Advanced Integration of Glutathione-Functionalized Optical Fiber SPR Sensor for Ultra-Sensitive Detection of Lead Ions
Abstract
:1. Introduction
2. Working Principle
3. Material and Methods
3.1. Experimental Materials and Chemical Reagents
3.2. Fabrication of AuNPs/GSH-Modified SPR Optical Fiber Sensor Probe
3.3. Instrument and Characterization
3.4. Device Testing
4. Results and Discussions
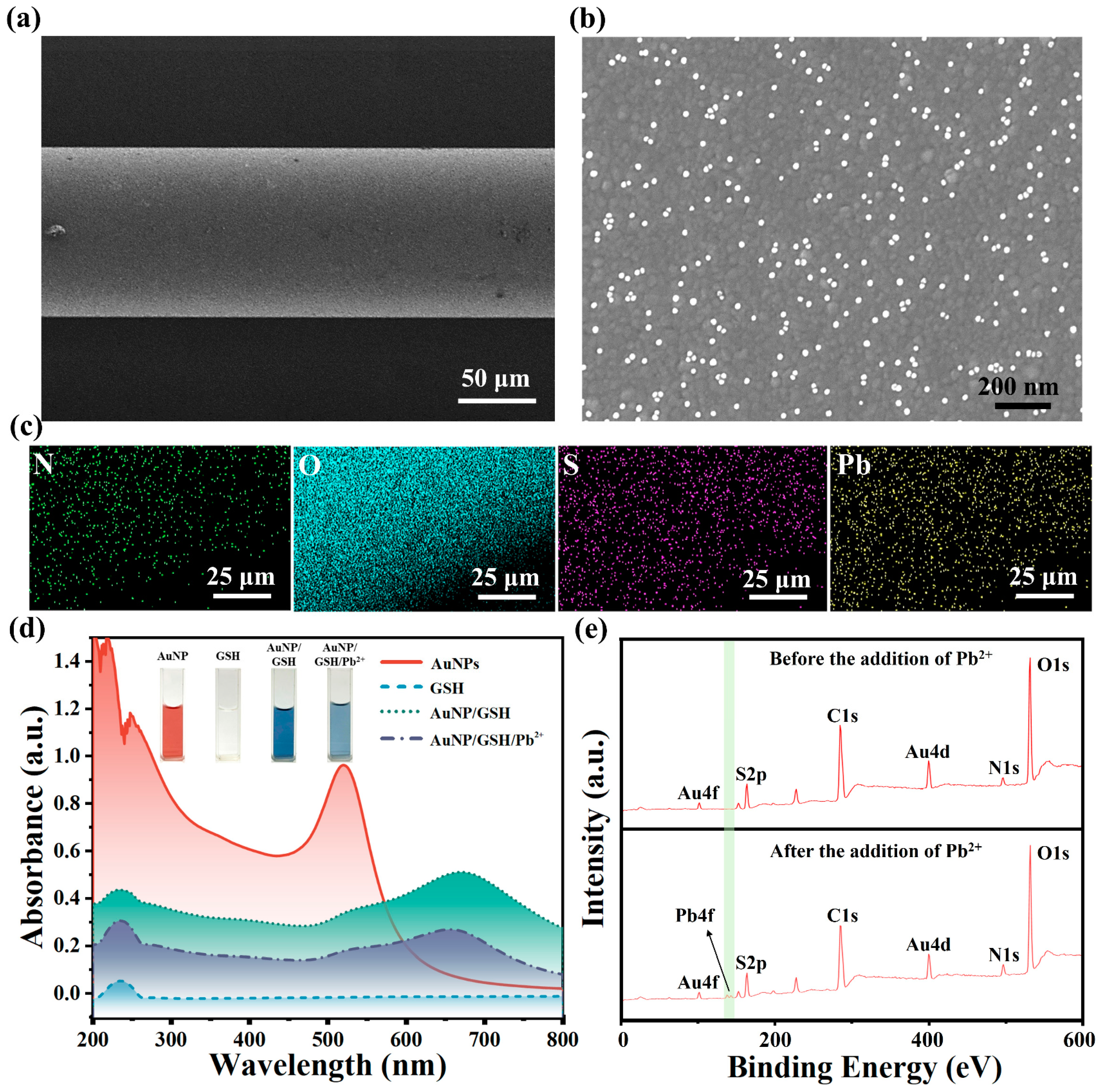
4.1. Characterization
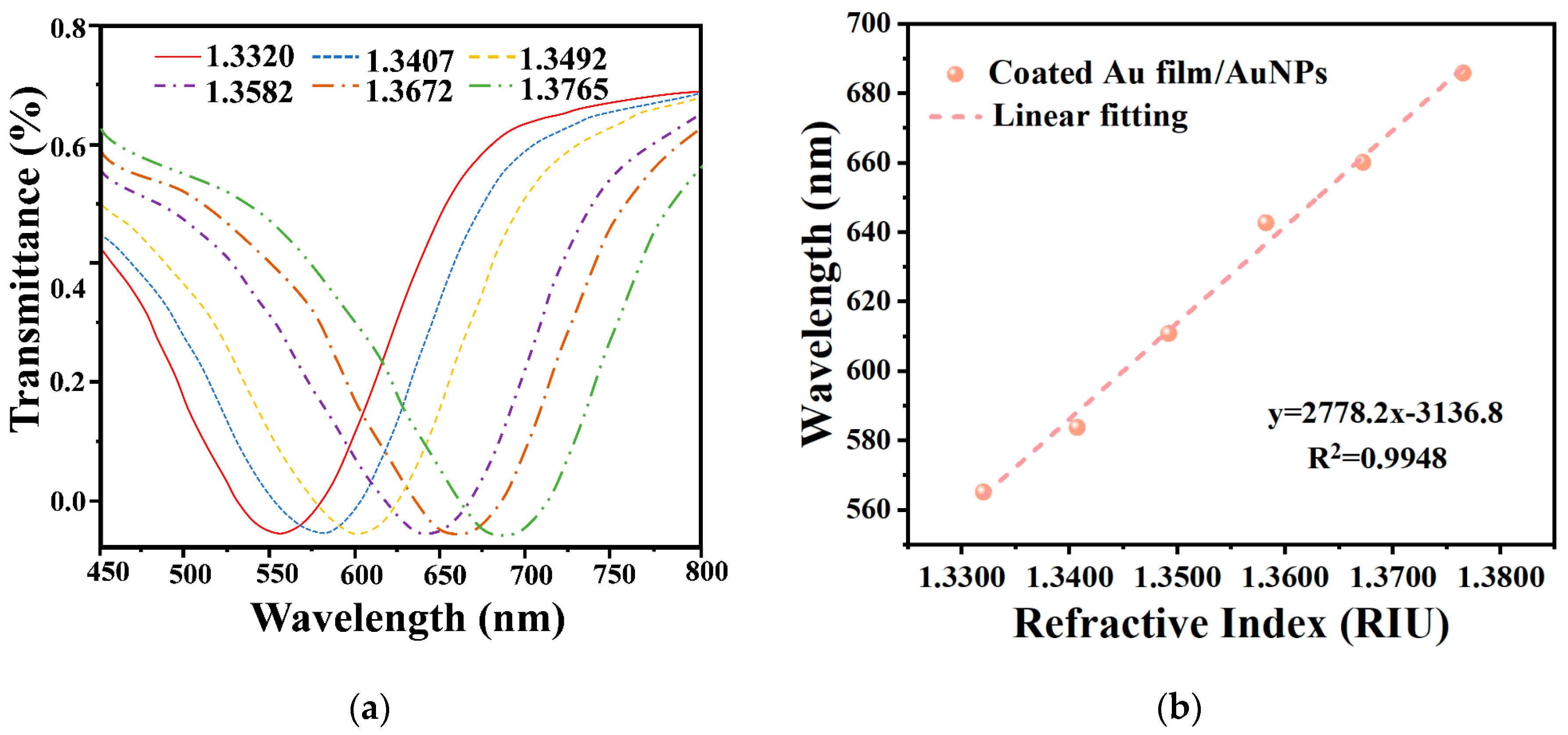
4.2. RI Sensitivity of Sensor
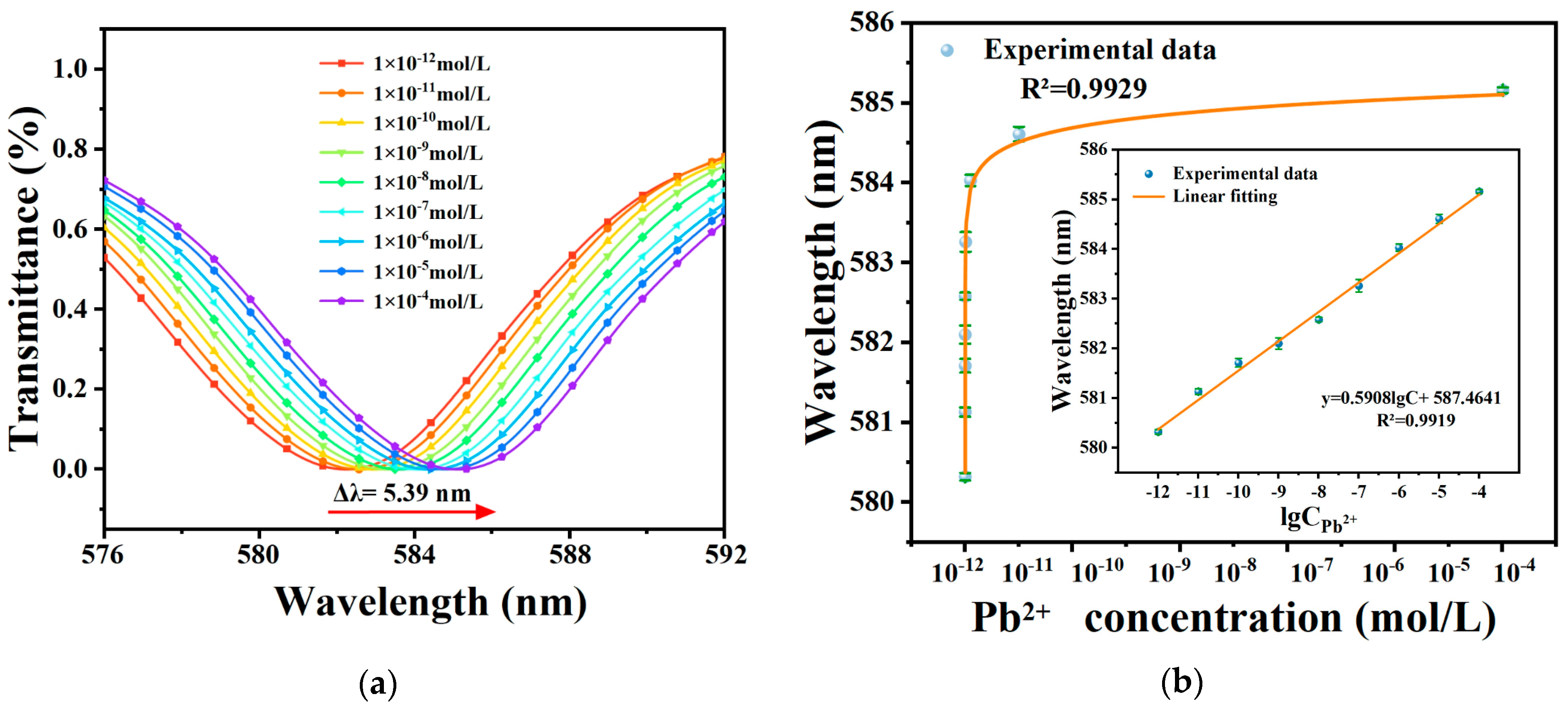
4.3. Detection of Pb2+
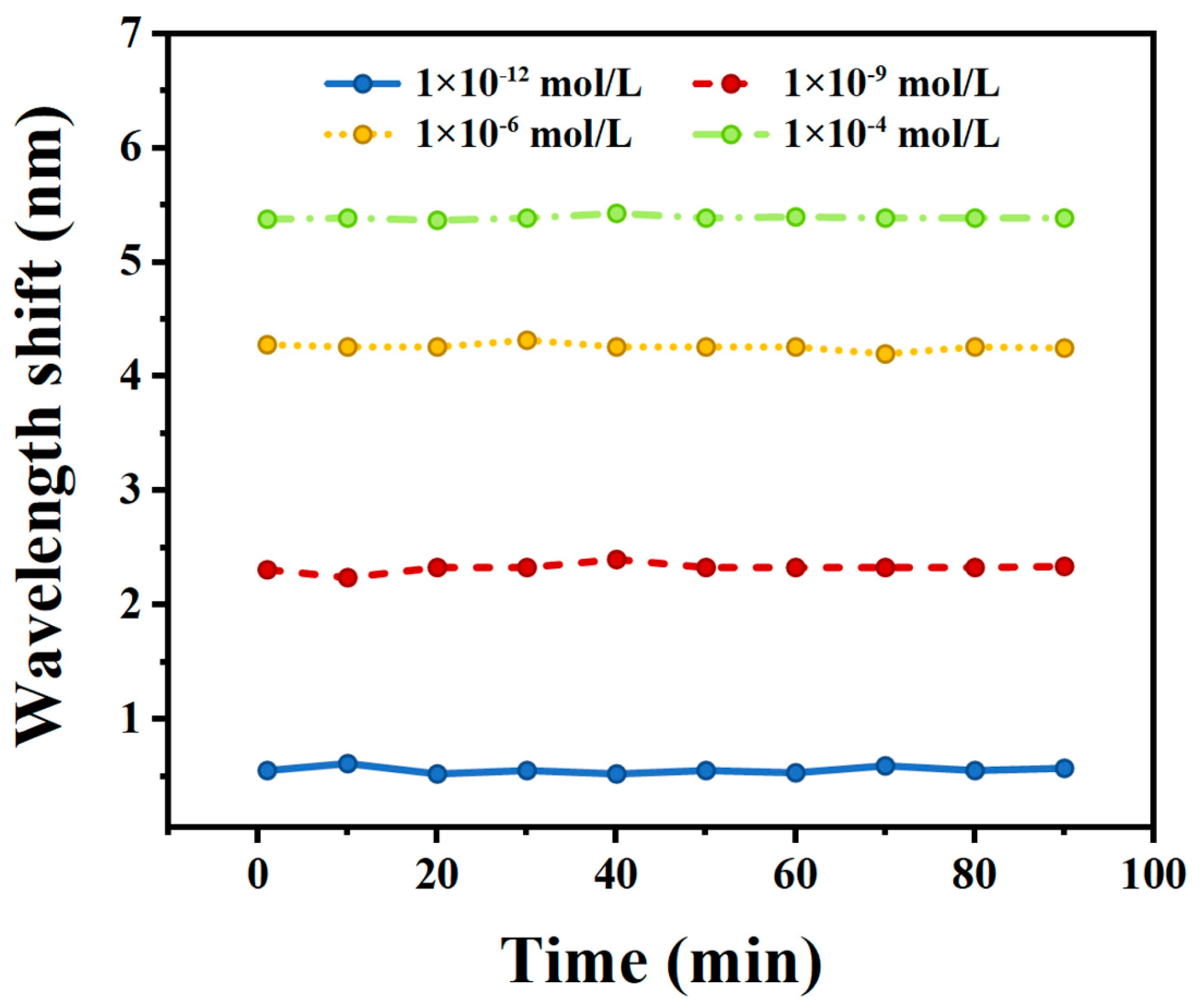
4.4. Stability of the Sensor
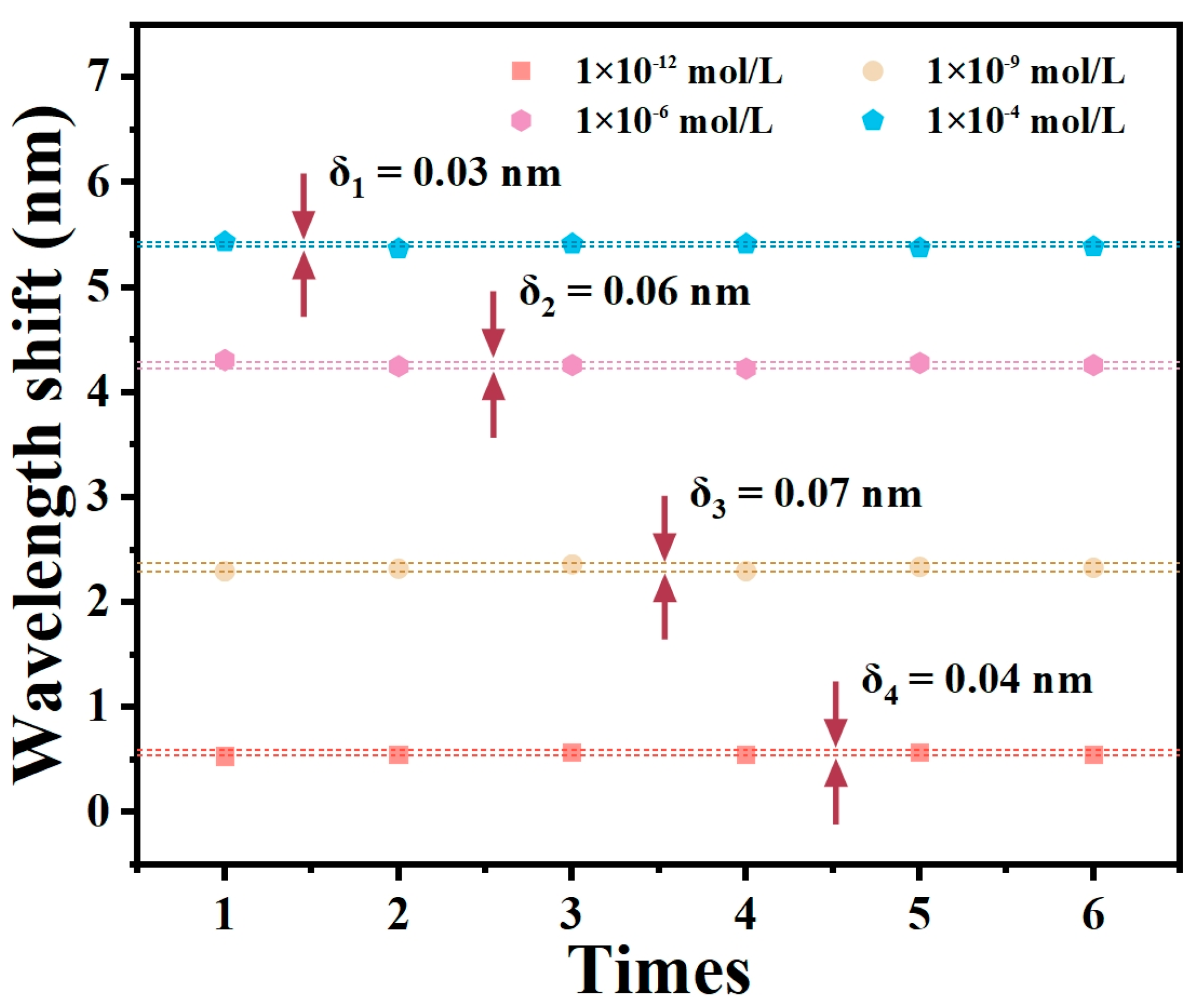
4.5. Repeatability of the Sensor
4.6. Specificity of the Sensor
4.7. Discussions
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Beardsley, C.A.; Fuller, K.Z.; Reilly, T.H.; Henry, C.S. Method for analysis of environmental lead contamination in soils. Analyst 2021, 146, 7520–7527. [Google Scholar] [CrossRef] [PubMed]
- Fouskaki, M.; Chaniotakis, N.A. Thick membrane, solid contact ion selective electrode for the detection of lead at picomolar levels. Anal. Chem. 2005, 77, 1780–1784. [Google Scholar] [CrossRef] [PubMed]
- Cui, W.; Abbasi, Z.; Ren, C. Real-time lead detection device based on nanomaterials modified microwave-microfluidic sensor. Sens. Actuators B-Chem. 2023, 362, 114652. [Google Scholar] [CrossRef]
- Larsen, B.; Sánchez-Triana, E. Global health burden and cost of lead exposure in children and adults: A health impact and economic modelling analysis. Lancet Planet. Health 2023, 7, e831–e840. [Google Scholar] [CrossRef] [PubMed]
- Bouida, L.; Rafatullah, M.; Kerrouche, A.; Qutob, M.; Alosaimi, A.M.; Alorfi, H.S.; Hussein, M.A. A review on cadmium and lead contamination: Sources, fate, mechanism, health effects and remediation methods. Water 2022, 14, 3432. [Google Scholar] [CrossRef]
- Lacerda, D.; Pestana, I.A.; dos Santos Vergílio, C.; de Rezende, C.E. Global decrease in blood lead concentrations due to the removal of leaded gasoline. Chemosphere 2023, 324, 138207. [Google Scholar] [CrossRef] [PubMed]
