 2020, 46, 14505–14515. [Google Scholar] [CrossRef]
- De Moraes, N.P.; de Siervo, A.; Silva, T.O.; da Silva Rocha, R.; Reddy, D.A.; Lianqing, Y.; de Vasconcelos Lanza, M.R.; Rodrigues, L.A. Kraft lignin-based carbon xerogel/zinc oxide composite for 4-chlorophenol solar-light photocatalytic degradation: Effect of pH, salinity, and simultaneous Cr(VI) reduction. Environ. Sci. Pollut. Res. 2023, 30, 8280–8296. [Google Scholar] [CrossRef]
- Sebastián, D.; Alegre, C.; Calvillo, L.; Pérez, M.; Moliner, R.; Lázaro, M.J. Carbon supports for the catalytic dehydrogenation of liquid organic hydrides as hydrogen storage and delivery system. Int. J. Hydrogen Energy 2014, 39, 4109–4115. [Google Scholar] [CrossRef]
- Bailón-García, E.; Carrasco-Marín, F.; Pérez-Cadenas, A.F.; Maldonado-Hódar, F.J. Microspheres of carbon xerogel: An alternative Pt-support for the selective hydrogenation of citral. Appl. Catal. A Gen. 2014, 482, 318–326. [Google Scholar] [CrossRef]
- Tang, D.; Sun, X.; Zhao, D.; Zhu, J.; Zhang, W.; Xu, X.; Zhao, Z. Nitrogen-Doped Carbon Xerogels Supporting Palladium Nanoparticles for Selective Hydrogenation Reactions: The Role of Pyridine Nitrogen Species. ChemCatChem 2018, 10, 1291–1299. [Google Scholar] [CrossRef]
