Green- and Blue-Emitting Tb3+-Activated Linde Type A Zeolite-Derived Boro-Aluminosilicate Glass for Deep UV Detection/Imaging
Abstract
:1. Introduction
2. Experimental Processes
2.1. Sample Preparation
2.2. Characterization
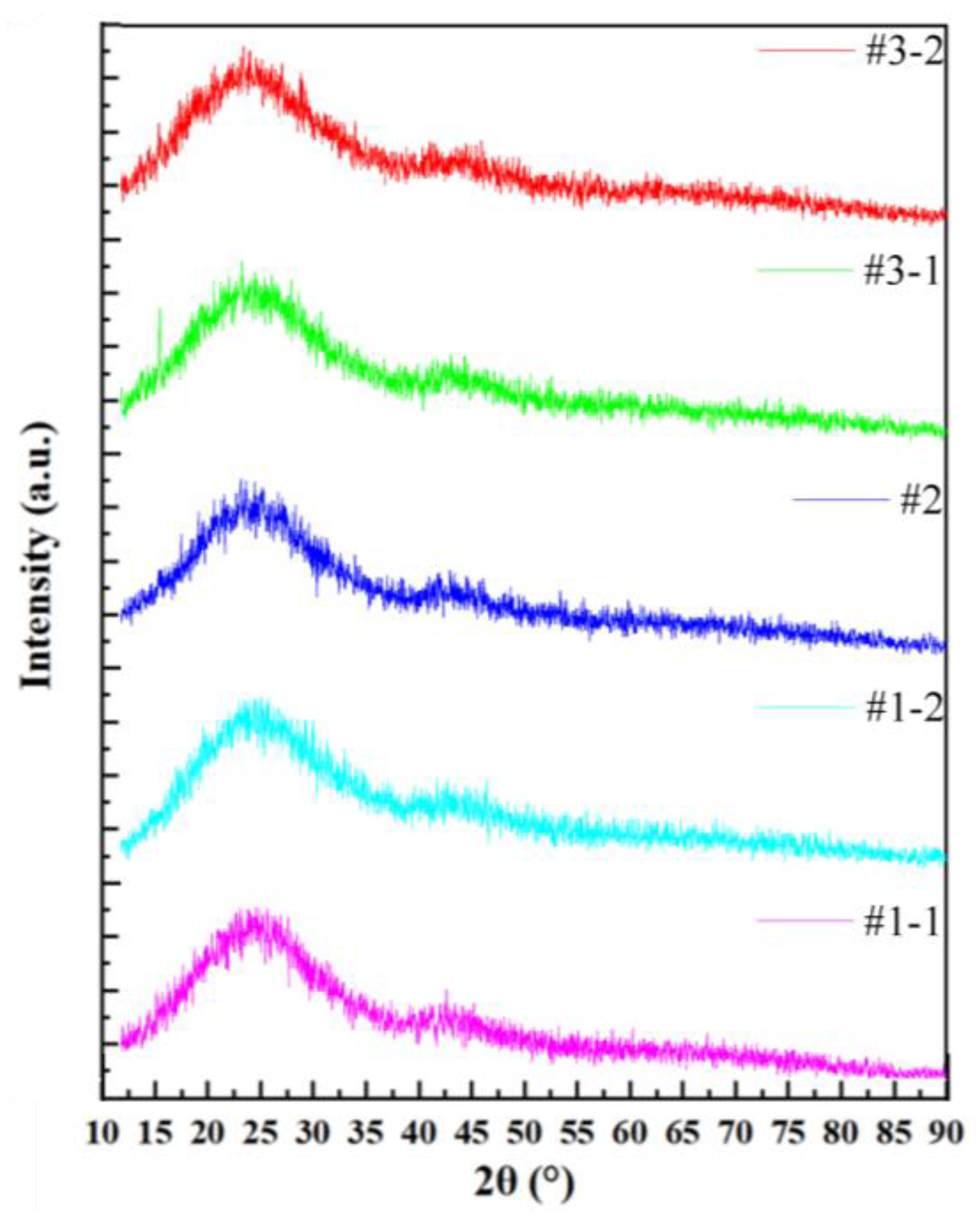
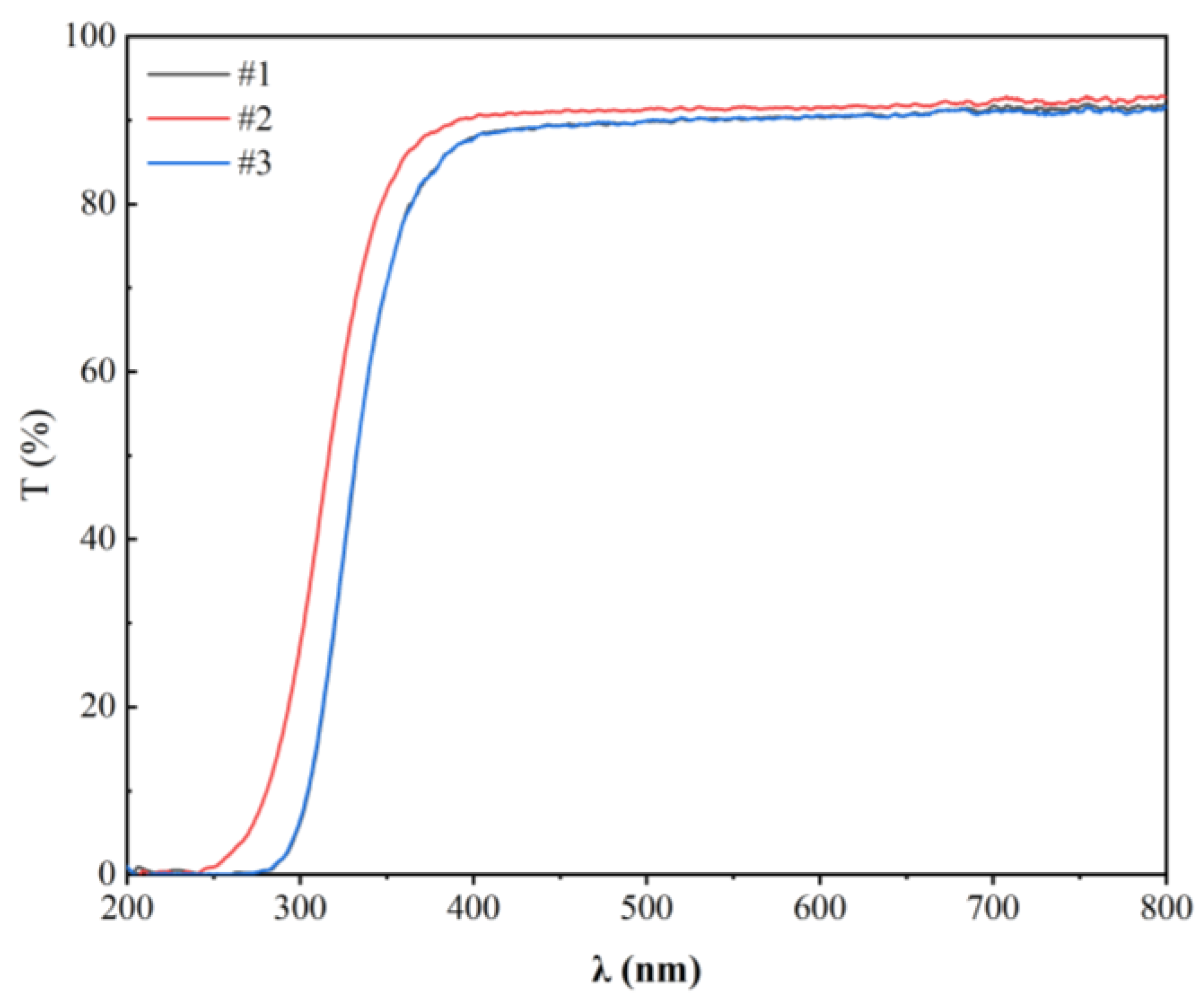
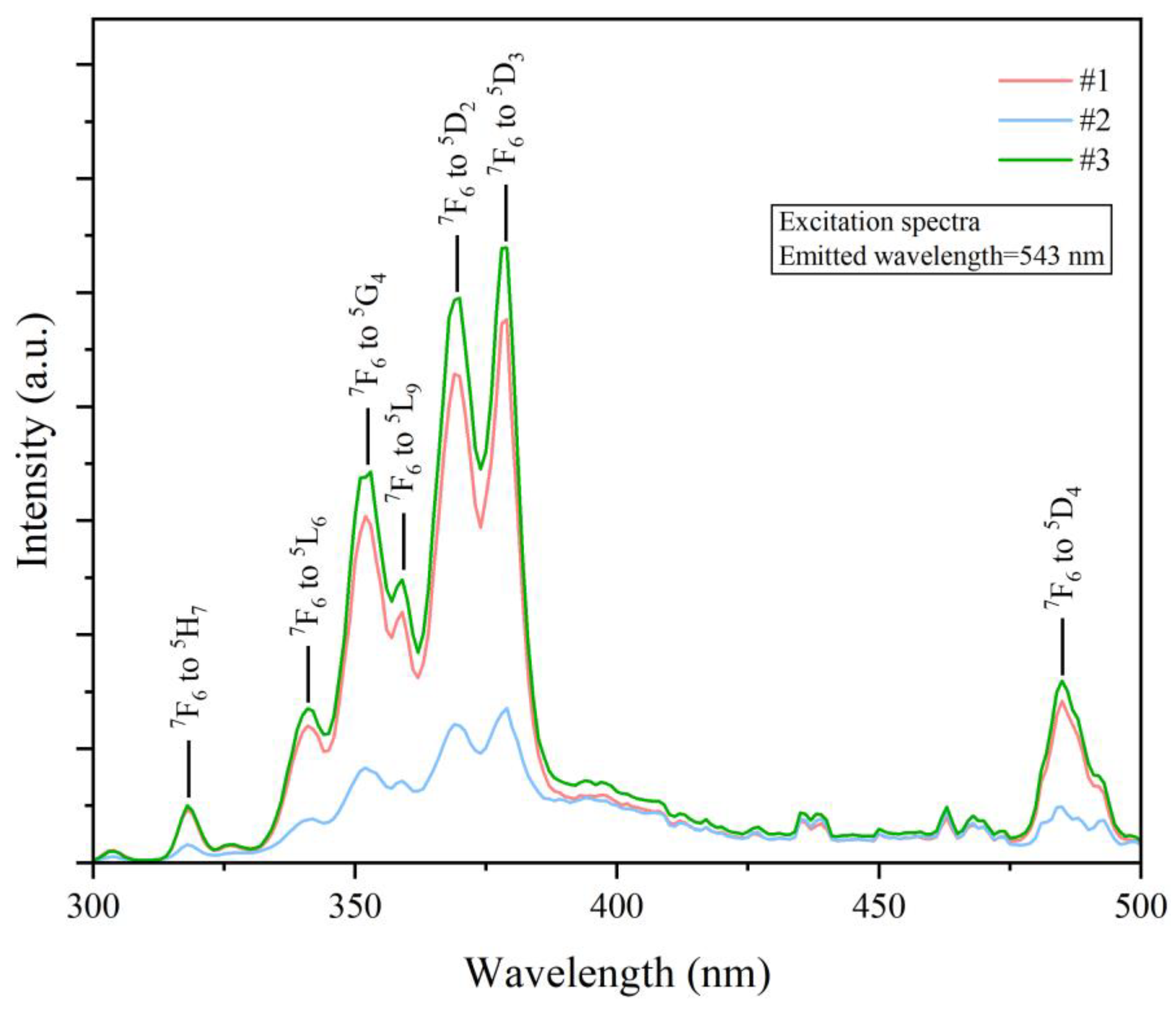
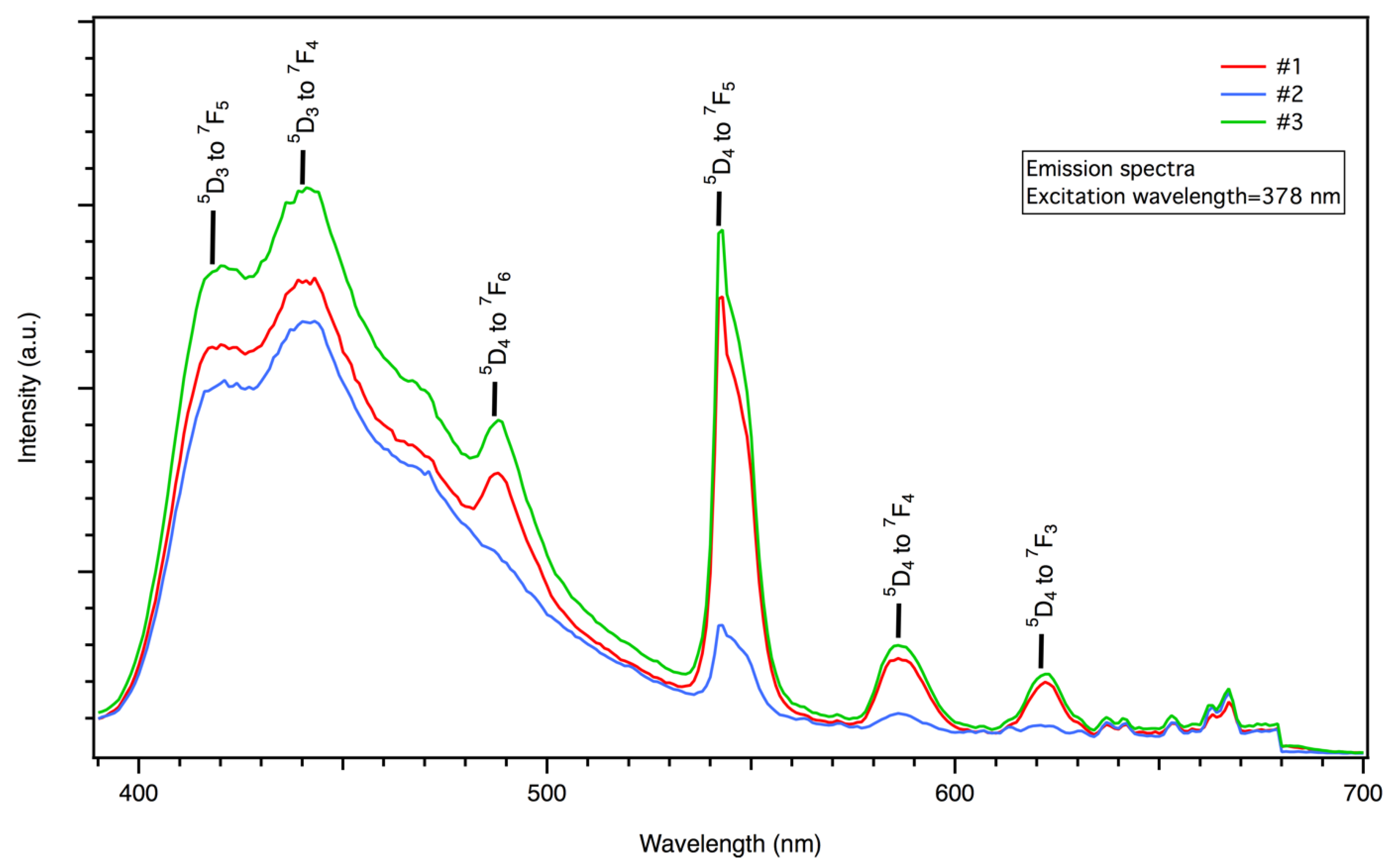
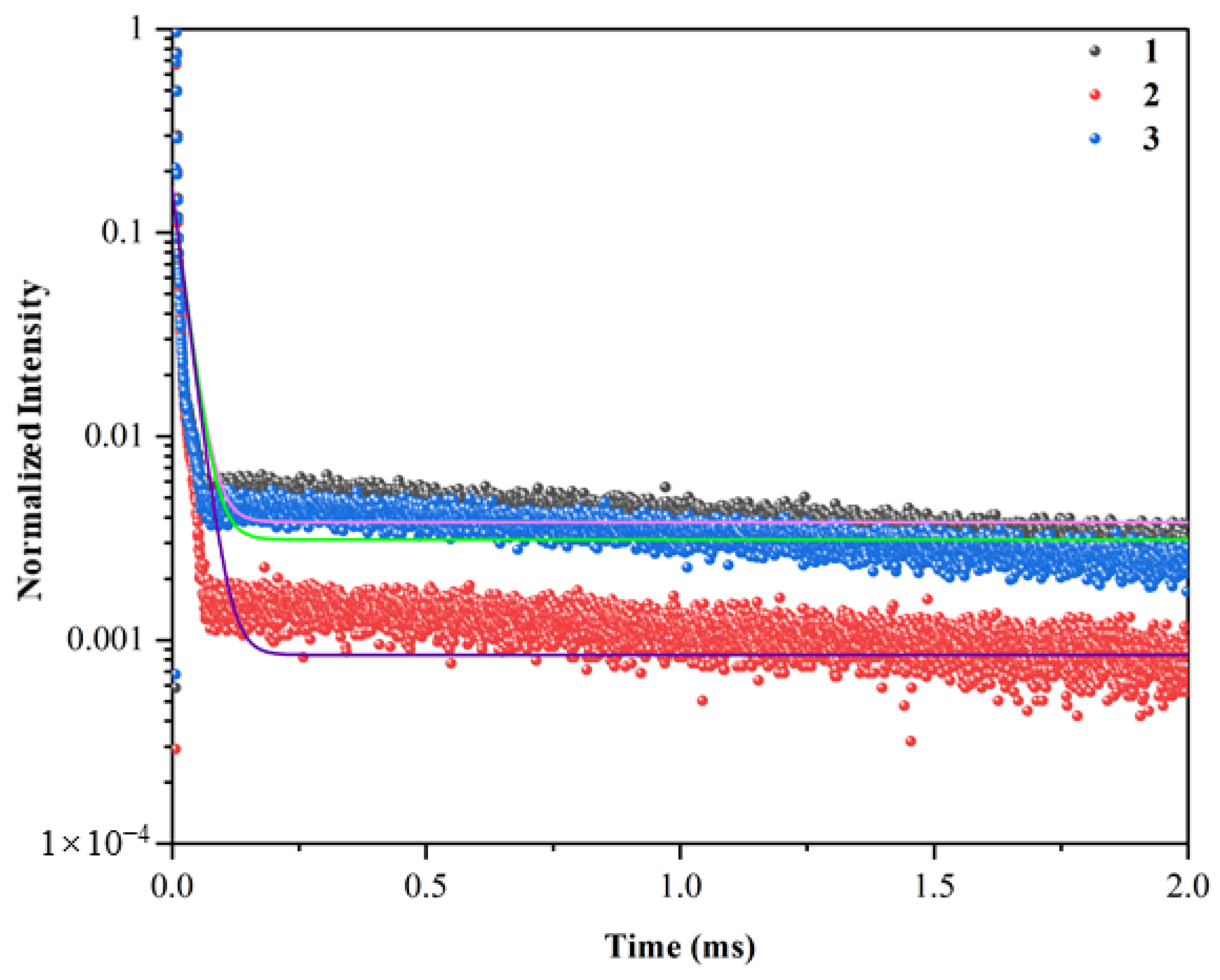
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Gupta, I.; Singh, S.; Bhagwan, S.; Singh, D. Rare earth (RE) doped phosphors and their emerging applications: A review. Ceram. Int. 2021, 47, 19282–19303. [Google Scholar] [CrossRef]
- Pisarski, W.A. Rare Earth Doped Glasses/Ceramics: Synthesis, Structure, Properties and Their Optical Applications. Materials 2022, 15, 8099. [Google Scholar] [CrossRef] [PubMed]
- Erol, E.; Vahedigharehchopogh, N.; Kıbrıslı, O.; Ersundu, M.Ç.; Ersundu, A.E. Recent progress in lanthanide-doped luminescent glasses for solid-state lighting applications-a review. J. Phys. Condens. Matter. 2021, 33, 483001. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Zhang, Y.; Geng, D.; Lian, J.; Zhang, G.; Hou, Z.; Lin, J. CaGdAlO4:Tb3+/Eu3+ as promising phosphors for full-color field emission displays. J. Mater. Chem. C 2014, 2, 9924–9933. [Google Scholar] [CrossRef]
- Kadyan, S.; Singh, S.; Sheoran, S.; Samantilleke, A.; Mari, B.; Singh, D. Optical and structural investigations of MLaAlO4:Eu3+ (M = Mg2+, Ca2+, Sr2+, and Ba2+) nanophosphors for full-color displays. J. Mater. Sci. Mater. Electron. 2020, 31, 414–422. [Google Scholar] [CrossRef]