- Shah, A.; Zahid, A.; Khan, A.; Iftikhar, F.J.; Nisar, J.; Fernandez, C.; Kraatz, H.B. Development of a highly sensitive electrochemical sensing platform for the trace level detection of lead ions. J. Electrochem. Soc. 2019, 166, B3136. [Google Scholar] [CrossRef]
- Fernández, L.; Espinoza-Montero, P.; Sánchez-Sarango, M.; Bolaños-Méndez, D.; Álvarez-Paguay, J.; Domínguez-Granda, L.; Rodríguez, A.; Romero, H.; Debut, A.; Ortiz, V. Simultaneous quantification of lead, cadmium and zinc in superficial marine sediments using a carbon-fiber microelectrode modified with bismuth film. Sci. Rep. 2023, 13, 20232. [Google Scholar] [CrossRef]
- Sitko, R.; Musielak, M.; Serda, M.; Talik, E.; Gagor, A.; Zawisza, B.; Malecka, M. Graphene oxide decorated with fullerenol nanoparticles for highly efficient removal of Pb (II) ions and ultrasensitive detection by total-reflection X-ray fluorescence spectrometry. Sep. Purif. Technol. 2021, 277, 119450. [Google Scholar] [CrossRef]
- Kim, H.N.; Ren, W.X.; Kim, J.S.; Yoon, J. Fluorescent and colorimetric sensors for detection of lead, cadmium, and mercury ions. Chem. Soc. Rev. 2012, 41, 3210–3244. [Google Scholar] [CrossRef]
- Alam, A.U.; Howlader, M.M.R.; Hu, N.; Deen, M.J. Electrochemical sensing of lead in drinking water using β-cyclodextrin-modified MWCNTs. Sens. Actuators B-Chem. 2019, 296, 126632. [Google Scholar] [CrossRef]
- Nguyen, T.T.H.; Pham, T.H.Y.; Doan, T.D.; Thi, N.H.; Oanh, H.T.; Nguyen, T.T.; Hoang, M.H. Silver nanowire/graphene oxide electrode for electrochemical detection of lead ions. Chem. Pap. 2022, 76, 5459–5469. [Google Scholar] [CrossRef]
- Solra, M.; Bala, R.; Wangoo, N.; Soni, G.K.; Kumar, M.; Sharma, R.K. Optical pico-biosensing of lead using plasmonic gold nanoparticles and a cationic peptide-based aptasensor. Chem. Commun. 2020, 56, 289–292. [Google Scholar] [CrossRef] [PubMed]
- Ali, M. Preconcentration and determination of trace amounts of heavy metals in water samples using membrane disk and flame atomic absorption spectrometry. Chin. J. Chem. 2007, 25, 640–644. [Google Scholar] [CrossRef]
