The Role of Carbon in Metal–Organic Chemical Vapor Deposition-Grown MoS2 Films
Abstract
:1. Introduction
2. Materials and Methods
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kim, S.; Konar, A.; Hwang, W.; Lee, J.H.; Lee, J.; Yang, J.; Jung, C.; Kim, H.; Yoo, J.; Choi, J.; et al. High-mobility and low-power thin-film transistors based on multilayer MoS2 crystals. Nat. Commun. 2012, 3, 1011. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Wakabayashi, K.; Xu, Y.; Nakaharai, S.; Komatsu, K.; Li, W.; Lin, Y.; Aparecido-Ferreira, A.; Tsukagoshi, K. Thickness-dependent interfacial coulomb scattering in atomically thin field-effect transistors. Nano Lett. 2013, 13, 3546–3552. [Google Scholar] [CrossRef] [PubMed]
- Lopez-Sanchez, O.; Lembke, D.; Kayci, M.; Radenovic, A.; Kis, A. Ultrasensitive photodetectors based on monolayer MoS2. Nat. Nanotechnol. 2013, 8, 497–501. [Google Scholar] [CrossRef] [PubMed]
- Pospischil, A.; Furchi, M.; Mueller, T. Solar-energy conversion and light emission in an atomic monolayer p-n diode. Nat. Nanotechnol. 2014, 9, 257–261. [Google Scholar] [CrossRef]
- Branny, A.; Kumar, S.; Proux, R.; Gerardot, B.D. Deterministic strain-induced arrays of quantum emitters in a two-dimensional semiconductor. Nat. Commun. 2017, 8, 15053. [Google Scholar] [CrossRef] [PubMed]
- Yu, W.J.; Liu, Y.; Zhou, H.; Yin, A.; Li, Z.; Huang, Y.; Duan, X. Highly efficient gate-tunable photocurrent generation in vertical heterostructures of layered materials. Nat. Nanotechnol. 2013, 8, 952–958. [Google Scholar] [CrossRef]
- Withers, F.; Del Pozo-Zamudio, O.; Mishchenko, A.; Rooney, A.P.; Gholinia, A.; Watanabe, K.; Taniguchi, T.; Haigh, S.J.; Geim, A.K.; Tartakovskii, A.I.; et al. Light-Emitting Diodes by Band-Structure Engineering in van der Waals Heterostructures. Nat. Mater. 2015, 14, 301–306. [Google Scholar] [CrossRef]
- Cheng, R.; Li, D.; Zhou, H.; Wang, C.; Yin, A.; Jiang, S.; Liu, Y.; Chen, Y.; Huang, Y.; Duan, X. Electroluminescence and photocurrent generation from atomically sharp WSe2/MoS2 heterojunction p-n diodes. Nano Lett. 2014, 14, 5590–5597. [Google Scholar] [CrossRef]
