Nitrogen- and Sulfur-Codoped Strong Green Fluorescent Carbon Dots for the Highly Specific Quantification of Quercetin in Food Samples
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals
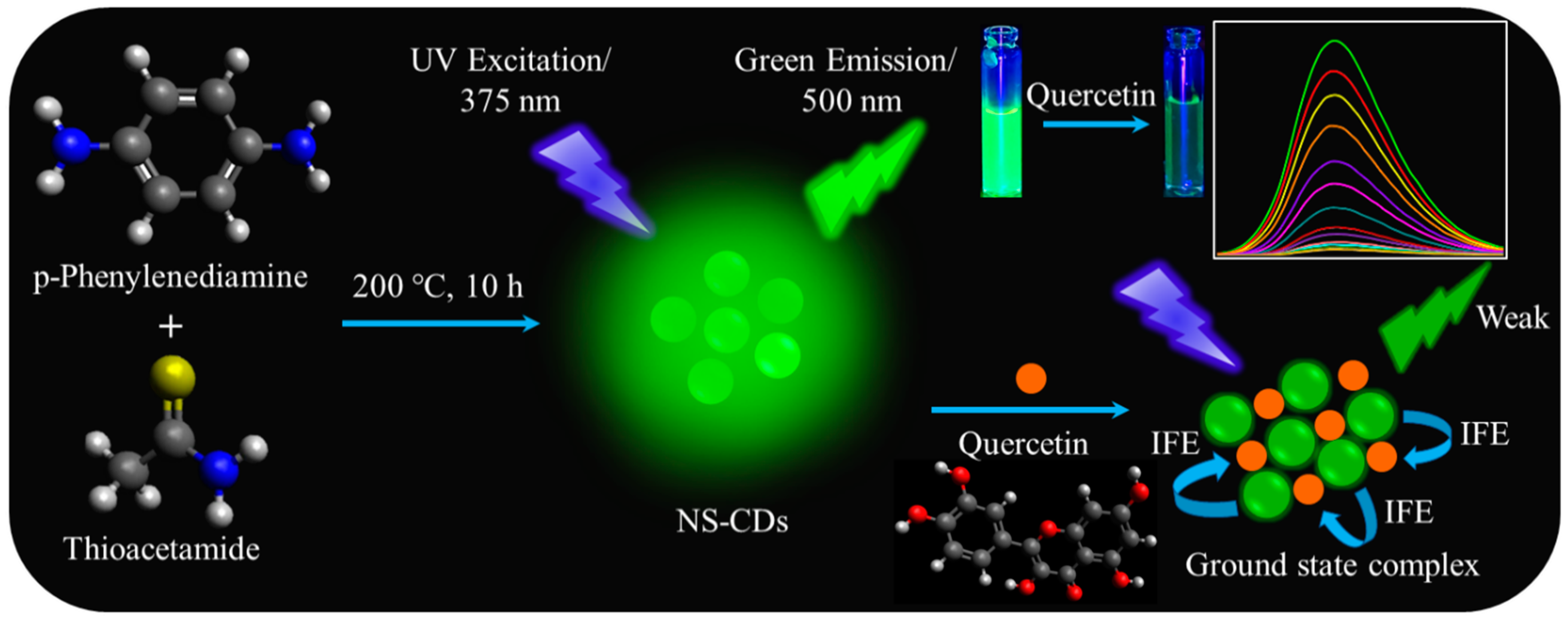
2.2. Preparation of NS-CDs
2.3. Characterization of NS-CDs
2.4. Quantum Yield (Ф) Measurement
2.5. Fluorescence Sensing of QT
2.6. Recovery Test in Food Samples
3. Results and Discussion
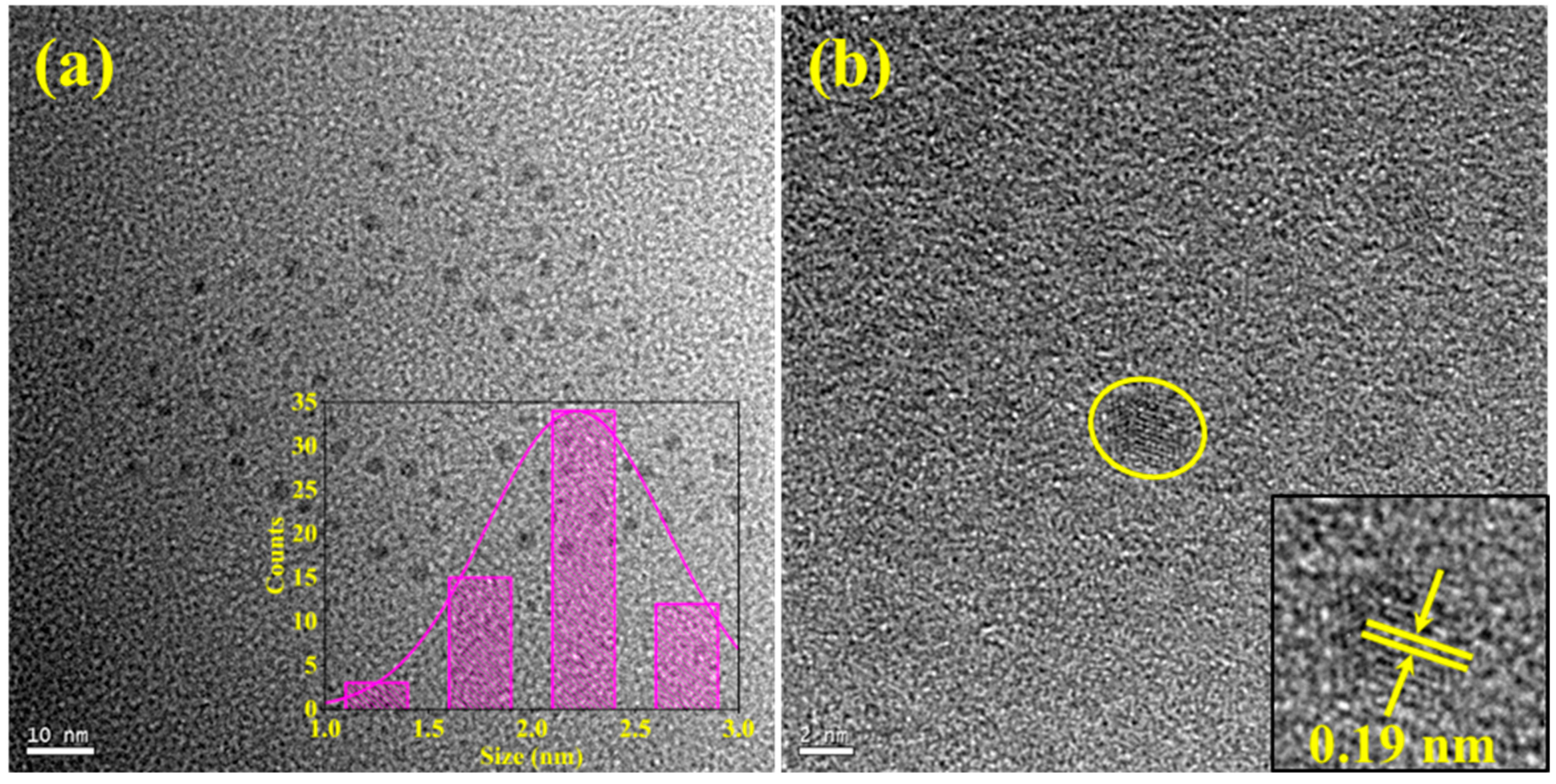
3.1. Analysis of Morphology and Chemical Compositions
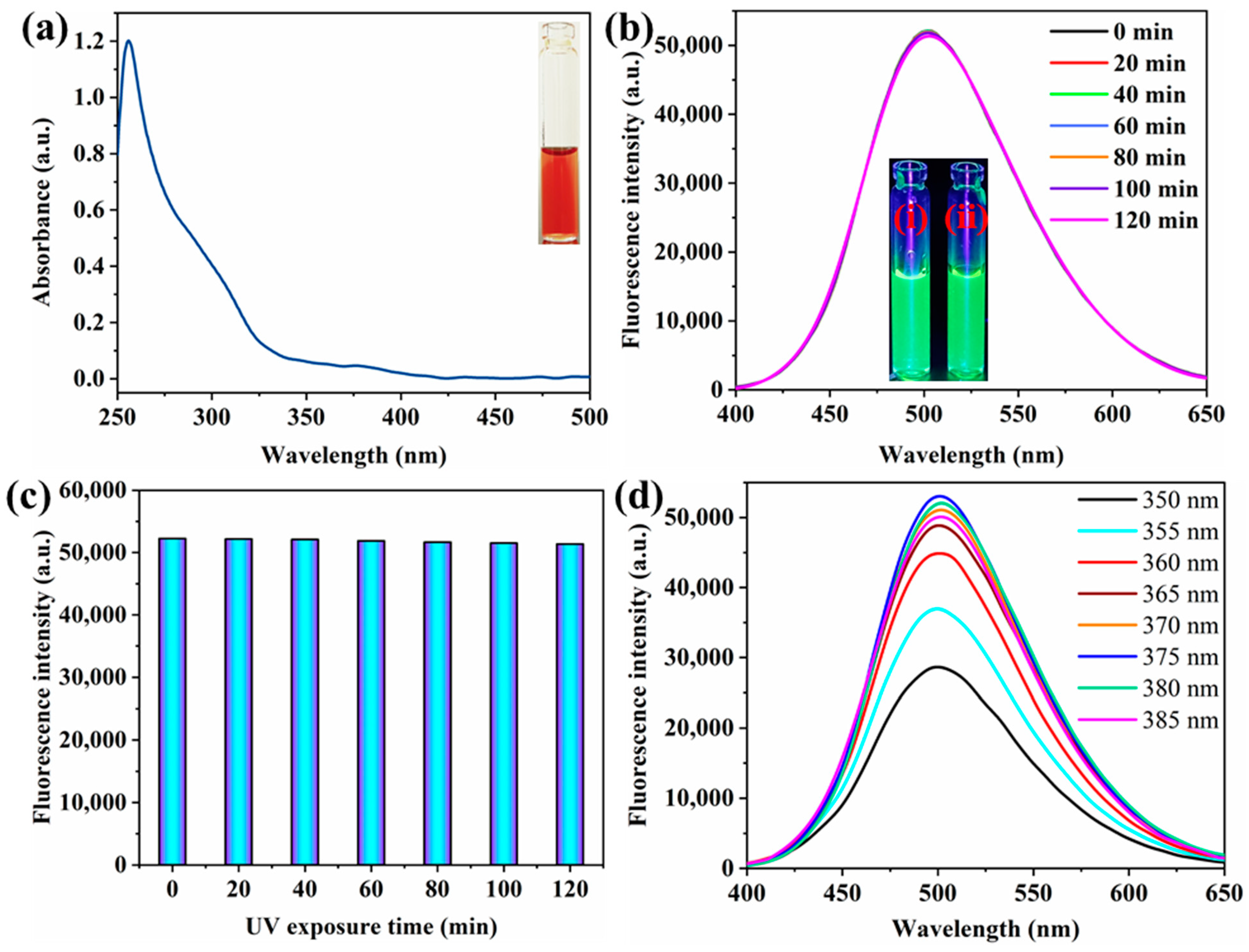
3.2. Optical Properties
3.3. Optimized Conditions
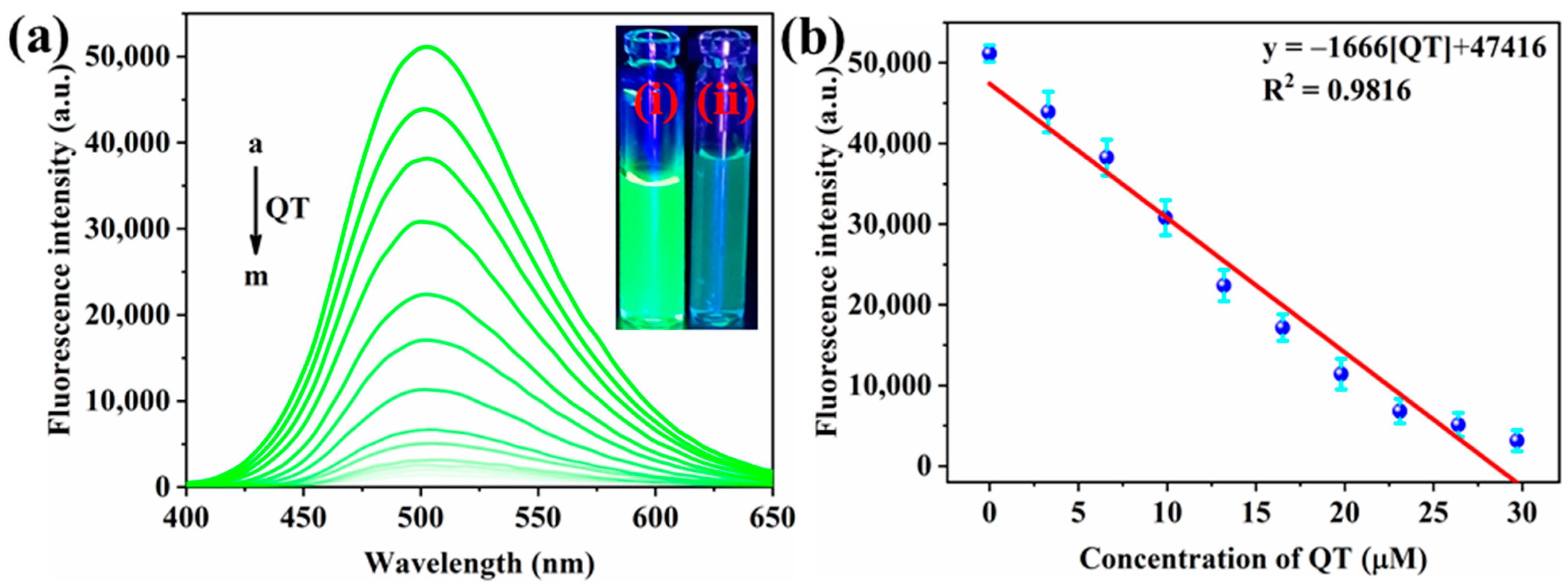
3.4. Sensitive Detection of QT
3.5. Fluorescence Quenching Mechanism
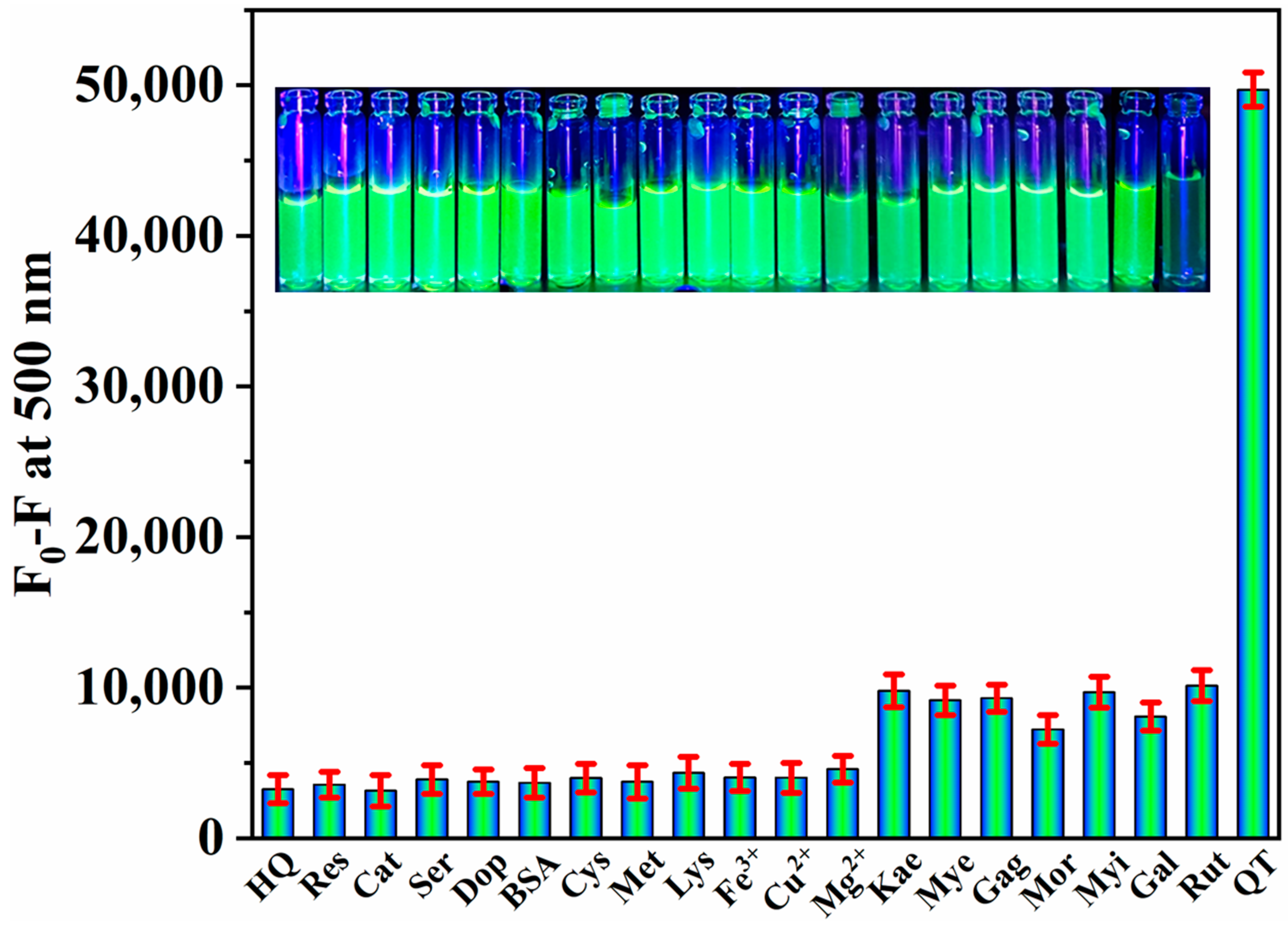
3.6. Specificity of QT Detection
3.7. Detection of QT in Food Samples
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Jakhar, R.; Paul, S.; Park, Y.R.; Han, J.; Kang, S.C. 3,5,7,3′,4′-Pentamethoxyflavone, a quercetin derivative protects DNA from oxidative challenges: Potential mechanism of action. J. Photochem. Photobiol. B 2014, 131, 96–103. [Google Scholar] [CrossRef]
- Cornard, J.P.; Merlin, J.C. Spectroscopic and structural study of complexes of quercetin with Al (III). J. Inorg. Biochem. 2002, 92, 19–27. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Zou, L.; Liu, W.; McClements, D.J. Potential of excipient emulsions for improving quercetin bioaccessibility and antioxidant activity: An in vitro study. J. Agric. Food. Chem. 2016, 64, 3653–3660. [Google Scholar] [CrossRef] [PubMed]
- Sivakumar, R.; Park, S.Y.; Lee, N.Y. Quercetin-mediated silver nanoparticle formation for the colorimetric detection of infectious pathogens coupled with loop-mediated isothermal amplification. ACS Sens. 2023, 8, 1422–1430. [Google Scholar] [CrossRef] [PubMed]
- Boots, A.W.; Haenen, G.R.; Bast, A. Health effects of quercetin: From antioxidant to nutraceutical. Eur. J. Pharmacol. 2008, 585, 325–337. [Google Scholar] [CrossRef] [PubMed]
- Vilian, A.E.; Puthiaraj, P.; Kwak, C.H.; Choe, S.R.; Huh, Y.S.; Ahn, W.S.; Han, Y.K. Electrochemical determination of quercetin based on porous aromatic frameworks supported Au nanoparticles. Electrochim. Acta 2016, 216, 181–187. [Google Scholar] [CrossRef]