- Jigam, A.A.; Dauda, B.E.N.; Tijani, J.O.; Yusuf, H.N.; Umar, Z.T. Determination of copper, zinc, lead and some biochemical parameters in fresh cow milk from different locations in Niger State. J. Food Sci. 2011, 5, 156–160. [Google Scholar]
- Menon, S.; Usha, S.P.; Manoharan, H.; Kishore, P.V.N.; Sai, V.V.R. Metal–organic framework-based fiber optic sensor for chromium (VI) detection. ACS Sens. 2023, 8, 684–693. [Google Scholar] [CrossRef]
- Chauhan, M.; Singh, V.K. Review on recent experimental SPR/LSPR based fiber optic analyte sensors. Opt. Fiber Technol. 2021, 64, 102580. [Google Scholar] [CrossRef]
- Pawar, D.; Kale, S.N. A review on nanomaterial-modified optical fiber sensors for gases, vapors and ions. Microchim. Microchim. Acta 2019, 186, 253. [Google Scholar] [CrossRef]
- Yap, S.H.K.; Chien, Y.H.; Tan, R.; bin Shaik Alauddi, A.R.; Ji, W.B.; Tjin, S.C.; Yong, K.T. An advanced hand-held microfiber-based sensor for ultrasensitive lead ion detection. ACS Sens. 2018, 3, 2506–2512. [Google Scholar] [CrossRef]
- Ghosh, S.; Dissanayake, K.; Asokan, S.; Sun, T.; Rahman, B.A.; Grattan, K.T. Lead (Pb2+) ion sensor development using optical fiber gratings and nanocomposite materials. Sens. Actuators B-Chem. 2022, 364, 131818. [Google Scholar] [CrossRef]
- Wang, F.; Zhang, Y.; Lu, M.; Du, Y.; Chen, M.; Meng, S.; Ji, W.; Sun, C.; Peng, W. Near-infrared band Gold nanoparticles-Au film “hot spot” model based label-free ultratrace lead (II) ions detection via fiber SPR DNAzyme biosensor. Sens. Actuators B-Chem. 2021, 337, 129816. [Google Scholar] [CrossRef]
- Du, J.; Cipot-Wechsler, J.; Lobez, J.M.; Loock, H.P.; Crudden, C.M. Periodic Mesoporous Organosilica Films: Key Components of Fiber-Optic-Based Heavy-Metal Sensors. Small 2010, 6, 1168–1172. [Google Scholar] [CrossRef] [PubMed]
- Biswas, R.; Bhuyan, R.; Boruah, B.S.; Mazumder, N. Assessing heavy metal ion contamination through functionalized D-shaped optical fiber. Opt. Fiber Technol. 2022, 72, 102996. [Google Scholar] [CrossRef]