- Xiong, Y.; Wang, Y.; Zhu, R.; Xu, H.; Wu, C.; Chen, J.; Ma, Y.; Liu, Y.; Chen, Y.; Watanabe, K.; et al. Twisted black phosphorus–based van der Waals stacks for fiber-integrated polarimeters. Sci. Adv. 2022, 8, eabo0375. [Google Scholar] [CrossRef]
- Zhu, Y.; Li, Y.; Arefe, G.; Burke, R.A.; Tan, C.; Hao, Y.; Liu, X.; Liu, X.; Yoo, W.J.; Dubey, M.; et al. Monolayer molybdenum disulfide transistors with single-atom-thick gates. Nano Lett. 2018, 18, 3807–3813. [Google Scholar] [CrossRef]
- Baugher, B.W.H.; Churchill, H.O.H.; Yang, Y.; Jarillo-Herrero, P. Optoelectronic Devices Based on Electrically Tunable p-n Diodes in a Monolayer Dichalcogenide. Nat. Nanotechnol. 2014, 9, 262–267. [Google Scholar] [CrossRef] [PubMed]
- Kang, K.; Xie, S.; Huang, L.; Han, Y.; Huang, P.Y.; Mak, K.F.; Kim, C.; Muller, D.; Park, J. High-mobility three-atom-thick semiconducting films with wafer-scale homogeneity. Nature 2015, 520, 656–660. [Google Scholar] [CrossRef] [PubMed]
- Ovchinnikov, D.; Allain, A.; Huang, Y.-S.; Dumcenco, D.; Kis, A. Electrical transport properties of single-layer WS2. ACS Nano 2014, 8, 8174–8181. [Google Scholar] [CrossRef] [PubMed]
- Splendiani, A.; Sun, L.; Zhang, Y.; Li, T.; Kim, J.; Chim, C.; Galli, G.; Wang, F. Emerging photoluminescence in monolayer MoS2. Nano Lett. 2010, 10, 1271–1275. [Google Scholar] [CrossRef]
- Eda, G.; Yamaguchi, H.; Voiry, D.; Fujita, T.; Chen, M.; Chhowalla, M. Photoluminescence from chemically exfoliated MoS2. Nano Lett. 2011, 11, 5111–5116. [Google Scholar] [CrossRef]
- Mak, K.F.; Lee, C.; Hone, J.; Shan, J.; Heinz, T.F. Atomically thin MoS2: A new direct-gap semiconductor. Phys. Rev. Lett. 2010, 105, 136805. [Google Scholar] [CrossRef]
- Xiao, D.; Liu, G.; Feng, W.; Xu, X.; Yao, W. Coupled spin and valley physics in monolayers of MoS2 and other group-VI dichalcogenides. Phys. Rev. Lett. 2012, 108, 196802. [Google Scholar] [CrossRef]