- Yanagida, T.; Kato, T.; Nakauchi, D.; Kawaguchi, N. Fundamental aspects, recent progress and future prospects of inorganic scintillators. Jpn. J. Appl. Phys. 2023, 62, 010508. [Google Scholar] [CrossRef]
- Righini, G.; Enrichi, F.; Zur, L.; Ferrari, M. Rare-earth doped glasses and light managing in solar cells. J. Phys. Conf. Ser. 2019, 1221, 012028. [Google Scholar] [CrossRef]
- Huang, X.; Han, S.; Huang, W.; Liu, X. Enhancing solar cell efficiency: The search for luminescent materials as spectral converters. Chem. Soc. Rev. 2013, 42, 173–201. [Google Scholar] [CrossRef]
- Tao, K.; Ye, Y.; Bai, H.; Wang, S. Synthesis and luminescence characteristics of Tb3+-doped fluorophosphate glass for UV detection. J. Non-Cryst. Solids 2021, 572, 121012. [Google Scholar] [CrossRef]
- Kuro, T.; Okada, G.; Kawaguchi, N.; Fujimoto, Y.; Masai, H.; Yanagida, T. Scintillation properties of rare-earth doped NaPO3-Al(PO3)3 glasses. Opt. Mater. 2016, 62, 561–568. [Google Scholar] [CrossRef]
- Silversmith, A.J.; Boye, D.M.; Brewer, K.S.; Gillespie, C.E.; Lu, Y.; Campbell, D.L. 5D3→7FJ emission in terbium-doped sol–gel glasses. J. Lumin. 2006, 121, 14–20. [Google Scholar] [CrossRef]
- Pankratov, V.; Popov, A.; Kotlov, A.; Feldmann, C. Luminescence of nano- and macrosized LaPO4:Ce,Tb excited by synchrotron radiation. Opt. Mater. 2011, 33, 1102–1105. [Google Scholar] [CrossRef]
- Beladi-Mousavi, M.; Walder, L. Photo-Electrochemical Device Enabling Luminescence Switching of LaPO4:Ce,Tb Nanoparticle Layers. Adv. Opt. Mater. 2020, 9, 2001891. [Google Scholar]