- Boruah, B.S.; Biswas, R. In-situ sensing of hazardous heavy metal ions through an ecofriendly scheme. Opt. Laser Technol. 2021, 137, 106813. [Google Scholar] [CrossRef]
- Boruah, B.S.; Biswas, R. An optical fiber based surface plasmon resonance technique for sensing of lead ions: A toxic water pollutant. Opt. Fiber Technol. 2018, 46, 152–156. [Google Scholar] [CrossRef]
- Yin, Y.; Li, S.; Wang, S.; Jia, S.; Ren, J.; Farrell, G.; Lewis, E.; Wang, P. Ultra-high-resolution detection of Pb2+ ions using a black phosphorus functionalized microfiber coil resonator. Photonics Res. 2019, 7, 622–629. [Google Scholar] [CrossRef]
- Liu, C.; Sun, Z.; Zhang, L.; Lv, J.; Yu, X.F.; Chen, X. Black phosphorus integrated tilted fiber grating for ultrasensitive heavy metal sensing. Sens. Actuators B-Chem. 2018, 257, 1093–1098. [Google Scholar] [CrossRef]
- Teng, P.; Jiang, Y.; Chang, X.; Shen, Y.; Liu, Z.; Copner, N.; Yang, J.; Li, K.; Bowkett, M.; Yuan, L.; et al. Highly sensitive on-line detection of trace Pb2+ based on tapered fiber integrated with black phosphorus. Opt. Fiber Technol. 2021, 66, 102668. [Google Scholar] [CrossRef]
- Riza, M.A.; Go, Y.I.; Maier, R.R.; Harun, S.W.; Anas, S.B.A. Enhanced fiber mounting and etching technique for optimized optical power transmission at critical cladding thickness for fiber-sensing application. Laser Phys. 2021, 31, 126201. [Google Scholar] [CrossRef]
- Al Noman, A.; Dash, J.N.; Cheng, X.; Tam, H.Y.; Yu, C. PCF based modal interferometer for lead ion detection. Opt. Express 2022, 30, 4895–4904. [Google Scholar] [CrossRef]
- Li, G.; Liu, Z.; Feng, J.; Zhou, G.; Huang, X. Pb2+ fiber optic sensor based on smart hydrogel coated Mach-Zehnder interferometer. Opt. Laser Technol. 2022, 145, 107453. [Google Scholar] [CrossRef]
- Dhara, P.; Kumar, R.; Binetti, L.; Nguyen, H.T.; Alwis, L.S.; Sun, T.; Grattan, K.T. Optical fiber-based heavy metal detection using the localized surface plasmon resonance technique. IEEE Sens. J. 2019, 19, 8720–8726. [Google Scholar] [CrossRef]
- Zhu, W.; Huang, Q.; Wang, Y.; Lewis, E.; Yang, M. Enhanced sensitivity of heterocore structure surface plasmon resonance sensors based on local microstructures. Opt. Eng. 2018, 57, 076105. [Google Scholar] [CrossRef]