- van Zanden, J.J.; Hamman, O.B.; van Iersel, M.L.; Boeren, S.; Cnubben, N.H.; Bello, M.L.; Vervoort, J.; van Bladeren, P.J.; Rietjens, I.M. Inhibition of human glutathione S-transferase P1-1 by the flavonoid quercetin. Chem. Biol. Interact. 2003, 145, 139–148. [Google Scholar] [CrossRef] [PubMed]
- Ravichandran, R.; Rajendran, M.; Devapiriam, D. Antioxidant study of quercetin and their metal complex and determination of stability constant by spectrophotometry method. Food Chem. 2014, 146, 472–478. [Google Scholar] [CrossRef]
- Gomez, F.J.V.; Espino, M.; de los Angeles Fernandez, M.; Raba, J.; Silva, M.F. Enhanced electrochemical detection of quercetin by natural deep eutectic solvents. Anal. Chim. Acta 2016, 936, 91–96. [Google Scholar] [CrossRef]
- Sun, Y.; Guo, T.; Sui, Y.; Li, F. Quantitative determination of rutin, quercetin, and adenosine in Flos Carthami by capillary electrophoresis. J. Sep. Sci. 2003, 26, 1203–1206. [Google Scholar] [CrossRef]
- Jones, D.J.L.; Lim, C.K.; Ferry, D.R.; Gescher, A. Determination of quercetin in human plasma by HPLC with spectrophotometric or electrochemical detection. Biomed. Chromatogr. 1998, 12, 232–235. [Google Scholar] [CrossRef]
- Wu, L.; Zhu, L.; Ma, J.; Li, J.; Liu, J.; Chen, Y. DNA enzyme mediated ratiometric fluorescence assay for Pb(II) ion using magnetic nanosphere-loaded gold nanoparticles and CdSe/ZnS quantum dots. Microchim. Acta 2020, 187, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Tran, V.T.; Seok, J.S.; Yoon, I.; Ju, H. Ultraviolet fluorescence-based quantitative detection of polycyclic aromatic hydrocarbons. Biochip J. 2021, 15, 356–361. [Google Scholar] [CrossRef]
- Tran, V.T.; Ju, H. Fluorescence based on surface plasmon coupled emission for ultrahigh sensitivity immunoassay of cardiac troponin I. Biomedicines 2021, 9, 448. [Google Scholar] [CrossRef] [PubMed]
- Rajamanikandan, R.; Ilanchelian, M. Protein-localized bright-red fluorescent gold nanoclusters as cyanide-selective colorimetric and fluorometric nanoprobes. ACS Omega 2018, 3, 14111–14118. [Google Scholar] [CrossRef] [PubMed]