- dos Santos, J.F.M.; Zanuto, V.S.; Soares, A.C.C.; Savi, E.; Nunes, L.A.D.O.; Baesso, M.L.; Catunda, T. Evaluating the link between blue-green luminescence and cross-relaxation processes in Tb3+-doped glasses. J. Lumin. 2021, 240, 118430. [Google Scholar] [CrossRef]
- de Graaf, D.; Stelwagen, S.; Hintzen, H.; de With, G. Tb3+ luminescence as a tool to study clustering of lanthanide ions in oxynitride glasses. J. Non-Cryst. Solids 2003, 325, 29–33. [Google Scholar] [CrossRef]
- Linganna, K.; Ju, S.; Basavapoornima, C.; Venkatramu, V.; Jayasankar, C.K. Luminescence and decay characteristics of Tb 3+-doped fluorophosphate glasses. J. Asian Ceram. Soc. 2018, 6, 82–87. [Google Scholar] [CrossRef]
- Sales, T.; Amjad, R.; Jacinto, C.; Dousti, M. Concentration dependent luminescence and cross-relaxation energy transfers in Tb3+ doped fluoroborate glasses. J. Lumin. 2019, 205, 282–286. [Google Scholar] [CrossRef]
- Kesavulu, C.; Silva, A.C.A.; Dousti, M.R.; Dantas, N.O.; de Camargo, A.; Catunda, T. Concentration effect on the spectroscopic behavior of Tb3+ ions in zinc phosphate glasses. J. Lumin. 2015, 165, 77–84. [Google Scholar] [CrossRef]
- dos Santos, J.F.M.; Astrath, N.G.C.; Baesso, M.L.; Nunes, L.A.D.O.; Catunda, T. The effect of silica content on the luminescence properties of Tb3+-doped calcium aluminosilicate glasses. J. Lumin. 2018, 202, 363–369. [Google Scholar] [CrossRef]