- Sakhraoui, H.E.E.Y.; Mazouz, Z.; Attia, G.; Fourati, N.; Zerrouki, C.; Maouche, N.; Nessark, B. Design of L-Cysteine and acrylic acid imprinted Polypyrrole sensors for picomolar detection of lead ions in simple and real media. IEEE Sens. J. 2019, 20, 4147–4155. [Google Scholar] [CrossRef]
- Faradilla, P.; Setiyanto, H.; Manurung, R.V.; Saraswaty, V. Electrochemical sensor based on screen printed carbon electrode–zinc oxide nano particles/molecularly imprinted-polymer (SPCE–ZnONPs/MIP) for detection of sodium dodecyl sulfate (SDS). RSC Adv. 2022, 12, 743–752. [Google Scholar] [CrossRef] [PubMed]
- Pan, K.; Tang, X.; Qu, G.; Tang, H.; Wei, K.; Lv, J. Mesoporous Silica/Iron Phthalocyanine Light-Driven Nanomaterials for Efficient Removal of Pb2+ Ions from Wastewater. ACS Appl. Nano Mater. 2023, 6, 12816–12827. [Google Scholar] [CrossRef]
- Gupta, A.; Verma, N.C.; Khan, S.; Tiwari, S.; Chaudhary, A.; Nandi, C.K. Paper strip based and live cell ultrasensitive lead sensor using carbon dots synthesized from biological media. Sens. Actuators B-Chem. 2016, 232, 107–114. [Google Scholar] [CrossRef]
- Venkateswarlu, S.; Kumar, B.N.; Prathima, B.; SubbaRao, Y.; Jyothi, N.V.V. A novel green synthesis of Fe3O4 magnetic nanorods using Punica Granatum rind extract and its application for removal of Pb (II) from aqueous environment. Arab. J. Chem. 2019, 12, 588–596. [Google Scholar] [CrossRef]
- Borges, V.C.; Nogueira, C.W. The role of thiol-reducing agents on modulation of glutamate binding induced by heavy metals in platelets. Toxicol. In Vitro 2008, 22, 438–443. [Google Scholar] [CrossRef]
- Cankurtaran, H.; Karadayi, E.B.; Sungur, S. Cosnductive composites of serigraphic inks and their usage in heavy metal sensor and biosensor. Prog. Org. Coat. 2016, 98, 6–9. [Google Scholar] [CrossRef]
- García-Santos, I.; Castiñeiras, A.; Mahmoudi, G.; Babashkina, M.G.; Zangrando, E.; Gomila, R.M.; Safin, D.A. Lead (ii) supramolecular structures formed through a cooperative influence of the hydrazinecarbothioamide derived and ancillary ligands. CrystEngComm 2022, 24, 368–378. [Google Scholar] [CrossRef]
