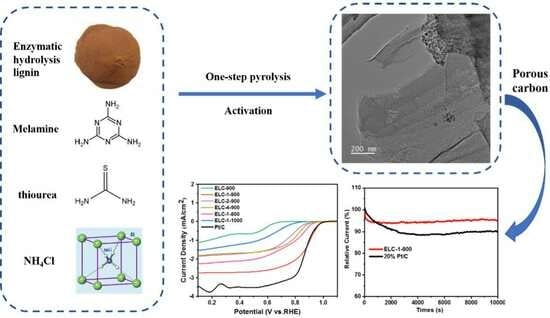
Enzymolytic Lignin-Derived N-S Codoped Porous Carbon Nanocomposites as Electrocatalysts for Oxygen Reduction Reactions
Abstract
:1. Introduction
2. Experimental Section
2.1. Experimental Materials
2.2. Experimental Procedure
2.3. Catalyst Structure Characterization
2.4. Electrochemical Measurements
3. Results and Discussion
3.1. Characterization of Different Catalysts
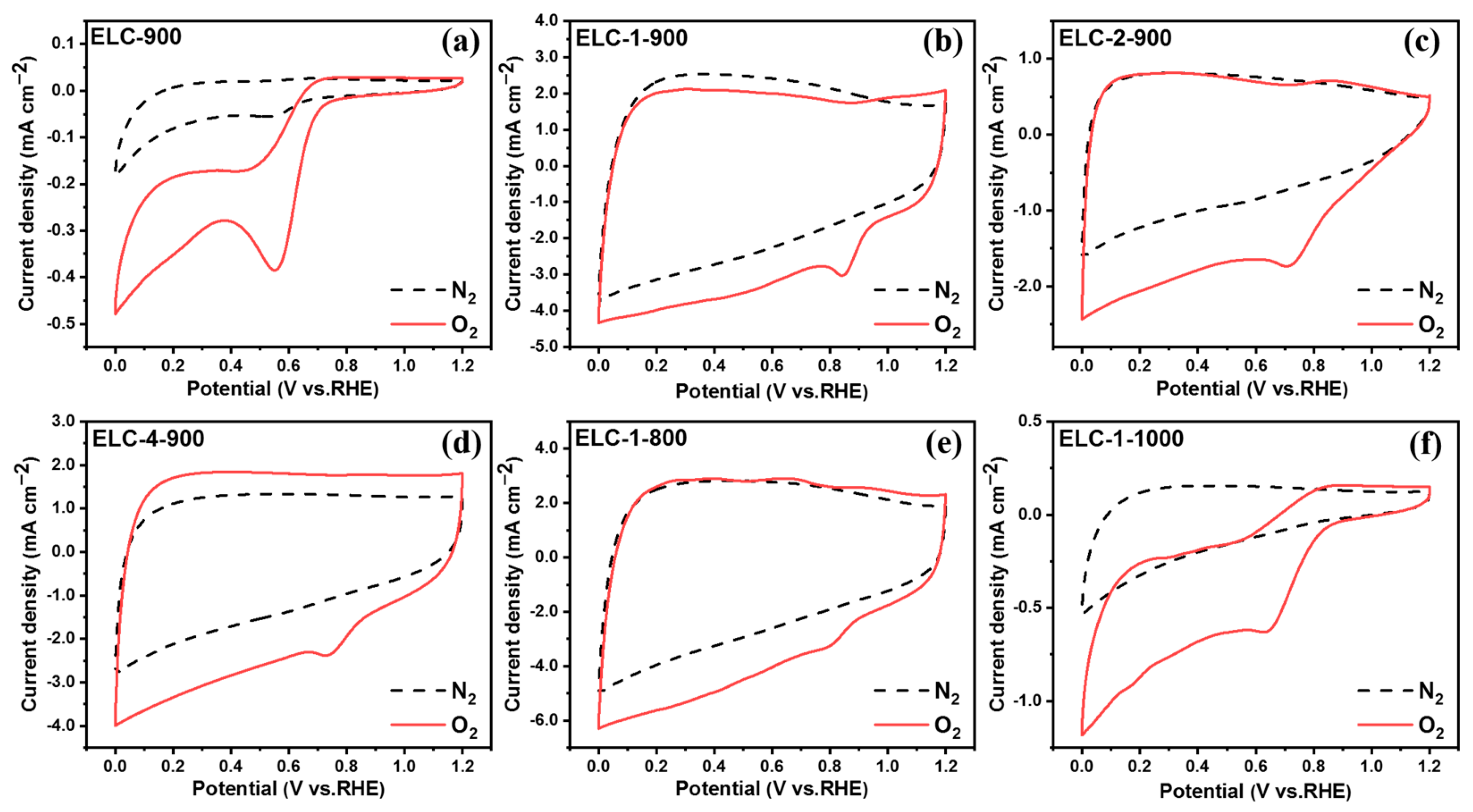
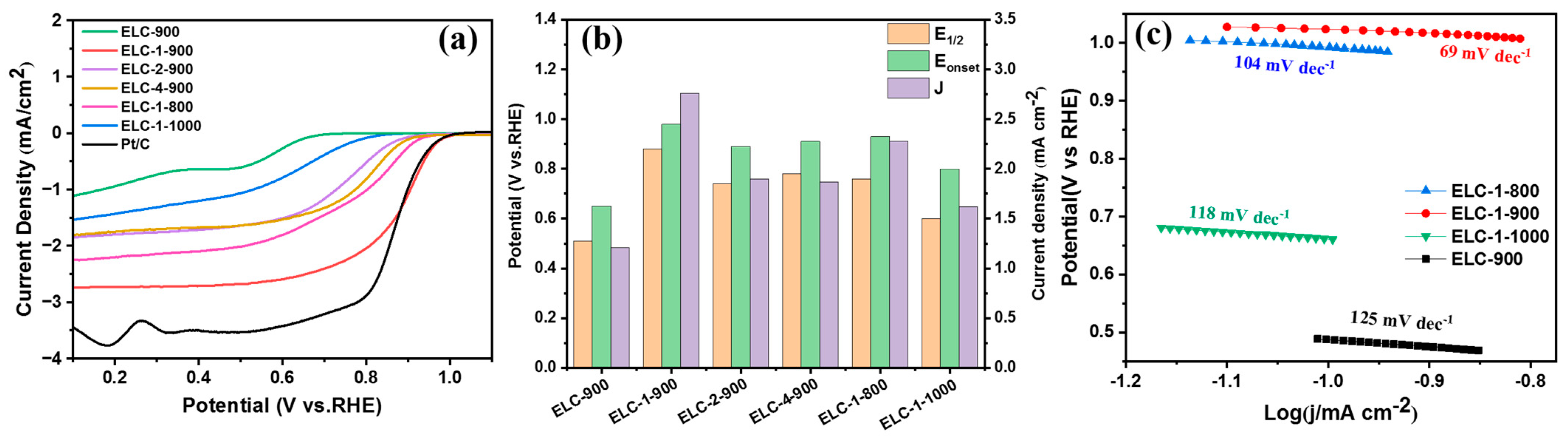
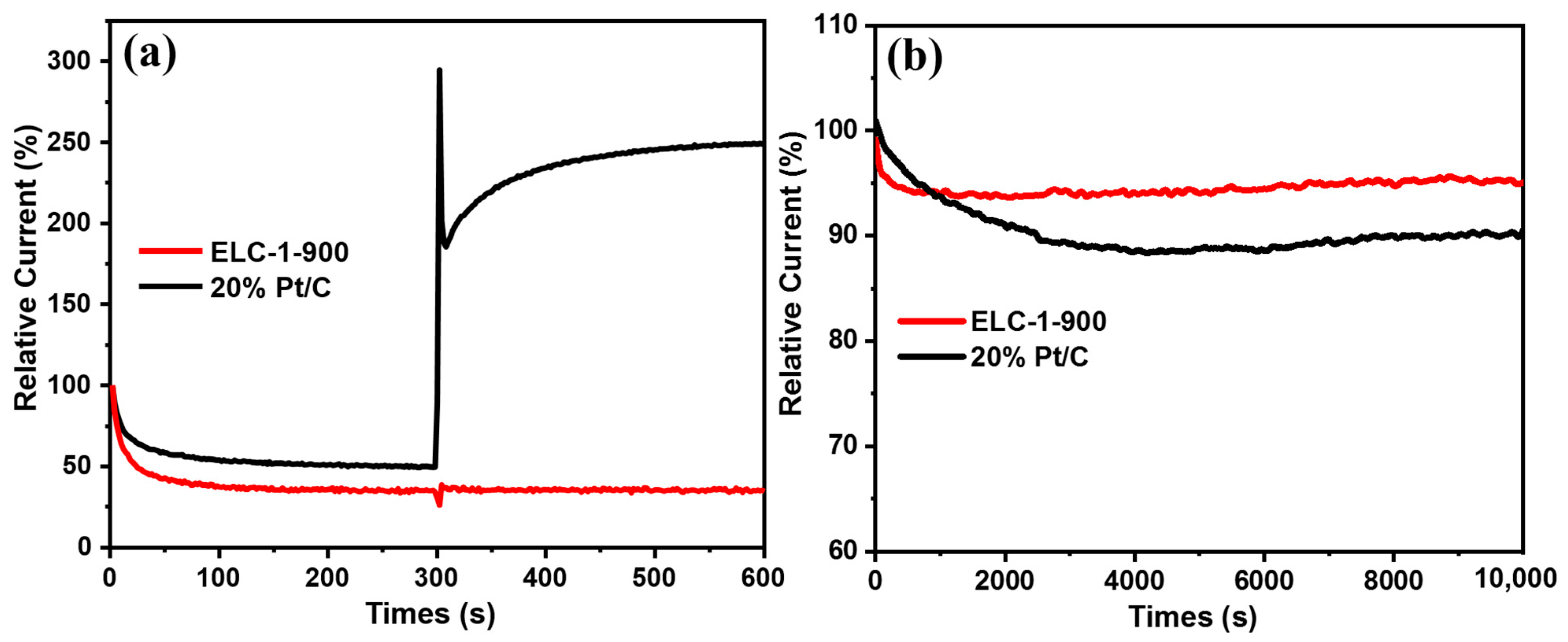
3.2. Electrocatalytic ORR Performance of Different Catalysts
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Borghei, M.; Lehtonen, J.; Liu, L.; Rojas, O.J. Advanced Biomass-Derived Electrocatalysts for the Oxygen Reduction Reaction. Adv. Mater. 2018, 30, 1703691. [Google Scholar] [CrossRef]
- Rupar, J.; Tekić, D.; Janošević Ležaić, A.; Upadhyay, K.K. ORR Catalysts Derived from Biopolymers. Catalysts 2023, 13, 80. [Google Scholar] [CrossRef]
- Ao, X.; Zhang, W.; Li, Z.; Li, J.-G.; Soule, L.; Huang, X.; Chiang, W.-H.; Chen, H.M.; Wang, C.; Liu, M.; et al. Markedly Enhanced Oxygen Reduction Activity of Single-Atom Fe Catalysts via Integration with Fe Nanoclusters. ACS Nano 2019, 13, 11853–11862. [Google Scholar] [CrossRef] [PubMed]
- Sekhon, S.S.; Lee, J.; Park, J.-S. Biomass-derived bifunctional electrocatalysts for oxygen reduction and evolution reaction: A review. J. Energy Chem. 2022, 65, 149–172. [Google Scholar] [CrossRef]