- Wu, L.; Zhou, S.; Yun, Y.; Zhu, L.; Li, B.; Zhang, W. A multifunctional probe for lead(II) sensing using CdSe/ZnS-luminol-conjugated Fe3O4 magnetic nanocomposites. Sens. Actuators B 2022, 356, 131124. [Google Scholar] [CrossRef]
- Yu, J.; Zhang, X.; Hao, X.; Zhang, X.; Zhou, M.; Lee, C.S.; Chen, X. Near-infrared fluorescence imaging using organic dye nanoparticles. Biomaterials 2014, 35, 3356–3364. [Google Scholar] [CrossRef]
- Luo, X.; Han, Y.; Chen, X.; Tang, W.; Yue, T.; Li, Z. Carbon dots derived fluorescent nanosensors as versatile tools for food quality and safety assessment: A review. Trends Food Sci. Technol. 2020, 95, 149–161. [Google Scholar] [CrossRef]
- Behi, M.; Gholami, L.; Naficy, S.; Palomba, S.; Dehghani, F. Carbon dots: A novel platform for biomedical applications. Nanoscale Adv. 2022, 4, 353–376. [Google Scholar] [CrossRef]
- Xu, X.; Ray, R.; Gu, Y.; Ploehn, H.J.; Gearheart, L.; Raker, K.; Scrivens, W.A. Electrophoretic analysis and purification of fluorescent single-walled carbon nanotube fragments. J. Am. Chem. Soc. 2004, 126, 12736–12737. [Google Scholar] [CrossRef]
- Egorova, M.; Tomskaya, A.; Smagulova, S.A. Optical properties of carbon dots synthesized by the hydrothermal method. Materials 2023, 16, 4018. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Li, R.; Yang, B. Carbon dots: A new type of carbon-based nanomaterial with wide applications. ACS Cent. Sci. 2020, 6, 2179–2195. [Google Scholar] [CrossRef] [PubMed]
- Lu, Y.; Wang, J.; Yuan, H.; Xiao, D. Separation of carbon quantum dots on a C18 column by binary gradient elution via HPLC. Anal. Methods 2014, 6, 8124–8128. [Google Scholar] [CrossRef]
- Sasikumar, K.; Rajamanikandan, R.; Ju, H. Fluorescent carbon dots for highly sensitive bilirubin sensing with excellent selectivity. J. Sci. Adv. Mater. Devices 2023, 8, 100599. [Google Scholar] [CrossRef]
- Xu, Q.; Kuang, T.; Liu, Y.; Cai, L.; Peng, X.; Sreeprasad, T.S.; Zhao, P.; Yu, Z.; Li, N. Heteroatom-doped carbon dots: Synthesis, characterization, properties, photoluminescence mechanism and biological applications. J. Mater. Chem. B 2016, 4, 7204–7219. [Google Scholar] [CrossRef] [PubMed]