- Liu, X.; Li, M.; Singh, R.; Wang, Y.; Xie, Y.; Su, X.; Gao, F.; Li, G.; Kumar, D.; Zhang, B.; et al. Feasibility analysis of an SMS-/MSM-/SMSMS-based optical fiber sensor structure. Appl. Opt. 2022, 61, 2327–2332. [Google Scholar] [CrossRef] [PubMed]
- Mustafa, D.E.; Yang, T.; Xuan, Z.; Chen, S.; Tu, H.; Zhang, A. Surface plasmon coupling effect of gold nanoparticles with different shape and size on conventional surface plasmon resonance signal. Plasmonics 2010, 5, 221–231. [Google Scholar] [CrossRef]
- Szunerits, S.; Boukherroub, R. Sensing using localised surface plasmon resonance sensors. Chem. Commun. 2012, 48, 8999–9010. [Google Scholar] [CrossRef] [PubMed]
- Yazdani, S.; Daneshkhah, A.; Diwate, A.; Patel, H.; Smith, J.; Reul, O.; Cheng, R.; Izadian, A.; Hajrasouliha, A.R. Model for gold nanoparticle synthesis: Effect of pH and reaction time. ACS Omega 2021, 6, 16847–16853. [Google Scholar] [CrossRef] [PubMed]
- Gong, C.; Li, Z.; Liu, G.; Wang, R.; Pu, S. A sensitive fluorescence “turn on” nanosensor for glutathione detection based on Ce-MOF and gold nanoparticles. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2022, 265, 120362. [Google Scholar] [CrossRef]
- Agrawal, H.; Shrivastav, A.M.; Gupta, B.D. Surface plasmon resonance based optical fiber sensor for atrazine detection using molecular imprinting technique. Sens. Actuators B-Chem. 2016, 227, 204–211. [Google Scholar] [CrossRef]
- Verma, R.; Gupta, B.D. Detection of heavy metal ions in contaminated water by surface plasmon resonance based optical fibre sensor using conducting polymer and chitosan. Food Chem. 2015, 166, 568–575. [Google Scholar] [CrossRef]
- Feng, B.; Zhu, R.; Xu, S.; Chen, Y.; Di, J. A sensitive LSPR sensor based on glutathione-functionalized gold nanoparticles on a substrate for the detection of Pb2+ ions. RSC Adv. 2018, 8, 4049–4056. [Google Scholar] [CrossRef]