- Nagappan, S.; Duraivel, M.; Han, S.; Yusuf, M.; Mahadadalkar, M.; Park, K.; Dhakshinamoorthy, A.; Prabakar, K.; Park, S.; Ha, C.-S.; et al. Electrocatalytic Oxygen Reduction Reaction of Graphene Oxide and Metal-Free Graphene in an Alkaline Medium. Nanomaterials 2023, 13, 1315. [Google Scholar] [CrossRef] [PubMed]
- Baik, S.; Lee, J.W. Effect of boron–nitrogen bonding on oxygen reduction reaction activity of BN Co-doped activated porous carbons. RSC Adv. 2015, 5, 24661–24669. [Google Scholar] [CrossRef]
- Bouleau, L.; Pérez-Rodríguez, S.; Quílez-Bermejo, J.; Izquierdo, M.T.; Xu, F.; Fierro, V.; Celzard, A. Best practices for ORR performance evaluation of metal-free porous carbon electrocatalysts. Carbon 2022, 189, 349–361. [Google Scholar] [CrossRef]
- Hanif, S.; Iqbal, N.; Shi, X.; Noor, T.; Ali, G.; Kannan, A.M. NiCo–N-doped carbon nanotubes based cathode catalyst for alkaline membrane fuel cell. Renew. Energy 2020, 154, 508–516. [Google Scholar] [CrossRef]
- Choi, E.Y.; Lee, S.; Kim, C.K. Hybridization Effects of Nitrogen-Doped Graphene-Carbon Nanotubes and Nano-Onion Carbons on the Electrocatalytic Activity of the Oxygen Reduction Reaction. ECS J. Solid State Sci. Technol. 2018, 7, M128–M137. [Google Scholar] [CrossRef]
- Borghei, M.; Laocharoen, N.; Kibena-Poldsepp, E.; Johansson, L.-S.; Campbell, J.; Kauppinen, E.; Tammeveski, K.; Rojas, O.J. Porous N,P-doped carbon from coconut shells with high electrocatalytic activity for oxygen reduction: Alternative to Pt-C for alkaline fuel cells. Appl. Catal. B Environ. 2017, 204, 394–402. [Google Scholar] [CrossRef]