- Pan, Y.; Fan, Y.; Lin, H.; Zhang, D.; Xu, X.; Yao, X. Preparation and characterization of green emitting NASO:Tb3+ and red emitting NASO:Eu3+ glasses derived from ions exchanged LTA zeolite. J. Lumin. 2022, 251, 119250. [Google Scholar] [CrossRef]
- Sontakke, A.D.; Biswas, K.; Annapurna, K. Concentration-dependent luminescence of Tb3+ ions in high calcium aluminosilicate glasses. J. Lumin. 2009, 129, 1347–1355. [Google Scholar] [CrossRef]
- Juárez-Batalla, J.; Meza-Rocha, A.N.; Muñoz, H.G.; Camarillo, I.; Caldiño, U. Luminescence properties of Tb3+-doped zinc phosphate glasses for green laser application. Opt. Mater. 2016, 58, 406–411. [Google Scholar] [CrossRef]
- Linganna, K.; Sreedhar, V.B.; Jayasankar, C.K. Luminescence properties of Tb3+ ions in zinc fluorophosphate glasses for green laser applications. Mater. Res. Bull. 2015, 67, 196–200. [Google Scholar] [CrossRef]
- Sun, X.Y.; Yu, X.G.; Wang, W.F.; Li, Y.N.; Zhang, Z.J.; Zhao, J.T. Luminescent properties of Tb3+-activated B2O3–GeO2–Gd2O3 scintillating glasses. J. Non-Cryst. Solids 2013, 379, 127–130. [Google Scholar] [CrossRef]
- Żur, L. Structural and luminescence properties of Eu3+, Dy3+ and Tb3+ ions in lead germanate glasses obtained by conventional high-temperature melt-quenching technique. J. Mol. Struct. 2013, 1041, 50–54. [Google Scholar] [CrossRef]
- Kawano, N.; Kawaguchi, N.; Okada, G.; Fujimoto, Y.; Yanagida, T. Photoluminescence, scintillation and TSL properties of Tb-doped strontium aluminoborate glasses. Radiat. Meas. 2019, 124, 69–73. [Google Scholar] [CrossRef]