- Yan, F.; Sun, Z.; Zhang, H.; Sun, X.; Jiang, Y.; Bai, Z. The fluorescence mechanism of carbon dots, and methods for tuning their emission color: A review. Microchim. Acta 2019, 186, 1–37. [Google Scholar] [CrossRef] [PubMed]
- Ding, H.; Li, X.H.; Chen, X.B.; Wei, J.S.; Li, X.B.; Xiong, H.M. Surface states of carbon dots and their influences on luminescence. J. Appl. Phys. 2020, 127, 231101. [Google Scholar] [CrossRef]
- Ge, G.; Li, L.; Wang, D.; Chen, M.; Zeng, Z.; Xiong, W.; Wu, X.; Guo, C. Carbon dots: Synthesis, properties and biomedical applications, J. Mater. Chem. B 2021, 9, 6553–6575. [Google Scholar] [CrossRef]
- Yuan, Y.H.; Liu, Z.X.; Li, R.S.; Zou, H.Y.; Lin, M.; Liu, H.; Huang, C.Z. Synthesis of nitrogen-doping carbon dots with different photoluminescence properties by controlling the surface states. Nanoscale 2016, 8, 6770–6776. [Google Scholar] [CrossRef]
- Wang, Y.; Zhuang, Q.; Ni, Y. Facile microwave-assisted solid-phase synthesis of highly fluorescent nitrogen-sulfur-codoped carbon quantum dots for cellular imaging applications. Chem. Eur. J. 2015, 21, 13004–13011. [Google Scholar] [CrossRef]
- Mohamed, R.M.; Mohamed, S.H.; Asran, A.M.; Alsohaimi, I.H.; Hassan, H.M.; Ibrahim, H.; El-Wekil, M.M. Bifunctional ratiometric sensor based on highly fluorescent nitrogen and sulfur biomass-derived carbon nanodots fabricated from manufactured dairy product as a precursor. Spectrochim. Acta A 2023, 293, 122444. [Google Scholar] [CrossRef] [PubMed]
- Gao, D.; Zhang, Y.; Liu, A.; Zhu, Y.; Chen, S.; Wei, D.; Sun, J.; Guo, Z.; Fan, H. Photoluminescence-tunable carbon dots from synergy effect of sulfur doping and water engineering. Chem. Eng. J. 2020, 388, 124199. [Google Scholar] [CrossRef]
- Qu, Z.B.; Zhou, X.; Gu, L.; Lan, R.; Sun, D.; Yu, D.; Shi, G. Boronic acid functionalized graphene quantum dots as a fluorescent probe for selective and sensitive glucose determination in microdialysate. Chem. Commun. 2013, 49, 9830–9832. [Google Scholar] [CrossRef] [PubMed]
- Rajamanikandan, R.; Ilanchelian, M.; Ju, H. Highly selective uricase-based quantification of uric acid using hydrogen peroxide sensitive poly-(vinylpyrrolidone) templated copper nanoclusters as a fluorescence probe. Chemosensors 2023, 11, 268. [Google Scholar] [CrossRef]