- Zeng, H.; Liu, G.; Dai, J.; Yan, Y.; Zhu, B.; He, R.; Xie, L.; Xu, S.; Chen, X.; Yao, W.; et al. Optical Signature of symmetry variations and spin-valley coupling in atomically thin tungsten dichalcogenides. Sci. Rep. 2013, 3, 1608. [Google Scholar] [CrossRef]
- Zhao, Z.; Hou, T.; Wu, N.; Jiao, S.; Zhou, K.; Yin, J.; Suk, J.W.; Cui, X.; Zhang, M.; Li, S.; et al. Polycrystalline few-layer graphene as a durable anticorrosion film for copper. Nano Lett. 2021, 21, 1161–1168. [Google Scholar] [CrossRef]
- Zhou, H.; Wang, C.; Shaw, J.C.; Cheng, R.; Chen, Y.; Huang, X.; Liu, Y.; Weiss, N.O.; Lin, Z.; Huang, Y.; et al. Large area growth and electrical properties of P-type WSe2 atomic layers. Nano Lett. 2015, 15, 709–713. [Google Scholar] [CrossRef]
- Ling, X.; Lee, Y.; Lin, Y.; Fang, W.; Yu, L.; Dresselhaus, M.S.; Kong, J. Role of the seeding promoter in MoS2 growth by chemical vapor deposition. Nano Lett. 2014, 14, 464–472. [Google Scholar] [CrossRef] [PubMed]
- Hao, Y.; Wang, L.; Liu, Y.; Chen, H.; Wang, X.; Tan, C.; Nie, S.; Suk, J.W.; Jiang, T.; Liang, T.; et al. Oxygen-activated growth and bandgap tunability of large single-crystal bilayer graphene. Nat. Nanotechnol. 2016, 11, 426–431. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Ugeda, M.M.; Jin, C.; Shi, S.-F.; Bradley, A.J.; MartínRecio, A.; Ryu, H.; Kim, J.; Tang, S.; Kim, Y.; et al. Electronic structure, surface doping, and optical response in epitaxial WSe2 thin films. Nano Lett. 2016, 16, 2485–2491. [Google Scholar] [CrossRef] [PubMed]
- Nakano, M.; Wang, Y.; Kashiwabara, Y.; Matsuoka, H.; Iwasa, Y. Layer-by-layer epitaxial growth of scalable WSe2 on Sapphire by molecular beam epitaxy. Nano Lett. 2017, 17, 5595–5599. [Google Scholar] [CrossRef] [PubMed]
- Ji, R.; Liao, J.; Li, L.; Wen, R.; Liu, M.; Ren, Y.; Wu, J.; Song, Y.; Qi, M.; Qiao, Z.; et al. Step-edge controlled fast growth of wafer-scale MoSe2 films by MOCVD. Nano Res. 2023, 16, 9577–9583. [Google Scholar] [CrossRef]
- Liu, M.; Liao, J.; Liu, Y.; Li, L.; Wen, R.; Hou, T.; Ji, R.; Wang, K.; Xing, Z.; Zheng, D.; et al. Periodical ripening for MOCVD growth of large 2D transition metal dichalcogenide domains. Adv. Funct. Mater. 2023, 33, 2212773. [Google Scholar] [CrossRef]