- Kumar, V.; Luo, Z. A Review on X-ray Excited Emission Decay Dynamics in Inorganic Scintillator Materials. Photonics 2021, 8, 71. [Google Scholar] [CrossRef]
- Zang, D.S.; Song, J.H.; Park, D.H.; Kim, Y.C.; Yoon, D.H. New fast-decaying green and red phosphors for 3D application of plasma display panels. J. Lumin. 2009, 129, 1088–1093. [Google Scholar] [CrossRef]
- Xia, F.; Liu, S.; Wang, Y.; Mao, J.; Li, X.; Wang, Y.; Chen, G. Fast and intense green emission of Tb3+ in borosilicate glass modified by Cu+. Sci. Rep. 2015, 5, 15387. [Google Scholar] [CrossRef] [PubMed]
- Teng, L.; Zhang, W.; Chen, W.; Cao, J.; Sun, X.; Guo, H. Highly efficient luminescence in bulk transparent Sr2GdF7:Tb3+ glass ceramic for potential X-ray detection. Ceram. Int. 2020, 46, 10718–10722. [Google Scholar] [CrossRef]
- Struebing, C.; Lee, G.; Wagner, B.; Kang, Z. Synthesis and luminescence properties of Tb doped LaBGeO5 and GdBGeO5 glass scintillators. J. Alloys Compd. 2016, 686, 9–14. [Google Scholar] [CrossRef] [PubMed]
- Greaves, G.N.; Meneau, F.; Sapelkin, A.; Colyer, L.M.; Ap Gwynn, I.; Wade, S.; Sankar, G. The rheology of collapsing zeolites amorphized by temperature and pressure. Nat. Mater. 2003, 2, 622–629. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Lin, H.; Xu, L.; Hong, R.; Zhang, D.; Zhou, S. White emitting aluminosilicate glass phosphors derived from Dy3+, Ag+ co-exchanged LTA zeolite. Ceram. Int. 2020, 46, 28933–28938. [Google Scholar] [CrossRef]
- Fan, Y.; Lin, H.; Liu, H.; Zhang, L.; Wang, J.; Xie, Q.; Zhou, L.; Hong, R.; Zhang, D.; Tian, Y.; et al. Ion-exchanged LTA zeolite derived nepheline phase NaAlSiO4:Eu2+ ceramic phosphor for laser illumination. Ceram. Int. 2021, 47, 30514–30522. [Google Scholar] [CrossRef]