- Kaare, K.; Yu, E.; Volperts, A.; Dobele, G.; Zhurinsh, A.; Dyck, A.; Niaura, G.; Tamasauskaite-Tamasiunaite, L.; Norkus, E.; Andrulevičius, M.; et al. Highly Active Wood-Derived Nitrogen-Doped Carbon Catalyst for the Oxygen Reduction Reaction. ACS Omega 2020, 5, 23578–23587. [Google Scholar] [CrossRef] [PubMed]
- Tsyganova, S.I.; Fetisova, O.Y.; Mazurova, E.N.; Taran, O.P.; Kuznetsov, B.N. Synthesis and Properties of Magnetically Susceptible Porous Carbon Materials Based on Hydrolysis Lignin Modified with ZnCl2 and FeCl3. Russ. J. Appl. Chem. 2022, 95, 408–416. [Google Scholar] [CrossRef]
- Rois, M.F.; Widiyastuti, W.; Setyawan, H.; Rahmatika, A.M.; Ogi, T. Preparation of activated carbon from alkali lignin using novel one-step process for high electrochemical performance application. Arab. J. Chem. 2021, 14, 103162. [Google Scholar] [CrossRef]
- Rois, M.F.; Ramadhani Alya Sasono, S.; Widiyastuti, W.; Nurtono, T.; Setyawan, H. High-performance electrocatalyst made from lignosulfonate nanofiber composited with manganese dioxide without carbonation process. Adv. Powder Technol. 2022, 33, 103572. [Google Scholar] [CrossRef]