- Kalanyan, B.; Kimes, W.A.; Beams, R.; Stranick, S.J.; Garratt, E.; Kalish, I.; Davydov, A.V.; Kanjolia, R.K.; Maslar, J.E. Rapid Wafer-Scale Growth of Polycrystalline 2H-MoS2 by Pulsed MetalOrganic Chemical Vapor Deposition. Chem. Mater. 2017, 29, 6279–6288. [Google Scholar] [CrossRef]
- Schaefer, C.M.; Caicedo Roque, J.M.; Sauthier, G.; Bousquet, J.; Hébert, C.; Sperling, J.R.; Pérez-Tomás, A.; Santiso, J.; Corro, E.d.; Garrido., J.A. Carbon Incorporation in MOCVD of MoS2 Thin Films Grown from an Organosulfide Precursor. Chem. Mater. 2021, 33, 4474–4487. [Google Scholar] [CrossRef]
- Mawlong, L.P.L.; Hoang, A.T.; Chintalapalli, J.; Ji, S.; Lee, K.; Kim, K.; Ahn, J.-H. Reduced Defect Density in MOCVD-Grown MoS2 by Manipulating the Precursor Phase. ACS Appl. Mater. Interfaces 2023, 15, 47359–47367. [Google Scholar] [CrossRef]
- Yang, X.; Li, S.; Ikeda, N.; Ohtake, A.; Sakuma, Y. Scalable growth of atomically thin MoS2 layers in a conventional MOCVD system using molybdenum dichloride dioxide as the molybdenum source. Appl. Surf. Sci. 2023, 636, 157756. [Google Scholar] [CrossRef]
- Zhang, X.; Balushi, Z.Y.A.; Zhang, F.; Choudhury, T.H.; Eichfeld, S.M.; Alem, N.; Jackson, T.N.; Robinson, J.A.; Redwing, J.M. Influence of carbon in metalorganic chemical vapor deposition of few-layer WSe2 thin films. J. Electron. Mater. 2016, 45, 6273–6279. [Google Scholar] [CrossRef]
- Zhang, F.; Lu, Y.F.; Schulman, D.S.; Zhang, T.Y.; Fujisawa, K.; Lin, Z.; Lei, Y.; Elias, A.L.; Das, S.; Sinnott, S.B.; et al. Carbon doping of WS2 monolayers: Bandgap reduction and p-type doping transport. Sci. Adv. 2019, 5, eaav5003. [Google Scholar] [CrossRef]
- Cochrane, K.A.; Zhang, T.; Kozhakhmetov, A.; Lee, J.-H.; Zhang, F.; Dong, C.; Neaton, J.B.; Robinson, J.A.; Terrones, M.; Bargioni, A.W.; et al. Intentional carbon doping reveals CH as an abundant charged impurity in nominally undoped synthetic WS2 and WSe2. 2D Materials 2020, 7, 031003. [Google Scholar] [CrossRef]