- Bao, H.; Lin, H.; Zhang, D.; Hong, R.; Tao, C.; Han, Z.; Yin, X.; Pan, Y.; Zhou, S.; Zhang, Z.-J.; et al. SrAlSiN3:Eu~(2+) containing phosphor-in-glass: A color converter for solid state laser lighting. Opt. Mater. 2022, 126, 112169. [Google Scholar] [CrossRef]
- Cao, J.; Wang, X.; Li, X.; Wei, Y.; Chen, L.; Guo, H. Enhanced emissions in Tb3+-doped oxyfluoride scintillating glass ceramics containing KLu2F7 nano-crystals. J. Lumin. 2016, 170, 207–211. [Google Scholar] [CrossRef]
- Papynov, E.; Belov, A.; Shichalin, O.; Buravlev, I.Y.; Azon, S.; Golub, A.; Gerasimenko, A.; Parotkina, Y.; Zavjalov, A.; Tananaev, I.; et al. SrAl2Si2O8 Ceramic Matrices for 90Sr Immobilization Obtained via Spark Plasma Sintering-Reactive Synthesis. Nucl. Eng. Technol. 2021, 53, 2289–2294. [Google Scholar] [CrossRef]
- Shichalin, O.; Papynov, E.; Ivanov, N.; Balanov, M.; Dran’Kov, A.; Shkuratov, A.; Zarubina, N.; Fedorets, A.; Mayorov, V.; Lembikov, A.; et al. Study of adsorption and immobilization of Cs+, Sr2+, Co2+, Pb2+, La3+ ions on Na-Faujasite zeolite transformed in solid state matrices. Sep. Purif. Technol. 2024, 332, 125662. [Google Scholar] [CrossRef]
- Dran’Kov, A.; Shichalin, O.; Papynov, E.; Nomerovskii, A.; Mayorov, V.; Pechnikov, V.; Ivanets, A.; Buravlev, I.; Yarusova, S.; Zavjalov, A.; et al. Hydrothermal synthesis, structure and sorption performance to cesium and strontium ions of nanostructured magnetic zeolite composites. Nucl. Eng. Technol. 2022, 54, 1991–2003. [Google Scholar] [CrossRef]