- Graglia, M.; Pampel, J.; Hantke, T.; Fellinger, T.-P.; Esposito, D. Nitro Lignin-Derived Nitrogen-Doped Carbon as an Efficient and Sustainable Electrocatalyst for Oxygen Reduction. ACS Nano 2016, 10, 4364–4371. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.L.; Yu, D.L.; Zhang, Y.Q.; Guo, W.H.; Ma, X.X.; He, X.Q. Nitrogen- and sulfur-doped carbon nanoplatelets via thermal annealing of alkaline lignin with urea as efficient electrocatalysts for oxygen reduction reaction. RSC Adv. 2016, 6, 104183–104192. [Google Scholar] [CrossRef]
- Yan, D.; Han, Y.; Ma, Z.; Wang, Q.; Wang, X.; Li, Y.; Sun, G. Magnesium lignosulfonate-derived N, S co-doped 3D flower-like hierarchically porous carbon as an advanced metal-free electrocatalyst towards oxygen reduction reaction. Int. J. Biol. Macromol. 2022, 209, 904–911. [Google Scholar] [CrossRef]
- Xiong, D.; Li, X.; Fan, L.; Bai, Z. Three-Dimensional Heteroatom-Doped Nanocarbon for Metal-Free Oxygen Reduction Electrocatalysis: A Review. Catalysts 2018, 8, 301. [Google Scholar] [CrossRef]
- Ma, Z.; Han, Y.; Wang, X.; Sun, G.; Li, Y. Lignin-derived hierarchical porous flower-like carbon nanosheets decorated with biomass carbon quantum dots for efficient oxygen reduction. Colloids Surf. A Physicochem. Eng. Asp. 2022, 652, 129818. [Google Scholar] [CrossRef]
- Huang, B.; Jiang, J. N-doped carbon nanosheets derived from lignin as a novel bifunctional electrocatalyst for rechargeable zinc-air battery. Diam. Relat. Mater. 2022, 128, 109291. [Google Scholar] [CrossRef]