- Liang, T.; Habib, M.R.; Xiao, H.; Xie, S.; Kong, Y.; Yu, C.; Iwai, H.; Fujita, D.; Hanagata, N.; Chen, H.; et al. Intrinsically Substitutional Carbon Doping in CVD-Grown Monolayer MoS2 and the Band Structure Modulation. ACS Appl. Electron. Mater. 2020, 2, 1055–1064. [Google Scholar] [CrossRef]
- Jeon, J.; Park, Y.; Choi, S.; Lee, J.; Lim, S.S.; Lee, B.H.; Song, Y.J.; Cho, J.H.; Jang, Y.H.; Lee, S. Epitaxial Synthesis of Molybdenum Carbide and Formation of a Mo2C/MoS2 Hybrid Structure via Chemical Conversion of Molybdenum Disulfide. ACS Nano 2018, 12, 338–346. [Google Scholar] [CrossRef]
- Choudhury, T.H.; Simchi, H.; Boichot, R.; Chubarov, M.; Mohney, S.E.; Redwing, J.M. Chalcogen Precursor Effect on ColdWall Gas-Source Chemical Vapor Deposition Growth of WS2. Cryst. Growth Des. 2018, 18, 4357–4364. [Google Scholar] [CrossRef]
- Yang, P.; Zou, X.; Zhang, Z.; Hong, M.; Shi, J.; Chen, S.; Shu, J.; Zhao, L.; Jiang, S.; Zhou, X.; et al. Batch production of 6-inch uniform monolayer molybdenum disulfide catalyzed by sodium in glass. Nat. Commun. 2018, 9, 979. [Google Scholar] [CrossRef]
- Ganatra, R.; Zhang, Q. Few-layer MoS2: A promising layered semiconductor. ACS Nano 2014, 8, 4074–4099. [Google Scholar] [CrossRef]
- Lee, C.; Yan, H.; Brus, L.E.; Heinz, T.F.; Hone, J.; Ryu, S. Anomalous lattice vibrations of single-and few-layer MoS2. ACS Nano 2010, 4, 2695–2700. [Google Scholar] [CrossRef]
- Malard, L.M.; Pimenta, M.A.; Dresselhaus, G.; Dresselhaus, M.S. Raman spectroscopy in graphene. Phys. Rep. 2009, 473, 51–87. [Google Scholar] [CrossRef]
- Maultzsch, J.; Reich, S.; Thomsen, C. Double-resonant Raman scattering in graphite: Interference effects, selection rules, and phonon dispersion. Phys. Rev. B 2004, 70, 155403. [Google Scholar] [CrossRef]
- Ferrari, A.C.; Meyer, J.C.; Scardaci, V.; Casiraghi, C.; Lazzeri, M.; Mauri, F.; Piscanec, S.; Jiang, D.; Novoselov, K.S.; Roth, S.; et al. Raman spectrum of graphene and graphene layers. Phys. Rev. Lett. 2006, 97, 187401. [Google Scholar] [CrossRef] [PubMed]