- Bailón-García, E.; Drwal, E.; Grzybek, T.; Henriques, C.; Ribeiro, M.F. Catalysts based on carbon xerogels with high catalytic activity for the reduction of NOx at low temperatures. Catal. Today 2020, 356, 301–311. [Google Scholar] [CrossRef]
- Mazzone, S.; Goklany, T.; Zhang, G.; Tan, J.; Papaioannou, E.I.; García-García, F.R. Ruthenium-based catalysts supported on carbon xerogels for hydrogen production via ammonia decomposition. Appl. Catal. A Gen. 2022, 632, 118484. [Google Scholar] [CrossRef]
- Barros, R.A.M.; Cristóvão, R.O.; Carabineiro, S.A.C.; Neves, M.C.; Freire, M.G.; Faria, J.L.; Santos-Ebinuma, V.C.; Tavares, A.P.M.; Silva, C.G. Immobilization and Characterization of L-Asparaginase over Carbon Xerogels. BioTech 2022, 11, 10. [Google Scholar] [CrossRef]

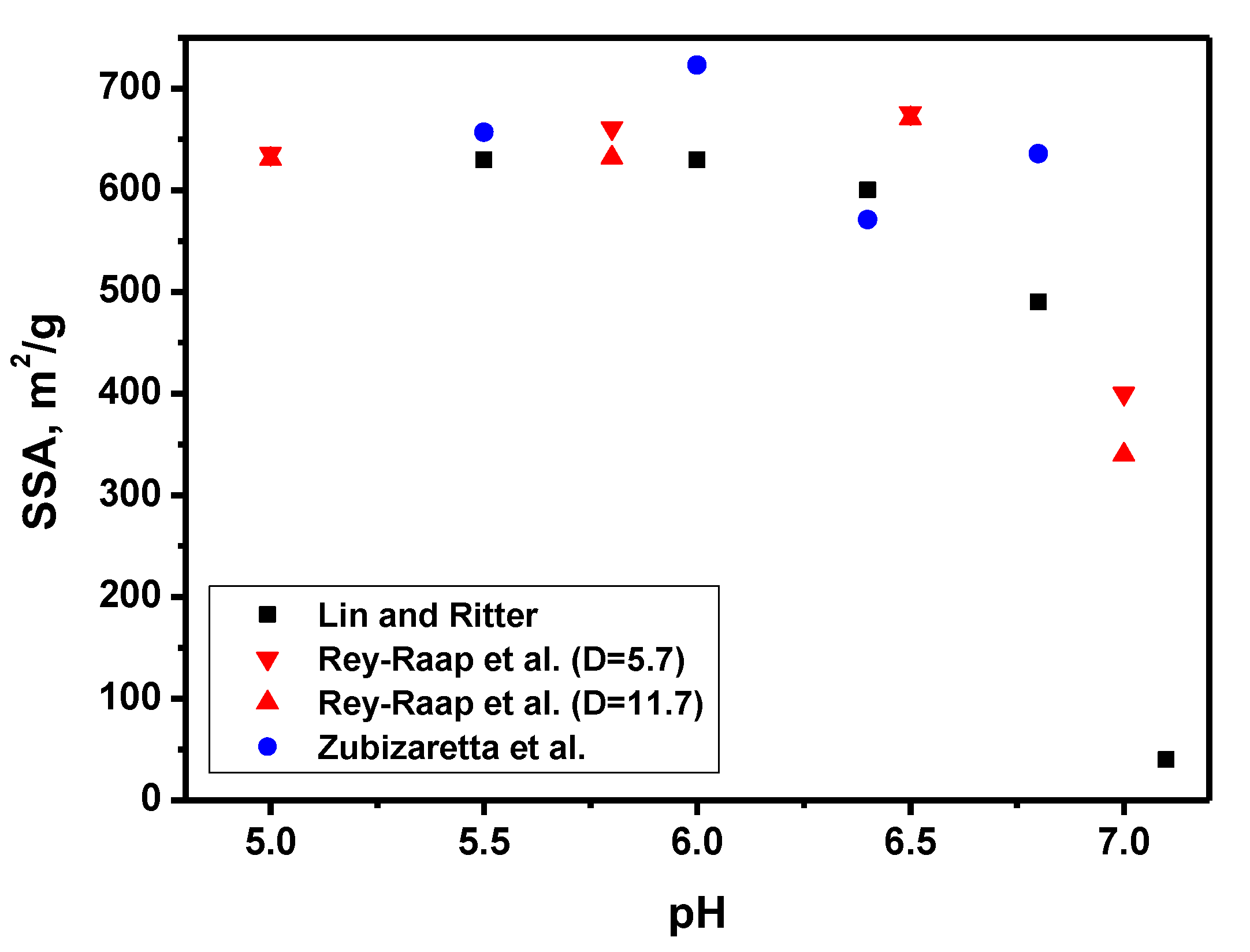
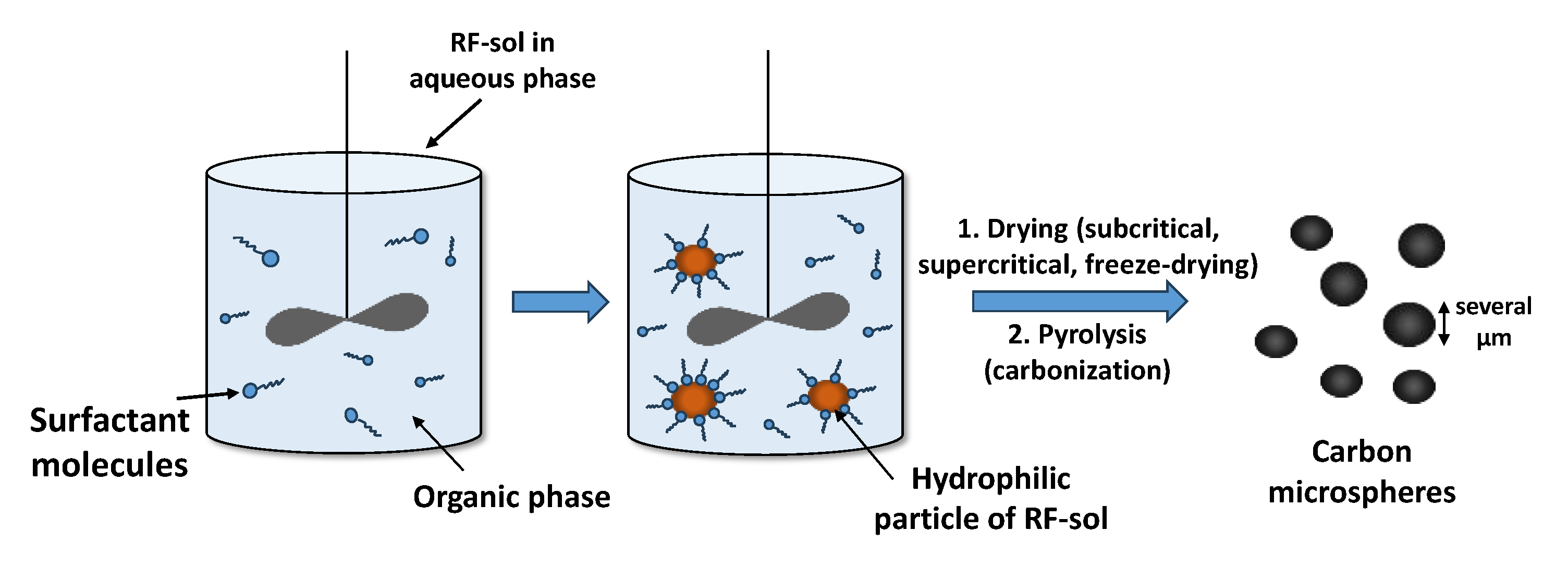
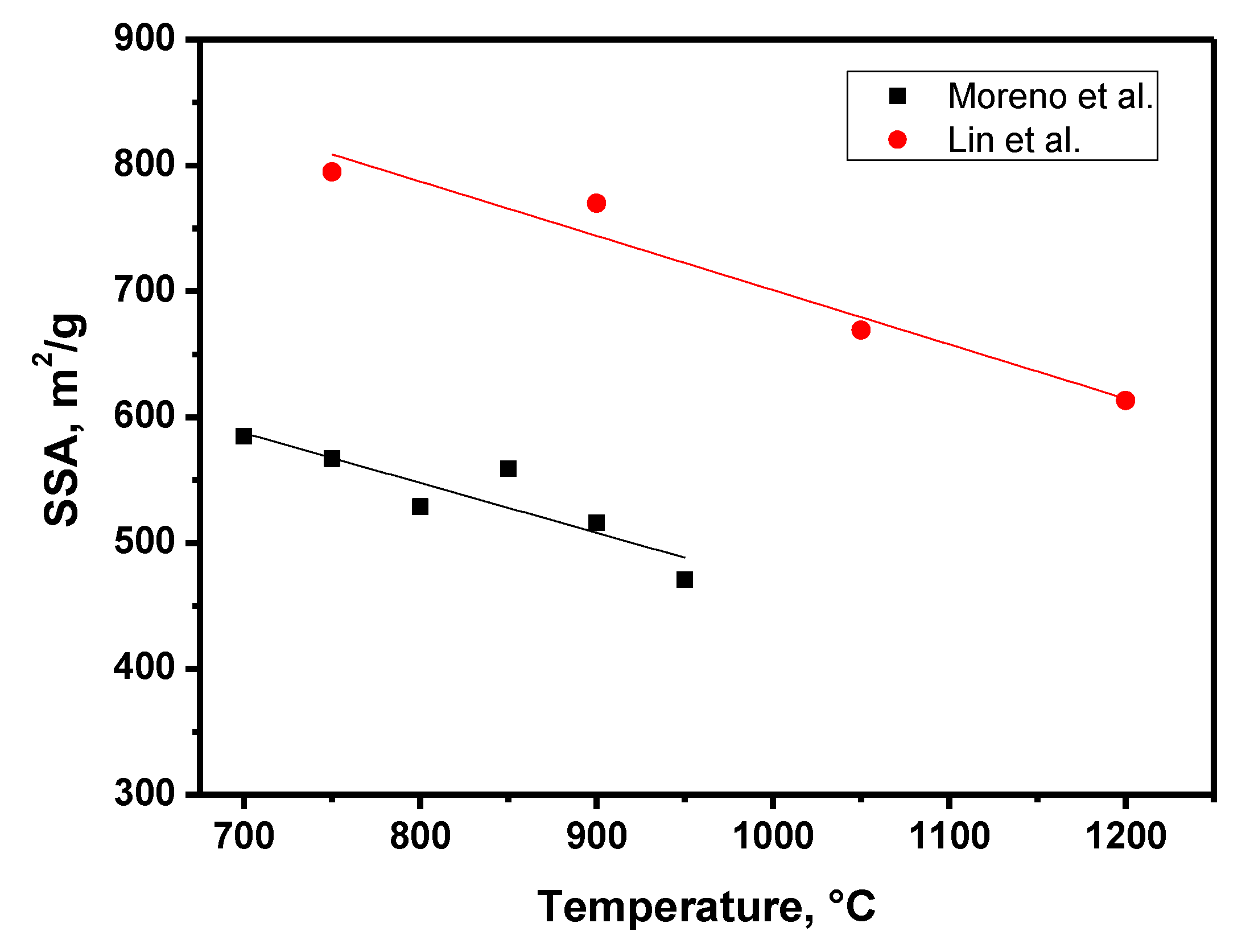
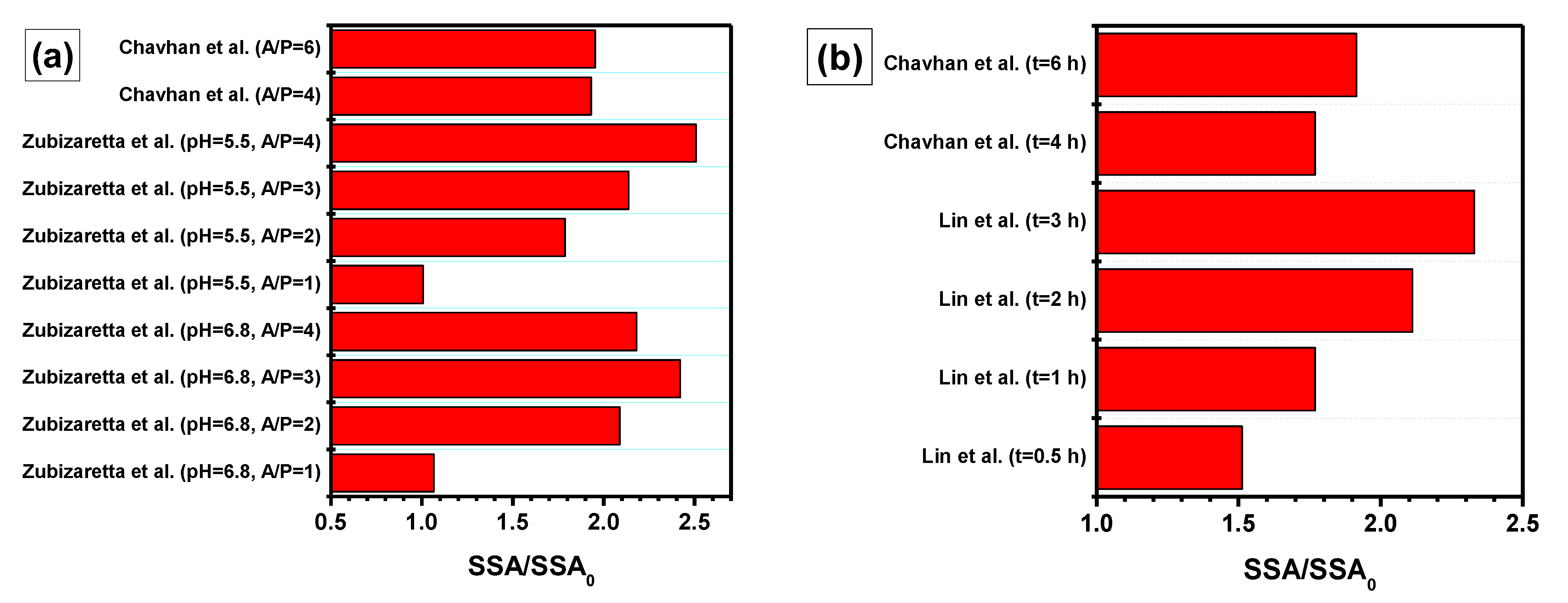
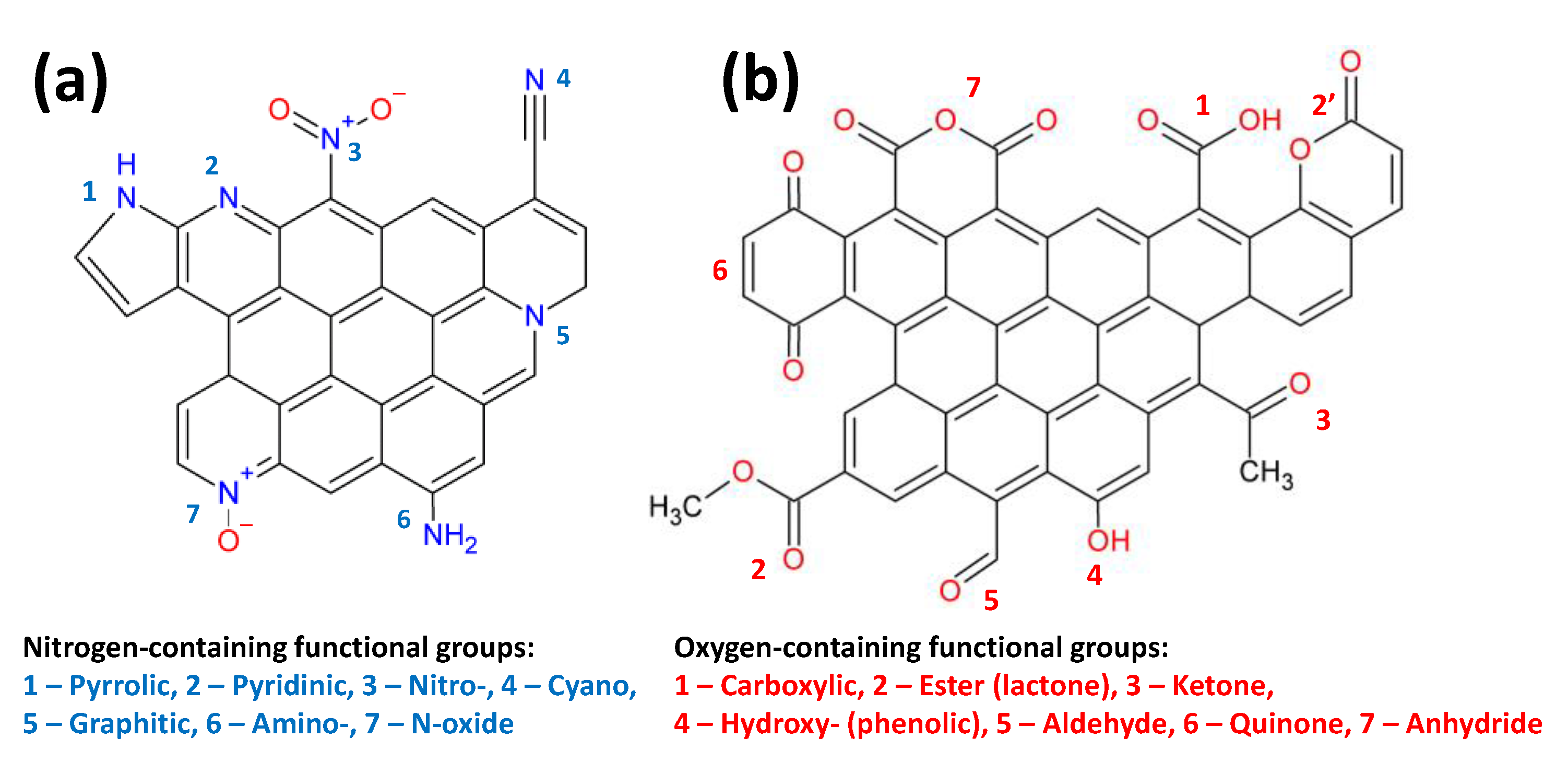
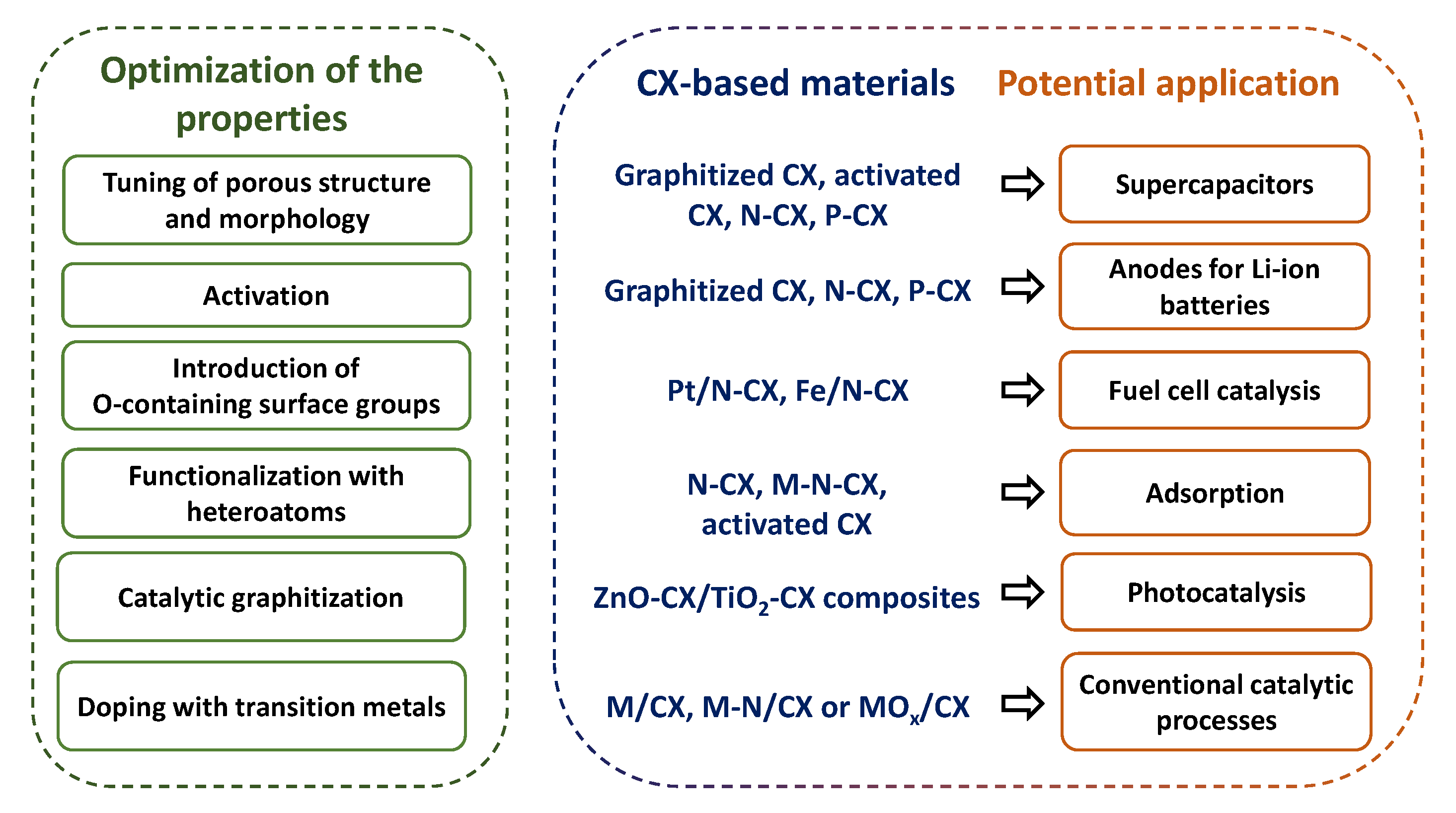
| Factor | Effect |
|---|---|
| Increase in the pyrolysis temperature | Decrease in the oxygen concentration |
| Decrease in the specific surface area (if particle size exceeds 212 microns) and pore volume | |
| Increase in macroporosity | |
| Increase in microporosity at low R/C values (high pH) | |