- Shen, Y.; Peng, F.; Cao, Y.; Zuo, J.; Wang, H.; Yu, H. Preparation of nitrogen and sulfur co-doped ultrathin graphitic carbon via annealing bagasse lignin as potential electrocatalyst towards oxygen reduction reaction in alkaline and acid media. J. Energy Chem. 2019, 34, 33–42. [Google Scholar] [CrossRef]
- Lin, S.-Y.; Xia, L.-X.; Cao, Y.; Meng, H.-L.; Zhang, L.; Feng, J.-J.; Zhao, Y.; Wang, A.-J. Electronic Regulation of ZnCo Dual-Atomic Active Sites Entrapped in 1D@2D Hierarchical N-Doped Carbon for Efficient Synergistic Catalysis of Oxygen Reduction in Zn-Air Battery. Small 2022, 18, 2107141. [Google Scholar] [CrossRef] [PubMed]
- Yan, L.; Xu, Y.; Chen, P.; Zhang, S.; Jiang, H.; Yang, L.; Wang, Y.; Zhang, L.; Shen, J.; Zhao, X.; et al. A Freestanding 3D Heterostructure Film Stitched by MOF-Derived Carbon Nanotube Microsphere Superstructure and Reduced Graphene Oxide Sheets: A Superior Multifunctional Electrode for Overall Water Splitting and Zn-Air Batteries. Adv. Mater. 2020, 32, 2003313. [Google Scholar] [CrossRef] [PubMed]
- Deng, X.; Shi, W.; Zhong, Y.; Zhou, W.; Liu, M.; Shao, Z. Facile Strategy to Low-Cost Synthesis of Hierarchically Porous, Active Carbon of High Graphitization for Energy Storage. ACS Appl. Mater. Interfaces 2018, 10, 21573–21581. [Google Scholar] [CrossRef] [PubMed]
- Guo, H.; Feng, Q.; Zhu, J.; Xu, J.; Li, Q.; Liu, S.; Xu, K.; Zhang, C.; Liu, T. Cobalt nanoparticle-embedded nitrogen-doped carbon/carbon nanotube frameworks derived from a metal–organic framework for tri-functional ORR, OER and HER electrocatalysis. J. Mater. Chem. A 2019, 7, 3664–3672. [Google Scholar] [CrossRef]