- Parvin, N.; Mandal, T.K. Synthesis of a highly fluorescence nitrogen-doped carbon quantum dots bioimaging probe and its in vivo clearance and printing applications. RSC Adv. 2016, 6, 18134–18140. [Google Scholar] [CrossRef]
- Liu, Q.; Niu, X.; Xie, K.; Yan, Y.; Ren, B.; Liu, R.; Li, Y.; Li, L. Fluorescent carbon dots as nanosensors for monitoring and imaging Fe3+ and [HPO4]2– ions. ACS Appl. Nano Mater. 2020, 4, 190–197. [Google Scholar] [CrossRef]
- Barhum, H.; Alon, T.; Attrash, M.; Machnev, A.; Shishkin, I.; Ginzburg, P. Multicolor phenylenediamine carbon dots for metal-ion detection with picomolar sensitivity. ACS Appl. Nano Mater. 2021, 4, 9919–9931. [Google Scholar] [CrossRef]
- Wang, B.; Liu, X.; Duan, W.; Dai, S.; Lu, H. Visual and ratiometric fluorescent determination of Al3+ by a red-emission carbon dot-quercetin system. Microchem. J. 2020, 156, 104807. [Google Scholar] [CrossRef]
- Zhang, Z.; Pei, K.; Yang, Q.; Dong, J.; Yan, Z.; Chen, J. A nanosensor made of sulfur-nitrogen co-doped carbon dots for “off-on” sensing of hypochlorous acid and Zn(II) and its bioimaging properties. New J. Chem. 2018, 42, 15895–15904. [Google Scholar] [CrossRef]
- Zhao, Y.; Geng, X.; Shi, X.; Guo, Y.; Sun, Y.; Qu, L.; Li, Z. A fluorescence-switchable carbon dot for the reversible turn-on sensing of molecular oxygen. J. Mater. Chem. C 2021, 9, 4300–4306. [Google Scholar] [CrossRef]
- Hu, Y.; Yang, Z.; Lu, X.; Guo, J.; Cheng, R.; Zhu, L.; Wang, C.F.; Chen, S. Facile synthesis of red dual-emissive carbon dots for ratiometric fluorescence sensing and cellular imaging. Nanoscale 2020, 12, 5494–5500. [Google Scholar] [CrossRef] [PubMed]
- Lin, J.S.; Tsai, Y.W.; Dehvari, K.; Huang, C.C.; Chang, J.Y. A carbon dot based theranostic platform for dual-modal imaging and free radical scavenging. Nanoscale 2019, 11, 20917–20931. [Google Scholar] [CrossRef] [PubMed]