| Rise in the electrochemical capacity at temperatures up to 850 °C (decrease at higher temperatures) | |
| Decrease in the interspace distance (d002) | |
| Rise in the electric conduction within a temperature range of 500–700 °C (no effect within a range of 700–900 °C) | |
| Increase in the pyrolysis duration | Increase in the specific surface area, pore diameter, and volume |
| Rise in the electrochemical capacity at times up to 3 h (decrease at longer times) | |
| Increase in the heating rate | Insignificant increase in the specific surface area |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Veselov, G.B.; Vedyagin, A.A. Resorcinol–Formaldehyde-Derived Carbon Xerogels: Preparation, Functionalization, and Application Aspects. Materials 2023, 16, 6566. https://doi.org/10.3390/ma16196566
Veselov GB, Vedyagin AA. Resorcinol–Formaldehyde-Derived Carbon Xerogels: Preparation, Functionalization, and Application Aspects. Materials. 2023; 16(19):6566. https://doi.org/10.3390/ma16196566
Chicago/Turabian StyleVeselov, Grigory B., and Aleksey A. Vedyagin. 2023. "Resorcinol–Formaldehyde-Derived Carbon Xerogels: Preparation, Functionalization, and Application Aspects" Materials 16, no. 19: 6566. https://doi.org/10.3390/ma16196566