- Thangasamy, P.; Oh, S.; Randriamahazaka, H.; Nam, S.; Oh, I.-K. Mechanistic insight into collectively exhaustive CoPi-NPC nanosheets for oxygen reduction reaction and Zn-air battery. Appl. Catal. B Environ. 2022, 316, 121656. [Google Scholar] [CrossRef]
- Yang, G.; Zhang, Z.; Kang, X.; Li, L.; Li, Y.; Sun, Y. Fe–N–C Composite Catalyst Derived from Solid Digestate for the Oxygen Reduction Reaction in Microbial Fuel Cells. ACS Appl. Energy Mater. 2020, 3, 11929–11938. [Google Scholar] [CrossRef]
- Dong, Q.; Mo, Z.; Wang, H.; Ji, S.; Wang, X.; Linkov, V.; Wang, R. N-Doped Carbon Networks Containing Inserted FeNx@NC Nanospheroids and Bridged by Carbon Nanotubes as Enhanced Catalysts for the Oxygen Reduction Reaction. ACS Sustain. Chem. Eng. 2020, 8, 6979–6989. [Google Scholar] [CrossRef]
- Chen, Y.-P.; Lin, S.-Y.; Sun, R.-M.; Wang, A.-J.; Zhang, L.; Ma, X.; Feng, J.-J. FeCo/FeCoP encapsulated in N, Mn-codoped three-dimensional fluffy porous carbon nanostructures as highly efficient bifunctional electrocatalyst with multi-components synergistic catalysis for ultra-stable rechargeable Zn-air batteries. J. Colloid Interface Sci. 2022, 605, 451–462. [Google Scholar] [CrossRef]
- Ait El Fakir, A.; Anfar, Z.; Enneiymy, M.; Jada, A.; El Alem, N. Conjugated polymers templated carbonization to design N, S co-doped finely tunable carbon for enhanced synergistic catalysis. Appl. Catal. B Environ. 2022, 300, 120732. [Google Scholar] [CrossRef]