- Duan, J.; Yu, J.; Feng, S.; Su, L. A rapid microwave synthesis of nitrogen–sulfur co-doped carbon nanodots as highly sensitive and selective fluorescence probes for ascorbic acid. Talanta 2016, 153, 332–339. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Cui, P.; Zhang, F.; Feng, X.; Wang, Y.; Yang, Y.; Liu, X. Fluorescent probes for “off–on” highly sensitive detection of Hg2+ and L-cysteine based on nitrogen-doped carbon dots. Talanta 2016, 152, 288–300. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; He, Y.H.; Cui, P.P.; Feng, X.T.; Chen, L.; Yang, Y.Z.; Liu, X.G. Water-soluble, nitrogen-doped fluorescent carbon dots for highly sensitive and selective detection of Hg2+ in aqueous solution. RSC Adv. 2015, 5, 40393–40401. [Google Scholar] [CrossRef]
- Guo, J.; Lu, W.; Meng, Y.; Wang, H.; Dong, C.; Shuang, S. Facile synthesis of multifunctional red-emissive carbon dots for fluorescent quercetin and pH sensing. Dyes Pigm. 2022, 208, 110766. [Google Scholar] [CrossRef]
- Gao, M.; He, M.; Xing, R.; Wang, X.; Wang, Z. Borate-modified carbon dots as a probe for quercetin in plants. Analyst 2021, 146, 590–596. [Google Scholar] [CrossRef]
- Li, T.; Guo, G.; Xing, H.; Wang, Y.; Luo, X.; Wang, L.; Gu, C.; Hou, Y.; Chen, D. Energy transfer mediated rapid and visual discrimination of tetracyclines and quercetin in food by using N, Cu Co-doped carbon dots. Anal. Chim. Acta 2023, 1239, 340706. [Google Scholar] [CrossRef]
- Montes, C.; Villamayor, N.; Villaseñor, M.J.; Rios, A. Distinctive sensing nanotool for free and nanoencapsulated quercetin discrimination based on S,N co-doped graphene dots. Anal. Chim. Acta 2022, 1230, 340406. [Google Scholar] [CrossRef]
- Kadian, S.; Manik, G. Sulfur doped graphene quantum dots as a potential sensitive fluorescent probe for the detection of quercetin. Food Chem. 2020, 317, 126457. [Google Scholar] [CrossRef]
- Zou, Y.; Yan, F.; Zheng, T.; Shi, D.; Sun, F.; Yang, N.; Chen, L. Highly luminescent organosilane-functionalized carbon dots as a nanosensor for sensitive and selective detection of quercetin in aqueous solution. Talanta 2015, 135, 145–148. [Google Scholar] [CrossRef] [PubMed]
- Du, F.; Shuang, S.; Guo, Z.; Gong, X.; Dong, C.; Xian, M.; Yang, Z. Rapid synthesis of multifunctional carbon nanodots as effective antioxidants, antibacterial agents, and quercetin nanoprobes. Talanta 2020, 206, 120243. [Google Scholar] [CrossRef] [PubMed]