- Shrivas, K.; Sahu, B.; Deb, M.K.; Thakur, S.S.; Sahu, S.; Kurrey, R.; Kant, T.; Patle, T.K.; Jangde, R. Colorimetric and paper-based detection of lead using PVA capped silver nanoparticles: Experimental and theoretical approach. Microchem. J. 2019, 150, 104156. [Google Scholar] [CrossRef]
- Roto, R.; Mellisani, B.; Kuncaka, A.; Mudasir, M.; Suratman, A. Colorimetric sensing of Pb2+ ion by using ag nanoparticles in the presence of dithizone. Chemosensors 2019, 7, 28. [Google Scholar] [CrossRef]
- Abdullah, S.; Azeman, N.H.; Mobarak, N.N.; Zan, M.S.D.; Bakar, A.A.A. Sensitivity enhancement of localized SPR sensor towards Pb (II) ion detection using natural bio-polymer based carrageenan. Optik 2018, 168, 784–793. [Google Scholar] [CrossRef]
- Qiu, G.; Ng, S.P.; Liang, X.; Ding, N.; Chen, X.; Wu, C.M.L. Label-free LSPR detection of trace lead (II) ions in drinking water by synthetic poly (mPD-co-ASA) nanoparticles on gold nanoislands. Anal. Chem. 2017, 89, 1985–1993. [Google Scholar] [CrossRef] [PubMed]
- Qiu, G.Y.; Law, A.H.; Ng, S.P.; Wu, C.L. Label-free detection of lead (II) ion using differential phase modulated localized surface plasmon resonance sensors. Procedia Eng. 2016, 168, 533–536. [Google Scholar] [CrossRef]
- Nawi, N.M.; Abdullah, S.; Bakar, A.A.A. Gold nanoparticles/graphene oxide/polyaniline nanocomposites film as sensitive LSPR-based sensor for Pb (II) ions detection. In Proceedings of the 2014 IEEE 5th International Conference on Photonics (ICP), Kuala Lumpur, Malaysia, 2–4 September 2014; pp. 188–190. [Google Scholar]



| Structure/ Method | LOD (pM) | Sensitivity (nm/M) | Dynamic Range (pM) | Sensing Layer | Ref. |
|---|---|---|---|---|---|
| Fiber Bragg grating | 1.80 × 103 | / | 1.80 × 103–1.80 × 104 | L-glutathione | [19] |
| Fiber Bragg grating | 500.00 | 2.55 × 109 | 500–1 × 109 | CCS-NGO/PAA nanocomposite | [20] |
| Fiber Bragg grating | 8.56 | / | 10–106 | DNAzyme/AuNPs | [21] |
| Fiber Bragg grating | 9.65 × 104 | 2.10 × 106 | 4.49 × 105–4.63 × 108 | PMO/BTESPTS | [22] |
| Tapered fiber | 102.47 | / | 360–1.80 × 108 | Black phosphorus | [26] |
| Tapered fiber | 8.60 | / | 8.60–3019 | Black phosphorus | [27] |
| Tapered fiber | 62.20 | 1.23 × 107 | 3.02 × 102–3.02 × 108 | Black phosphorus | [28] |