| Nominal (wt%) | Experimental (wt%) | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Na | Al | Si | O | B | Tb | Na | Al | Si | O | B | Tb | Y | |
| #1 | 8.5 | 10.0 | 10.4 | 58.7 | 9.8 | 0.2 | 6.2 | 15.1 | 25.3 | 47.9 | 0 | 2.4 | 3.2 |
| #2 | 8.5 | 10.0 | 10.3 | 58.3 | 9.8 | 0.9 | 6.8 | 16.95 | 21.2 | 45.1 | 0 | 3.5 | 6.5 |
| #3 | 7.8 | 9.1 | 9.5 | 54.8 | 9.0 | 7.7 | 10.1 | 12.65 | 13.8 | 45.1 | 0 | 6.7 | 11.8 |
| Tb3+-Doped Glass Material | Composition | Decay Time (ms) | Refs. |
|---|---|---|---|
| LTA zeolite-derived boro-aluminosilicate | xTb2O3-68(Na2O-Al2O3-SiO2)–32B2O3 (x = 0.2, 1.0 and 10 extra wt%) | τ1~1.2 μs, τ2~12 μs τm = 18, 14, 17 μs | Present study |
| calcium aluminosilicate | 47.2CaO-41.3Al2O-4.1MgO-7.0SiO2-xTb4O7 (x = 0.04–15) (in wt%) and 33.2CaO-27.7Al2O3-4.1MgO-34SiO2-0.5Tb4O7 (in wt%) | 1.9, 2.3 | [14,19] |
| fluorophosphate | 44P2O5-17K2O-(29 − x) SrF2-9Al2O3-x Tb4O7 (x = 0.1–4) (in mol%) | 2.65–2.94 | [16] |
| fluoroborate | (50 − x)B2O3-20ZnF2-30BaF2-xTbF3 (x = 0.1–4.0) (in mol%) | 3.33–4.57 | [17] |
| zinc phosphate | 60P2O5-15ZnO-5Al2O3-10BaO-10PbO-xTb2O3 (in mol%) (x = 1.0–5.0) (in wt%) | 2.62–2.94 | [18] |
| LTA zeolite-derived aluminosilicate | Na+ was ion-exchanged with Tb3+ in Na12Al12Si12O48 | 2.398 | [19] |
| calcium aluminosilicate | (100 − x)(58SiO2-23CaO-5Al2O3-4MgO-0NaF in mol%)-xTb2O3 (x = 0.25–40 in wt%) | 2.32–3.38 | [20] |
| zinc phosphate | (100.0 − x)Zn(PO3)2-xTb2O3 (x = 0.6–5.0) (in mol%) | 2.76–2.97 | [22] |