- Koc, Ö.K.; Üzer, A.; Apak, R. High quantum yield nitrogen-doped carbon quantum dot-based fluorescent probes for selective sensing of 2,4,6-trinitrotoluene. ACS Appl. Nano Mater. 2022, 5, 5868–5881. [Google Scholar] [CrossRef]
- Chang, H.C.; Ho, J.A.A. Gold nanocluster-assisted fluorescent detection for hydrogen peroxide and cholesterol based on the inner filter effect of gold nanoparticles. Anal. Chem. 2015, 87, 10362–10367. [Google Scholar] [CrossRef] [PubMed]
- Singh, P.; Kumar, S.; Kumar, P.; Kataria, N.; Bhankar, V.; Kumar, K.; Kumar, R.; Hsieh, C.T.; Khoo, K.S. Assessment of biomass-derived carbon dots as highly sensitive and selective templates for the sensing of hazardous ions. Nanoscale 2023, 15, 16241–16267. [Google Scholar] [CrossRef] [PubMed]
- Lakowicz, J.R. Principles of Fluorescence Spectroscopy, 3rd ed.; Springer: New York, NY, USA, 2006. [Google Scholar]
- Rajamanikandan, R.; Ilanchelian, M. Fluorescence sensing approach for high specific detection of 2,4,6-trinitrophenol using bright cyan blue color-emittive poly (vinylpyrrolidone)-supported copper nanoclusters as a fluorophore. ACS Omega 2018, 3, 18251–18257. [Google Scholar] [CrossRef]
- Das, R.; Rajender, G.; Giri, P.K. Anomalous fluorescence enhancement and fluorescence quenching of graphene quantum dots by single walled carbon nanotubes. Phys. Chem. Chem. Phys. 2018, 20, 4527–4537. [Google Scholar] [CrossRef]
- Wang, Y.; Yue, Q.; Hu, Y.; Liu, C.; Tao, L.; Zhang, C. Synthesis of N-doped carbon dots and application in vanillin detection based on collisional quenching. RSC Adv. 2019, 9, 40222–40227. [Google Scholar] [CrossRef]
- Pearson, R.G. Hard and soft acids and bases. J. Am. Chem. Soc. 1963, 85, 3533–3539. [Google Scholar] [CrossRef]


| Fluorescence Probe | Chemical Precursors | Linear Range (μM) | LOD (nM) | Ref. |
|---|---|---|---|---|
| Red-emissive CDs | Neutral red + catechol | 0.66–111.33 | 190.0 | [46] |
| Boron-functionalized CDs | Ethylenediamine + 4-boronobenzoyl chloride | 0–28.5 | 28.8 | [47] |
| N,Cu-codoped CDs | Ethylenediaminetetraacetic acid + CuCl2 | 0–100.0 | 75.4 | [48] |