| Interferometric fiber | 3019.00 | 1.03 × 107 | 3019–1.51 × 105 | Chitosan-PVA/ GSH/AuNPs | [30] |
| Interferometric fiber | 2.45 × 108 | 8.16 × 105 | 2 × 105–1.20 × 106 | Hydroxyethyl methacrylate crosslinked hydrogel | [31] |
| End reflection fiber | 8.00 × 108 | 280.00 | 8 × 108–1 × 1010 | AuNPs/1,1-Mercaptoundecanoic acid | [32] |
| Plastic clad silica optical fiber | 158.00 | 2.10 × 109 | 3.60 × 104–7.20 × 105 | Pyrrole/CS/ITO/Ag | [48] |
| ITO glass | 50.00 | / | 100–1 × 107 | AuNPs/GSH | [49] |
| Colorimetry | 9.60 × 104 | / | 9.60 × 104–4.80 × 106 | Paper-based/AgNPs/PVA | [50] |
| Colorimetry | 3100.00 | 5.90 × 107 | 2.40 × 103–4.80 × 104 | AgNPs/dithizone | [51] |
| Optical sensor | / | 2.10 × 109 | / | AuNPs/kappa-carrageenan | [52] |
| Optical sensor | 53.00 | / | 53–2.40 × 104 | AuNIs/Poly (m-phenylenediamine-co-aniline-2-sulfonic acid) copolymer nanoparticles | [53] |
| Optical sensor | 720.00 | / | / | AuNIs/Poly (m-phenylenediamine-co-Aniline-2- sulfonic acids) copolymer | [54] |
| LSPR sensor | 1.40 × 105 | / | 1.40 × 105–1.40 × 107 | AuNPs/GO/PANI | [55] |
| Multimode-singlemode-multimode fiber | 0.43 | 2.32 × 1011 | 1–1 × 108 | AuNPs/GSH | This Work |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, J.; Niu, K.; Hou, J.; Zhuang, Z.; Zhu, J.; Jing, X.; Wang, N.; Xia, B.; Lei, L. Advanced Integration of Glutathione-Functionalized Optical Fiber SPR Sensor for Ultra-Sensitive Detection of Lead Ions. Materials 2024, 17, 98. https://doi.org/10.3390/ma17010098
Wang J, Niu K, Hou J, Zhuang Z, Zhu J, Jing X, Wang N, Xia B, Lei L. Advanced Integration of Glutathione-Functionalized Optical Fiber SPR Sensor for Ultra-Sensitive Detection of Lead Ions. Materials. 2024; 17(1):98. https://doi.org/10.3390/ma17010098
Chicago/Turabian StyleWang, Jiale, Kunpeng Niu, Jianguo Hou, Ziyang Zhuang, Jiayi Zhu, Xinyue Jing, Ning Wang, Binyun Xia, and Lei Lei. 2024. "Advanced Integration of Glutathione-Functionalized Optical Fiber SPR Sensor for Ultra-Sensitive Detection of Lead Ions" Materials 17, no. 1: 98. https://doi.org/10.3390/ma17010098