| zinc fluorophosphate | 44P2O5-17K2O-9Al2O3-(29 − x)ZnF2-xTb4O7 (x = 0.1–2.0) (in mol%) | 3.12–3.78 | [23] |
| borogermanate | 25B2O3-40GeO2-(35 − x)Gd2O3-xTb2O3 (x = 0.25–16) (in mol%) | 1.0–1.8 | [24] |
| lead germanate | 45PbO-45GeO2-9.5Ga2O3-0.5Tb2O3 (in mol%) | 1.34 | [25] |
| strontium aluminoborate | 50B2O3-15Al2O3-35-xSrO-xTb4O7 (x = 0.1–5.0) (in mol%) | 2.2–2.6 | [26] |
| strontium fluoroaluminate | 70SiO2-7Al2O3-16SrF2-7GdF3-4TbF3 (in mol%) | ~3.1 | [30] |
| borogermanate | 50GeO2-25B2O3-(25 − x)La2O3/Gd2O3-xTb2O3 (x = 1–4) | 1.87–1.97 | [31] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xiao, Y.; Hou, S.; Yang, Z.; Huang, X.; Guo, Y.; Ji, S.; Huang, X.; Wang, F.; Hu, Q.; Guo, X. Green- and Blue-Emitting Tb3+-Activated Linde Type A Zeolite-Derived Boro-Aluminosilicate Glass for Deep UV Detection/Imaging. Materials 2024, 17, 671. https://doi.org/10.3390/ma17030671
Xiao Y, Hou S, Yang Z, Huang X, Guo Y, Ji S, Huang X, Wang F, Hu Q, Guo X. Green- and Blue-Emitting Tb3+-Activated Linde Type A Zeolite-Derived Boro-Aluminosilicate Glass for Deep UV Detection/Imaging. Materials. 2024; 17(3):671. https://doi.org/10.3390/ma17030671
Chicago/Turabian StyleXiao, Yongneng, Shaoyi Hou, Zhenhuai Yang, Xingxing Huang, Yuanjun Guo, Siyu Ji, Xiaochan Huang, Fengshuang Wang, Qiang Hu, and Xiaodong Guo. 2024. "Green- and Blue-Emitting Tb3+-Activated Linde Type A Zeolite-Derived Boro-Aluminosilicate Glass for Deep UV Detection/Imaging" Materials 17, no. 3: 671. https://doi.org/10.3390/ma17030671