- Jin Bae, E.; Hun Kang, Y.; Jang, K.-S.; Yun Cho, S. Enhancement of Thermoelectric Properties of PEDOT:PSS and Tellurium-PEDOT:PSS Hybrid Composites by Simple Chemical Treatment. Sci. Rep. 2016, 6, 18805. [Google Scholar] [CrossRef] [PubMed]
- Ding, X.-B.; Li, F.; Cao, Q.-C.; Wu, H.; Qin, Y.-H.; Yang, L.; Wang, T.; Zheng, X.; Wang, C.-W. Core-shell S-doped g-C3N4@P123 derived N and S co-doped carbon as metal-free electrocatalysts highly efficient for oxygen reduction reaction. Chem. Eng. J. 2022, 429, 132469. [Google Scholar] [CrossRef]
- Zhang, M.; Song, Y.; Tao, H.; Yan, C.; Masa, J.; Liu, Y.; Shi, X.; Liu, S.; Zhang, X.; Sun, Z. Lignosulfonate biomass derived N and S co-doped porous carbon for efficient oxygen reduction reaction. Sustain. Energy Fuels 2018, 2, 1820–1827. [Google Scholar] [CrossRef]
- Li, C.; Wu, Y.; Fu, M.; Zhao, X.; Zhai, S.; Yan, Y.; Zhang, L.; Zhang, X. Preparation of Fe/N Double Doped Carbon Nanotubes from Lignin in Pennisetum as Oxygen Reduction Reaction Electrocatalysts for Zinc-Air Batteries. ACS Appl. Energy Mater. 2022, 5, 4340–4350. [Google Scholar] [CrossRef]
- Wu, D.-H.; Huang, H.; Ul Haq, M.; Zhang, L.; Feng, J.-J.; Wang, A.-J. Lignin-derived iron carbide/Mn, N, S-codoped carbon nanotubes as a high-efficiency catalyst for synergistically enhanced oxygen reduction reaction and rechargeable zinc-air battery. J. Colloid Interface Sci. 2023, 647, 1–11. [Google Scholar] [CrossRef]