- Ferrari, A.; Basko, D. Raman spectroscopy as a versatile tool for studying the properties of graphene. Nat. Nanotechnol. 2013, 8, 235–246. [Google Scholar] [CrossRef] [PubMed]
- López-Díaz, D.; López Holgado, M.; García-Fierro, J.L.; Velázquez, M.M. Evolution of the Raman spectrum with the chemical composition of graphene oxide. J. Phys. Chem. C 2017, 121, 20489–20497. [Google Scholar] [CrossRef]
- Tonndorf, P.; Schmidt, R.; Böttger, P.; Zhang, X.; Börner, J.; Liebig, A.; Albrecht, M.; Kloc, C.; Gordan, O.; Zahn, D.R.T.; et al. Photoluminescence emission and Raman response of monolayer MoS2, MoSe2, and WSe2. Opt. Express 2013, 21, 4908–4916. [Google Scholar] [CrossRef]
- Najmaei, S.; Liu, Z.; Zhou, W.; Zou, X.; Shi, G.; Lei, S.; Yakobson, B.I.; Idrobo, J.-C.; Ajayan, P.M.; Lou, J. Vapour phase growth and grain boundary structure of molybdenum disulphide atomic layers. Nat. Mater. 2013, 12, 754–759. [Google Scholar] [CrossRef]
- van der Zande, A.M.; Huang, P.Y.; Chenet, D.A.; Berkelbach, T.C.; You, Y.; Lee, G.; Heinz, T.F.; Reichman, D.R.; Muller, D.A.; Hone, J.C. Grains and grain boundaries in highly crystalline monolayer molybdenum disulphide. Nat. Mater. 2013, 12, 554–561. [Google Scholar] [CrossRef]
- Krivanek, O.L.; Chisholm, M.F.; Nicolosi, V.; Pennycook, T.J.; Corbin, G.J.; Dellby, N.; Murfitt, M.F.; Own, C.S.; Szilagyi, Z.S.; Oxley, M.P.; et al. Atom-by-atom structural and chemical analysis by annular dark-field electron microscopy. Nature 2010, 464, 571–574. [Google Scholar] [CrossRef]
- Yoon, S.F.; Huang, Q.F.; Rusli; Yang, H.; Ahn, J.; Zhang, Q.; Blomfield, C.; Tielsch, B.; Tan, L.Y.C. X-ray photoelectron spectroscopy of molybdenum-containing carbon films. J. Appl. Phys. 1999, 86, 4871–4875. [Google Scholar] [CrossRef]
- Xiang, Z.C.; Zhang, Z.; Xu, X.; Zhang, Q.; Yuan, C. MoS2 nanosheets array on carbon cloth as a 3D electrode for highly efficient electrochemical hydrogen evolution. Carbon 2016, 98, 84–89. [Google Scholar] [CrossRef]
- Varga, M.; Izak, T.; Vretenar, V.; Kozak, H.; Holovsky, J.; Artemenko, A.; Hulman, M.; Skakalova, V.; Lee, D.S.; Kromka, A. Diamond/carbon nanotube composites: Raman, FTIR and XPS spectroscopic studies. Carbon 2017, 111, 54–61. [Google Scholar] [CrossRef]