| N,S-codoped graphene quantum dots | Citric acid + cysteamine hydrochloride | 0.17–33.09 | 56.25 | [49] |
| Sulfur-doped graphene quantum dots | Citric acid + 3-mercaptopropionic acid | 0–50.0 | 19.85 | [50] |
| Organosilane-functionalized CDs (SiCDs) | Citric acid + N-(b-aminoethyl)-g-aminopropyl methyldimethoxy silane | 0–40.0 | 79.0 | [51] |
| Carbon nanodots | Glucose + ethylenediamine + nitric acid | 1.0–47.0 | 172.4 | [52] |
| N,S-codoped CDs | p-Phenylenediamine + thioacetamide | 0–29.7 | 17.3 | This work |
| Samples | Amount of QT Spiked (μM) | Amount of QT Detected (μM) | Recovery (%) | RSD * (%) (n = 5) |
|---|---|---|---|---|
| Red wine | 0 | 4.38 ± 0.05 | --- | 1.08 |
| 5.0 | 9.24 ± 0.23 | 97.2 | 2.53 | |
| 10.0 | 13.97 ± 0.20 | 95.9 | 1.42 | |
| 15.0 | 18.46 ± 0.50 | 93.87 | 2.72 | |
| 30.0 | 33.91 ± 0.46 | 98.43 | 1.35 | |
| Onion | 0 | 1.50 ± 0.04 | --- | 2.56 |
| 5.0 | 6.22 ± 0.12 | 94.4 | 1.91 | |
| 10.0 | 11.13 ± 0.32 | 96.3 | 2.86 | |
| 15.0 | 16.84 ± 0.19 | 102.27 | 1.13 | |
| 30.0 | 30.59 ± 0.81 | 96.97 | 2.64 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sasikumar, K.; Rajamanikandan, R.; Ju, H. Nitrogen- and Sulfur-Codoped Strong Green Fluorescent Carbon Dots for the Highly Specific Quantification of Quercetin in Food Samples. Materials 2023, 16, 7686. https://doi.org/10.3390/ma16247686
Sasikumar K, Rajamanikandan R, Ju H. Nitrogen- and Sulfur-Codoped Strong Green Fluorescent Carbon Dots for the Highly Specific Quantification of Quercetin in Food Samples. Materials. 2023; 16(24):7686. https://doi.org/10.3390/ma16247686
Chicago/Turabian StyleSasikumar, Kandasamy, Ramar Rajamanikandan, and Heongkyu Ju. 2023. "Nitrogen- and Sulfur-Codoped Strong Green Fluorescent Carbon Dots for the Highly Specific Quantification of Quercetin in Food Samples" Materials 16, no. 24: 7686. https://doi.org/10.3390/ma16247686
APA StyleSasikumar, K., Rajamanikandan, R., & Ju, H. (2023). Nitrogen- and Sulfur-Codoped Strong Green Fluorescent Carbon Dots for the Highly Specific Quantification of Quercetin in Food Samples. Materials, 16(24), 7686. https://doi.org/10.3390/ma16247686