- Dong, R.; Yang, Z.; Fu, Y.; Chen, Z.; Hu, Y.; Zhou, Y.; Qin, H. Aminated lignin chelated metal derived bifunctional electrocatalyst with high catalytic performance. Appl. Surf. Sci. 2022, 580, 152205. [Google Scholar] [CrossRef]

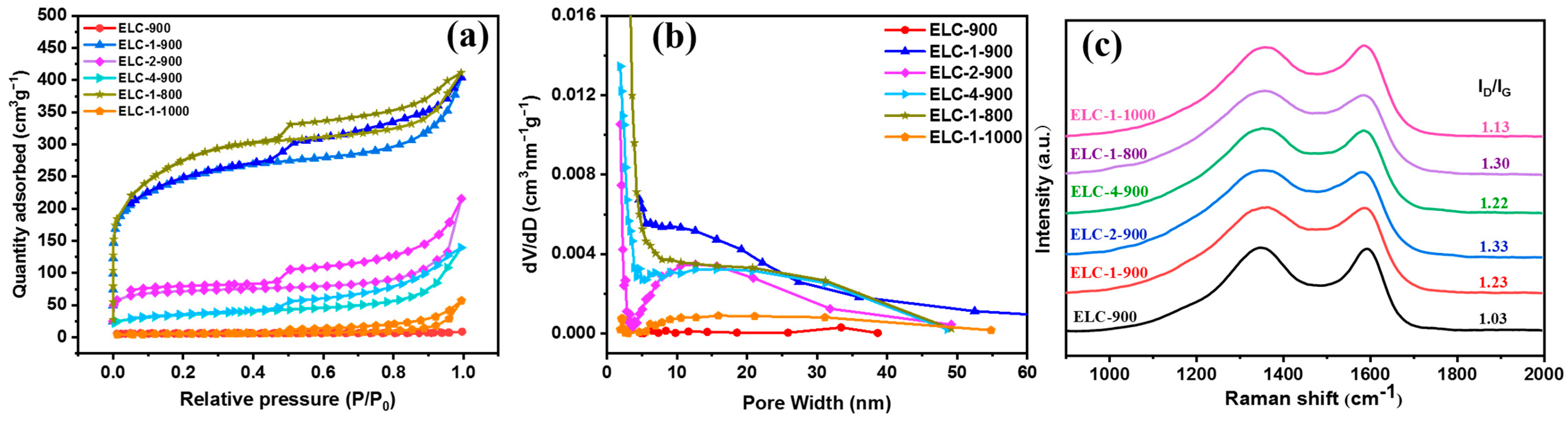
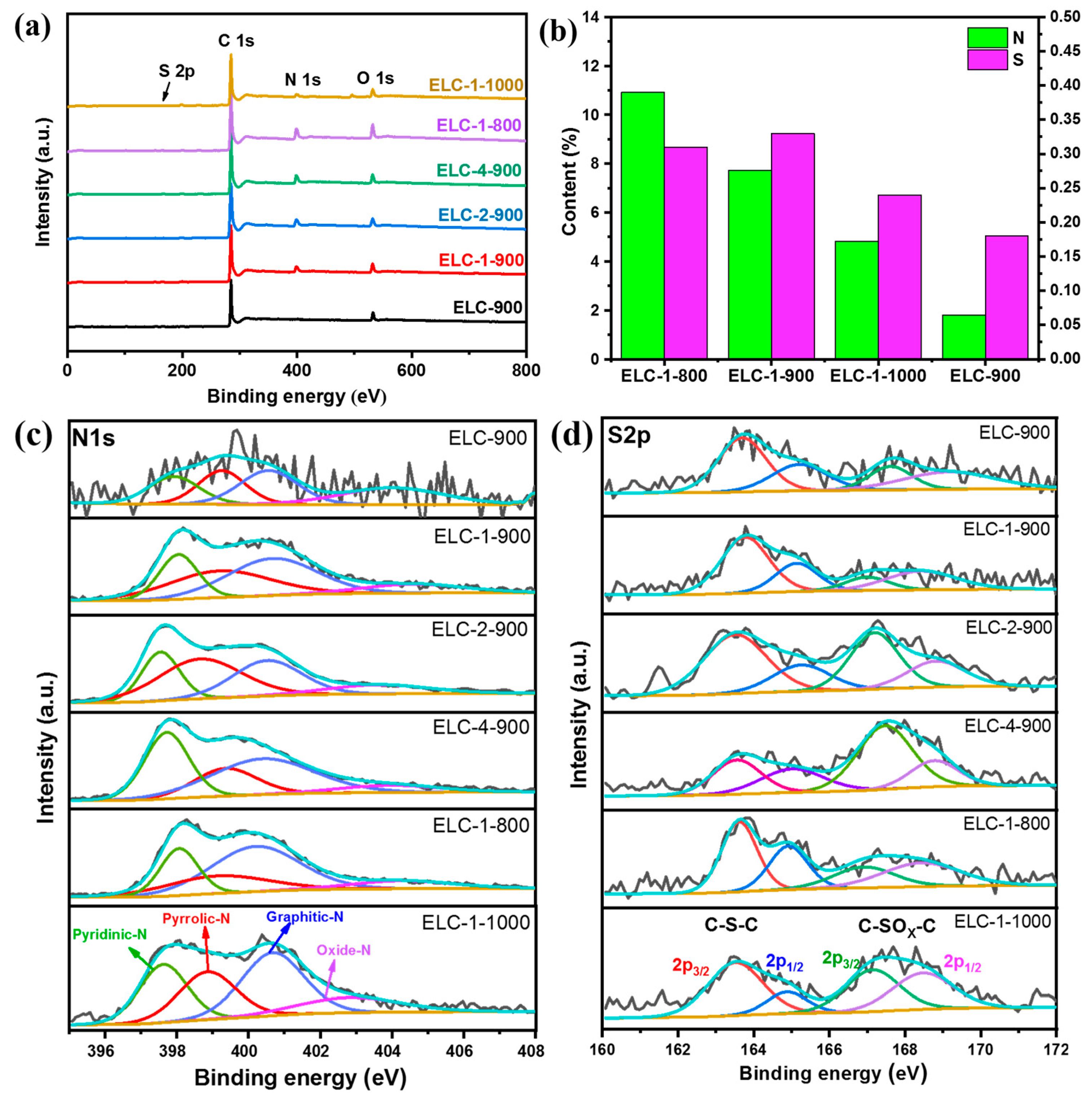
| Samples | SBET (m2 g−1) | Average Pore Diameter (nm) | Pore Volume (cm−3 g−1) |
|---|---|---|---|
| ELC-900 | 18 | 4.22 | 0.012 |
| ELC-1-900 | 844 | 4.36 | 0.587 |
| ELC-2-900 | 262 | 9.33 | 0.333 |
| ELC-4-900 | 123 | 9.08 | 0.215 |
| ELC-1-800 | 962 | 5.91 | 0.636 |
| ELC-1-1000 | 18 | 11.56 | 0.088 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, Z.; Qu, X.; Feng, Y.; Dong, L.; Yang, Y.; Lei, T.; Ren, S. Enzymolytic Lignin-Derived N-S Codoped Porous Carbon Nanocomposites as Electrocatalysts for Oxygen Reduction Reactions. Materials 2023, 16, 7614. https://doi.org/10.3390/ma16247614
Li Z, Qu X, Feng Y, Dong L, Yang Y, Lei T, Ren S. Enzymolytic Lignin-Derived N-S Codoped Porous Carbon Nanocomposites as Electrocatalysts for Oxygen Reduction Reactions. Materials. 2023; 16(24):7614. https://doi.org/10.3390/ma16247614
Chicago/Turabian StyleLi, Zheng, Xia Qu, Yuwei Feng, Lili Dong, Yantao Yang, Tingzhou Lei, and Suxia Ren. 2023. "Enzymolytic Lignin-Derived N-S Codoped Porous Carbon Nanocomposites as Electrocatalysts for Oxygen Reduction Reactions" Materials 16, no. 24: 7614. https://doi.org/10.3390/ma16247614