- Pimentel, J.V.; Polcar, T.; Cavaleiro, A. Structural, mechanical and tribological properties of Mo-S-C solid lubricant coating. Surf. Coat. Technol. 2011, 205, 3274–3279. [Google Scholar] [CrossRef]
- Wu, Y.P.; Fang, S.; Jiang, Y.; Holze, R. Effects of doped sulfur on electrochemical performance of carbon anode. J. Power Sources 2002, 108, 245–249. [Google Scholar] [CrossRef]
- Yang, C.; Yin, Y.; Ye, H.; Jiang, K.; Zhang, J.; Guo, Y. Insight into the effect of boron doping on sulfur/carbon cathode in lithium-sulfur batteries. ACS Appl. Mater. Interfaces 2014, 6, 8789–8795. [Google Scholar] [CrossRef] [PubMed]
- Hu, Q.; Paau, M.C.; Zhang, Y.; Gong, X.; Zhang, L.; Lu, D.; Liu, Y.; Liu, Q.; Yao, J.; Choi, M.M.F. Green synthesis of fluorescent nitrogen/sulfur doped carbon dots and investigation of their properties by HPLC coupled with mass spectrometry. RSC Adv. 2014, 4, 18065. [Google Scholar] [CrossRef]
- Wan, C.; Regmi, Y.N.; Leonard, B.M. Multiple phases of molybdenum carbide as electrocatalysts for the hydrogen evolution reaction. Angew. Chem. Int. Ed. 2014, 53, 6407–6410. [Google Scholar] [CrossRef]
- Schulman, D.S.; Arnold, A.J.; Das, S. Contact engineering for 2D materials and devices. Chem. Soc. Rev. 2018, 47, 3037–3058. [Google Scholar] [CrossRef]
- Chee, S.-S.; Seo, D.; Kim, H.; Jang, H.; Lee, S.; Moon, S.P.; Lee, K.H.; Kim, S.W.; Choi, H.; Ham, M.-H. Lowering the Schottky barrier height by graphene/Ag electrodes for high-mobility MoS2 field-effect transistors. Adv. Mater. 2019, 31, 1804422. [Google Scholar] [CrossRef]
- Chanana, A.; Mahapatra, S. Prospects of zero Schottky barrier height in a graphene-inserted MoS2-metal interface. J. Appl. Phys. 2016, 119, 014303. [Google Scholar] [CrossRef]
- Wang, J.-W.; Liu, Y.-P.; Chen, P.-H.; Chuang, M.-H.; Pezeshki, A.; Ling, D.-C.; Chen, J.-C.; Chen, Y.-F.; Lee, Y.-H. Controlled low-frequency electrical noise of monolayer MoS2 with Ohmic contact and tunable carrier concentration. Adv. Electron. Mater. 2018, 4, 1700340. [Google Scholar] [CrossRef]
- Wang, Q.; Kalantar-Zadeh, K.; Kis, A.; Coleman, J.N.; Strano, M.S. Electronics and optoelectronics of two-dimensional transition metal dichalcogenides. Nat. Nanotechnol. 2012, 7, 699–712. [Google Scholar] [CrossRef] [PubMed]
- O’Brien, M.; McEvoy, N.; Hanlon, D.; Hallam, T.; Coleman, J.N.; Duesberg, G.S. Mapping of low-frequency Raman modes in CVD-grown transition metal dichalcogenides: Layer number, stacking orientation and resonant effects. Sci. Rep. 2016, 6, 19476. [Google Scholar] [CrossRef] [PubMed]
- Ponomarev, E.; Gutiérrez-Lezama, I.; Ubrig, N.; Morpurgo, A.F. Ambipolar light-emitting transistors on chemical vapor deposited monolayer MoS2. Nano Lett. 2015, 15, 8289–8294. [Google Scholar] [CrossRef] [PubMed]
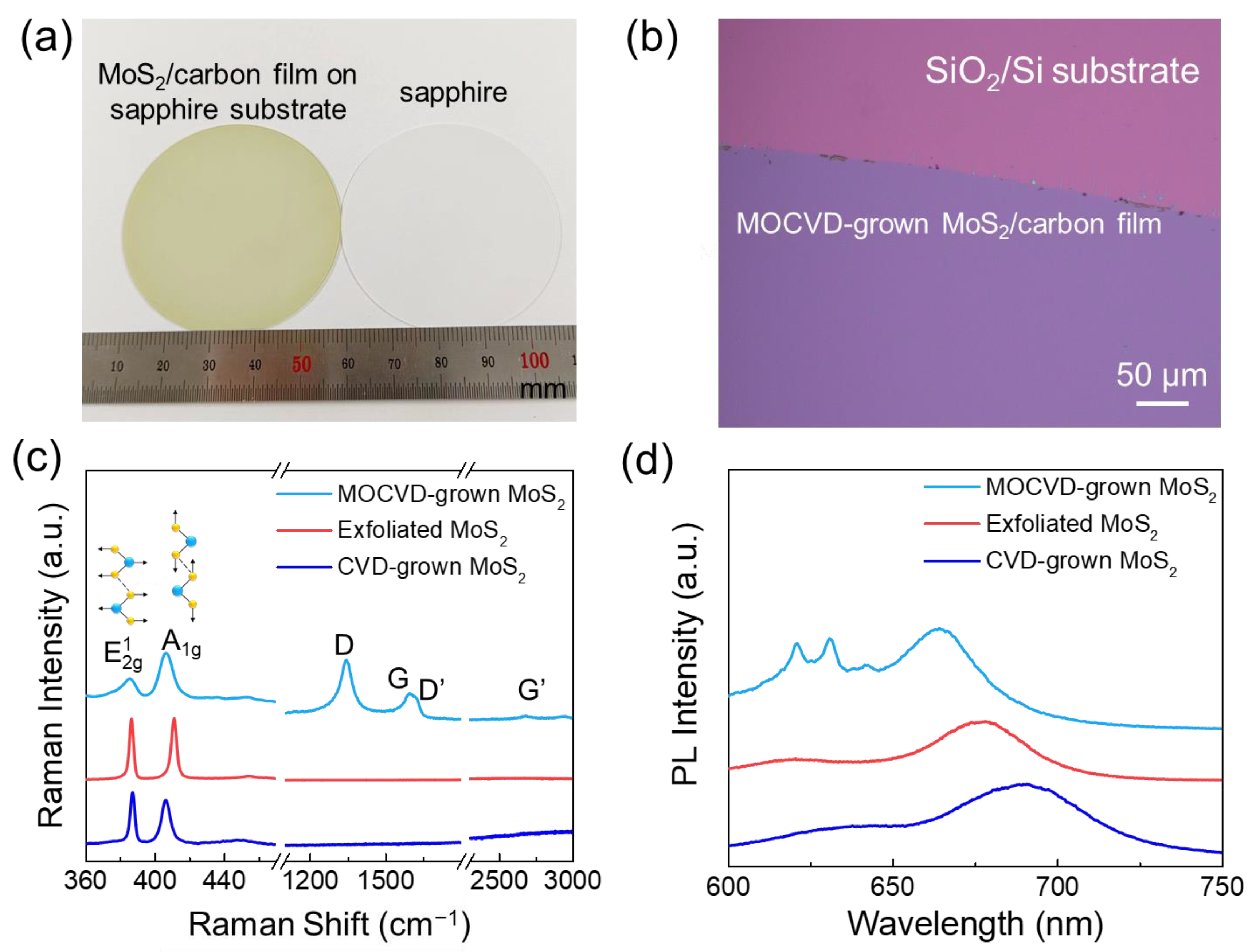
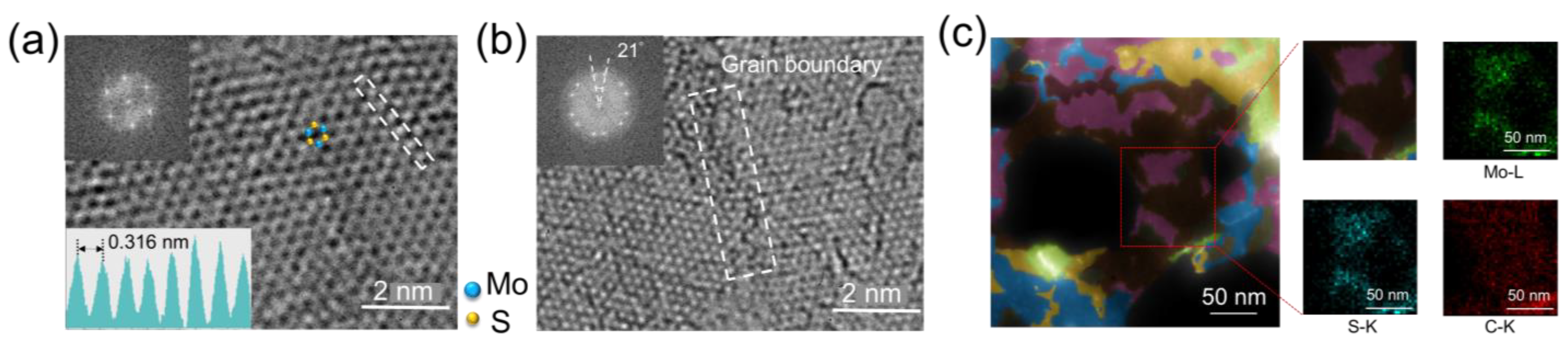

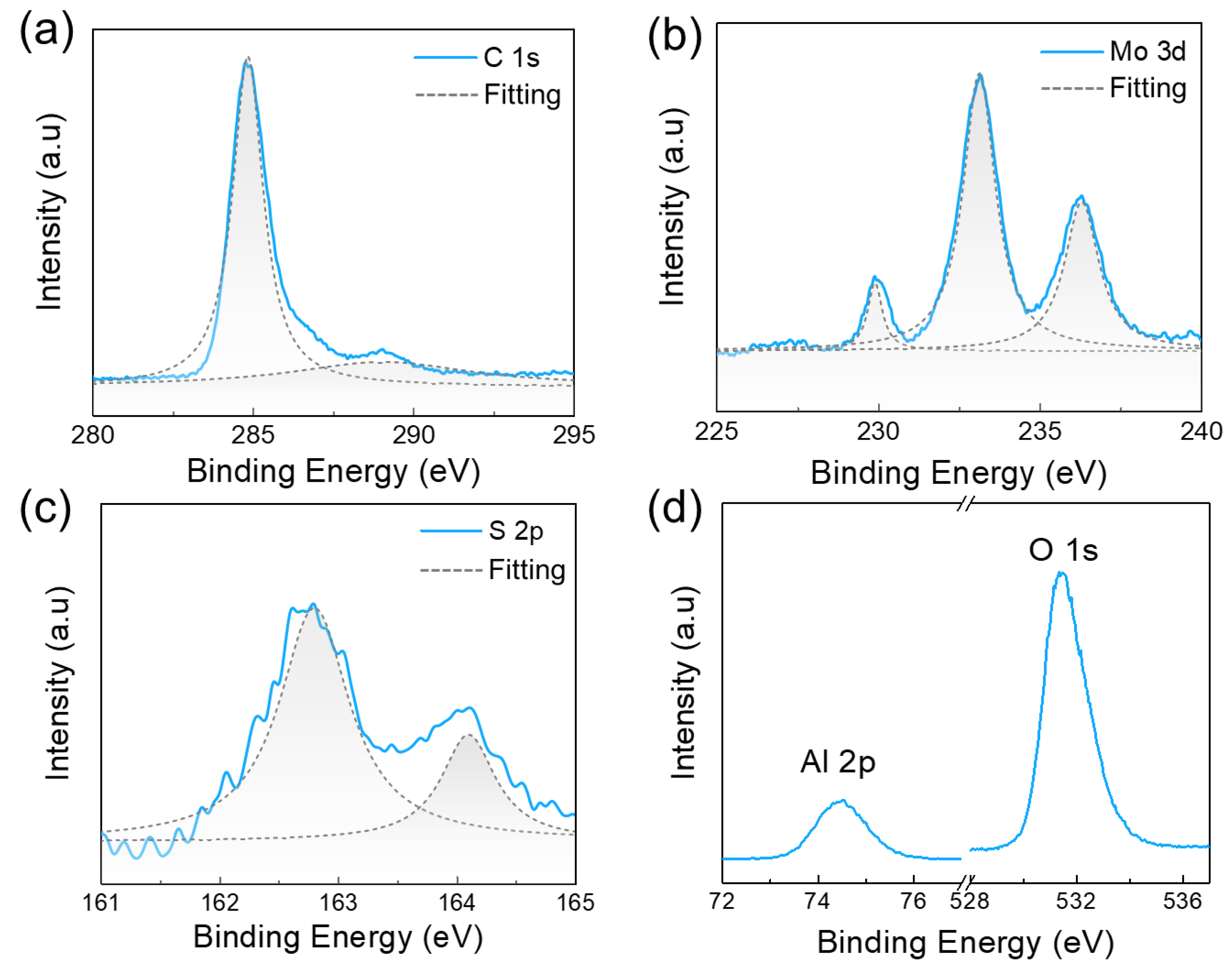
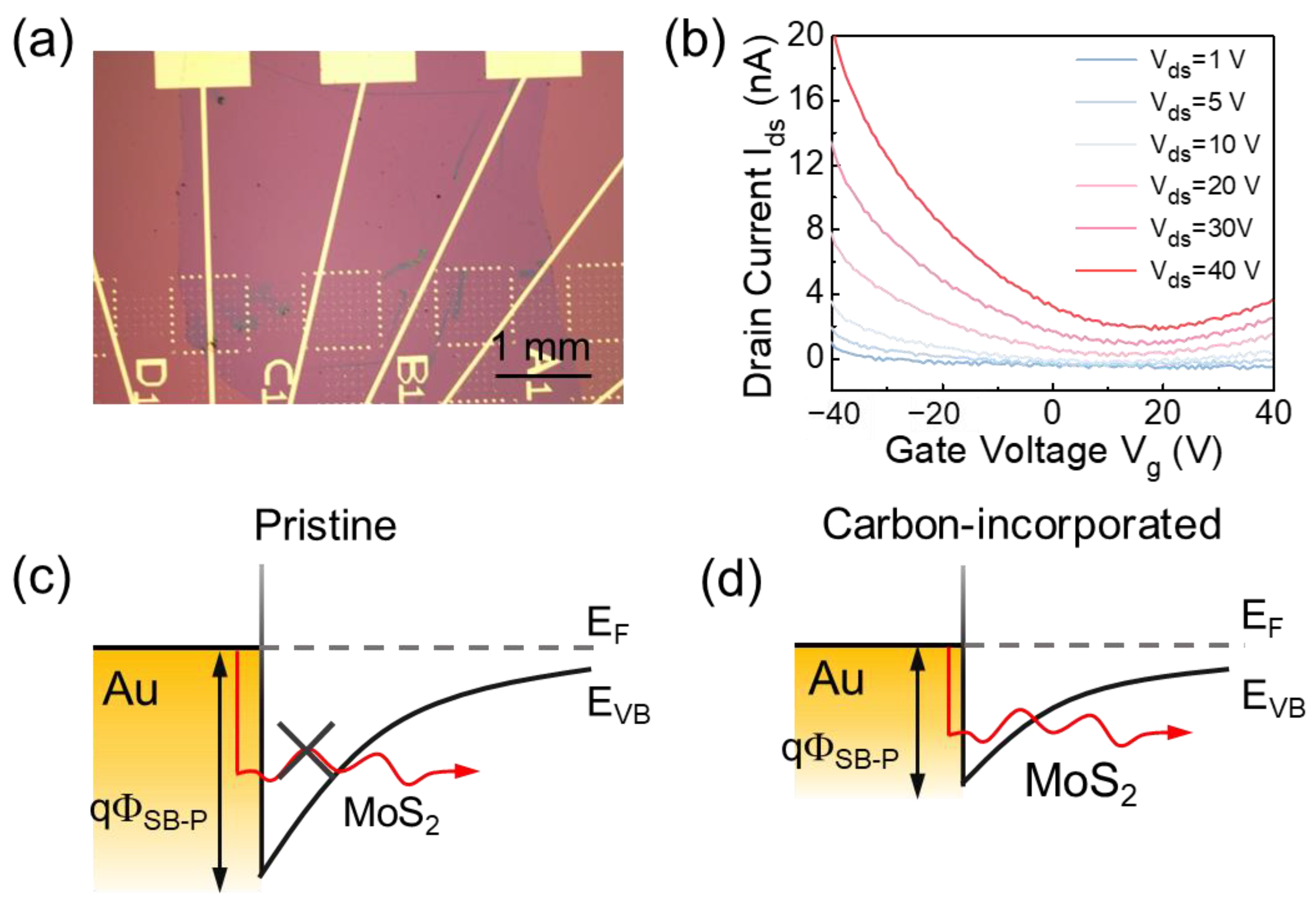
| Carbon Incorporated or Not | Precursors | Raman [61,62] | PL [61,63] | Semiconducting Property | |
|---|---|---|---|---|---|
| MOCVD-grown MoS2 | yes | Mo(CO)6, (CH3)3C)2S | (~385 cm−1), A1g (~405 cm−1) and some carbon related peaks | A (~660 nm), B (~640 nm) | p-type |
| CVD-grown MoS2 | no | MoO3 and S powders | (~385 cm−1), A1g (~403 cm−1) | A (~660 nm) | n-type |
| exfoliated MoS2 | no | bulk materials | (~385 cm−1), A1g (~403 cm−1) | A (~660 nm), B (~610 nm) | n-type |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hou, T.; Li, D.; Qu, Y.; Hao, Y.; Lai, Y. The Role of Carbon in Metal–Organic Chemical Vapor Deposition-Grown MoS2 Films. Materials 2023, 16, 7030. https://doi.org/10.3390/ma16217030
Hou T, Li D, Qu Y, Hao Y, Lai Y. The Role of Carbon in Metal–Organic Chemical Vapor Deposition-Grown MoS2 Films. Materials. 2023; 16(21):7030. https://doi.org/10.3390/ma16217030
Chicago/Turabian StyleHou, Tianyu, Di Li, Yan Qu, Yufeng Hao, and Yun Lai. 2023. "The Role of Carbon in Metal–Organic Chemical Vapor Deposition-Grown MoS2 Films" Materials 16, no. 21: 7030. https://doi.org/10.3390/ma16217030






